Abstract
The Bacillus sphaericus gene coding for penicillin V amidase, which catalyzes the hydrolysis of penicillin V to yield 6-aminopenicillanic acid and phenoxyacetic acid, has been isolated by molecular cloning in Escherichia coli. The gene is contained within a 2.2-kilobase HindIII-PstI fragment and is expressed when transferred into E. coli and Bacillus subtilis. The expression in B. subtilis carrying the recombinant plasmid is approximately two times higher than in the original B. sphaericus strain. A comparison of the purified enzyme from B. sphaericus and the expressed gene product in E. coli minicells suggests that the native enzyme consists of four identical subunits, each with a molecular weight of 35,000.
Full text
PDF
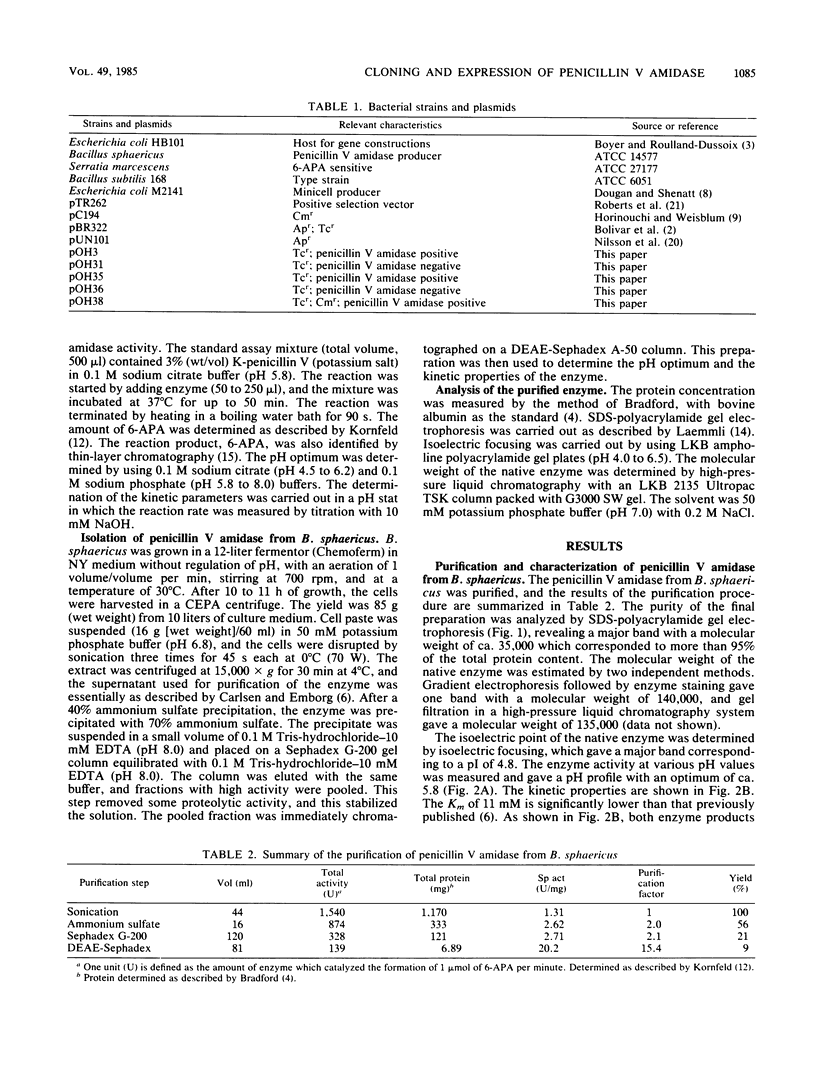
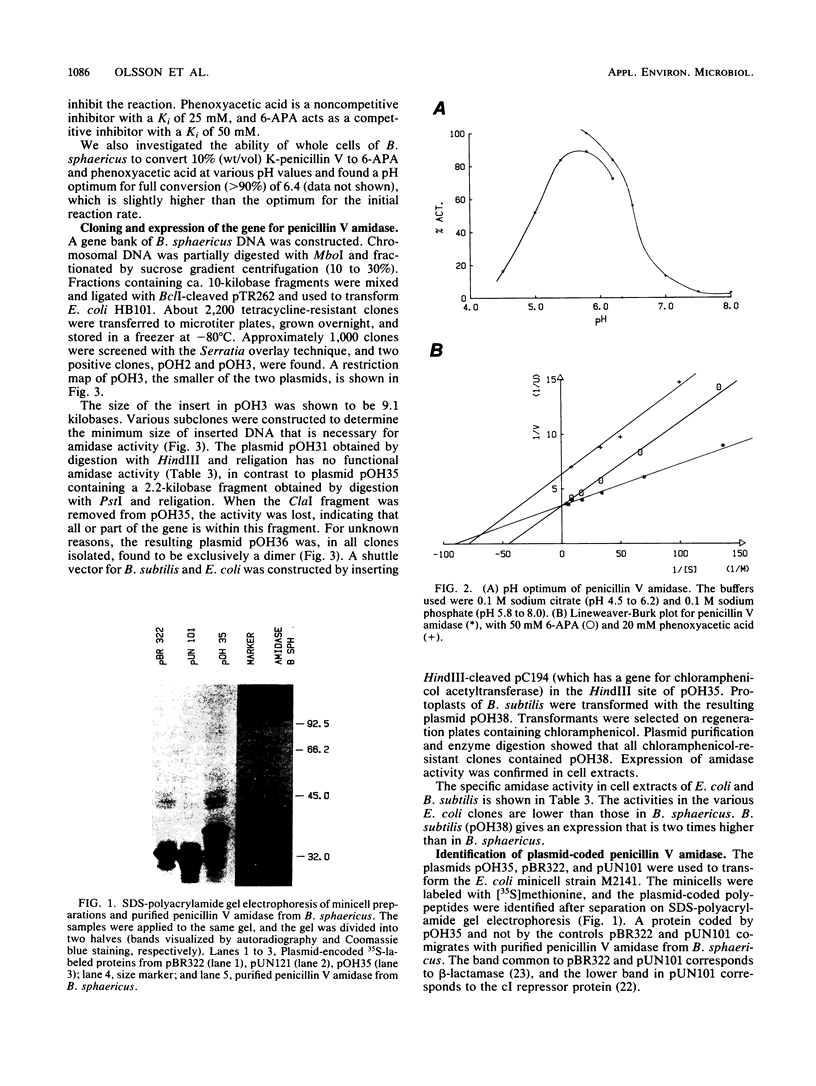
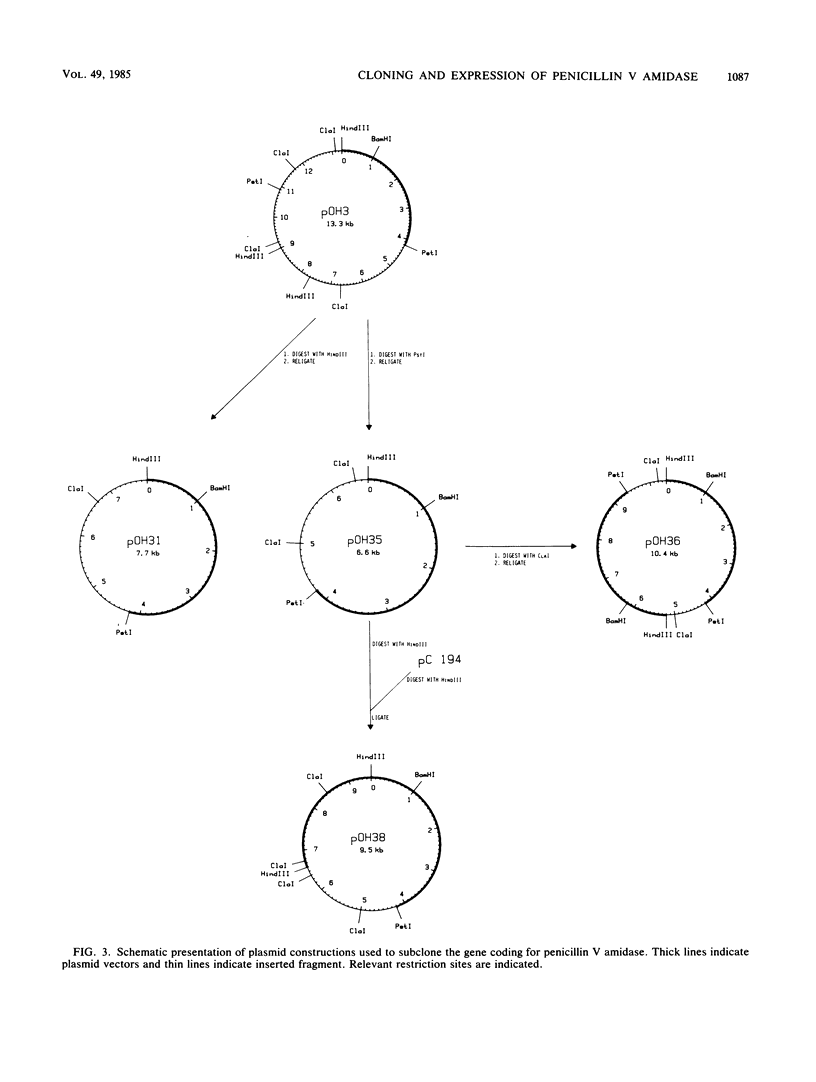


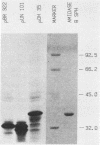
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chang S., Cohen S. N. High frequency transformation of Bacillus subtilis protoplasts by plasmid DNA. Mol Gen Genet. 1979 Jan 5;168(1):111–115. doi: 10.1007/BF00267940. [DOI] [PubMed] [Google Scholar]
- Dougan G., Sherratt D. The transposon Tn1 as a probe for studying ColE1 structure and function. Mol Gen Genet. 1977 Mar 7;151(2):151–160. doi: 10.1007/BF00338689. [DOI] [PubMed] [Google Scholar]
- Horinouchi S., Weisblum B. Nucleotide sequence and functional map of pC194, a plasmid that specifies inducible chloramphenicol resistance. J Bacteriol. 1982 May;150(2):815–825. doi: 10.1128/jb.150.2.815-825.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy N., Beutin L., Achtman M., Skurray R., Rahmsdorf U., Herrlich P. Conjugation proteins encoded by the F sex factor. Nature. 1977 Dec 15;270(5638):580–585. doi: 10.1038/270580a0. [DOI] [PubMed] [Google Scholar]
- Kieser T. Factors affecting the isolation of CCC DNA from Streptomyces lividans and Escherichia coli. Plasmid. 1984 Jul;12(1):19–36. doi: 10.1016/0147-619x(84)90063-5. [DOI] [PubMed] [Google Scholar]
- Kornfeld J. M. A new colorimetric method for the determination of 6-aminopenicillanic acid. Anal Biochem. 1978 May;86(1):118–126. doi: 10.1016/0003-2697(78)90324-x. [DOI] [PubMed] [Google Scholar]
- Kutzbach C., Rauenbusch E. Preparation and general properties of crystalline penicillin acylase from Escherichia coli ATCC 11 105. Hoppe Seylers Z Physiol Chem. 1974 Jan;355(1):45–53. doi: 10.1515/bchm2.1974.355.1.45. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Molin S., Stougaard P., Light J., Nordström M., Nordström K. Isolation and characterization of new copy mutants of plasmid R1, and identification of a polypeptide involved in copy number control. Mol Gen Genet. 1981;181(1):123–130. doi: 10.1007/BF00339015. [DOI] [PubMed] [Google Scholar]
- Morrison D. A. Transformation and preservation of competent bacterial cells by freezing. Methods Enzymol. 1979;68:326–331. doi: 10.1016/0076-6879(79)68023-0. [DOI] [PubMed] [Google Scholar]
- Nilsson B., Uhlén M., Josephson S., Gatenbeck S., Philipson L. An improved positive selection plasmid vector constructed by oligonucleotide mediated mutagenesis. Nucleic Acids Res. 1983 Nov 25;11(22):8019–8030. doi: 10.1093/nar/11.22.8019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts T. M., Swanberg S. L., Poteete A., Riedel G., Backman K. A plasmid cloning vehicle allowing a positive selection for inserted fragments. Gene. 1980 Dec;12(1-2):123–127. doi: 10.1016/0378-1119(80)90022-0. [DOI] [PubMed] [Google Scholar]
- Sauer R. T. DNA sequence of the bacteriophage gama cI gene. Nature. 1978 Nov 16;276(5685):301–302. doi: 10.1038/276301a0. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G. Nucleotide sequence of the ampicillin resistance gene of Escherichia coli plasmid pBR322. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3737–3741. doi: 10.1073/pnas.75.8.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandamme E. J., Voets J. P. Microbial penicillin acylases. Adv Appl Microbiol. 1974;17(0):311–369. doi: 10.1016/s0065-2164(08)70563-x. [DOI] [PubMed] [Google Scholar]