Abstract
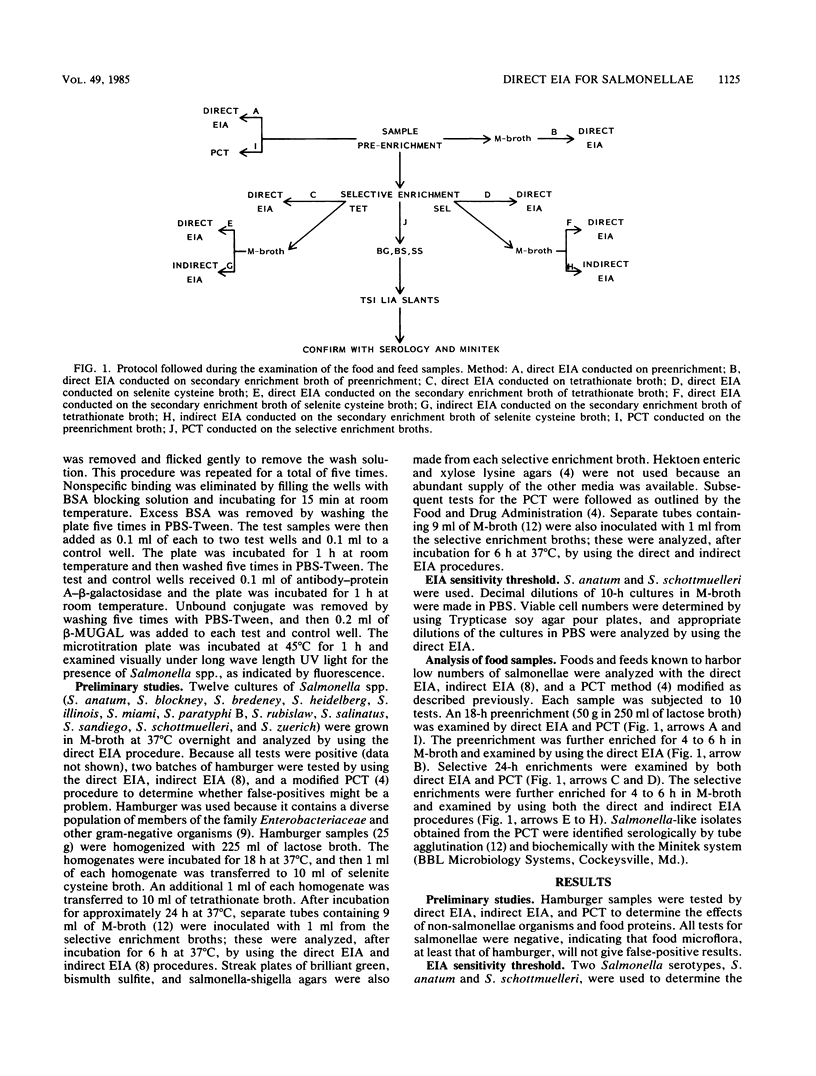
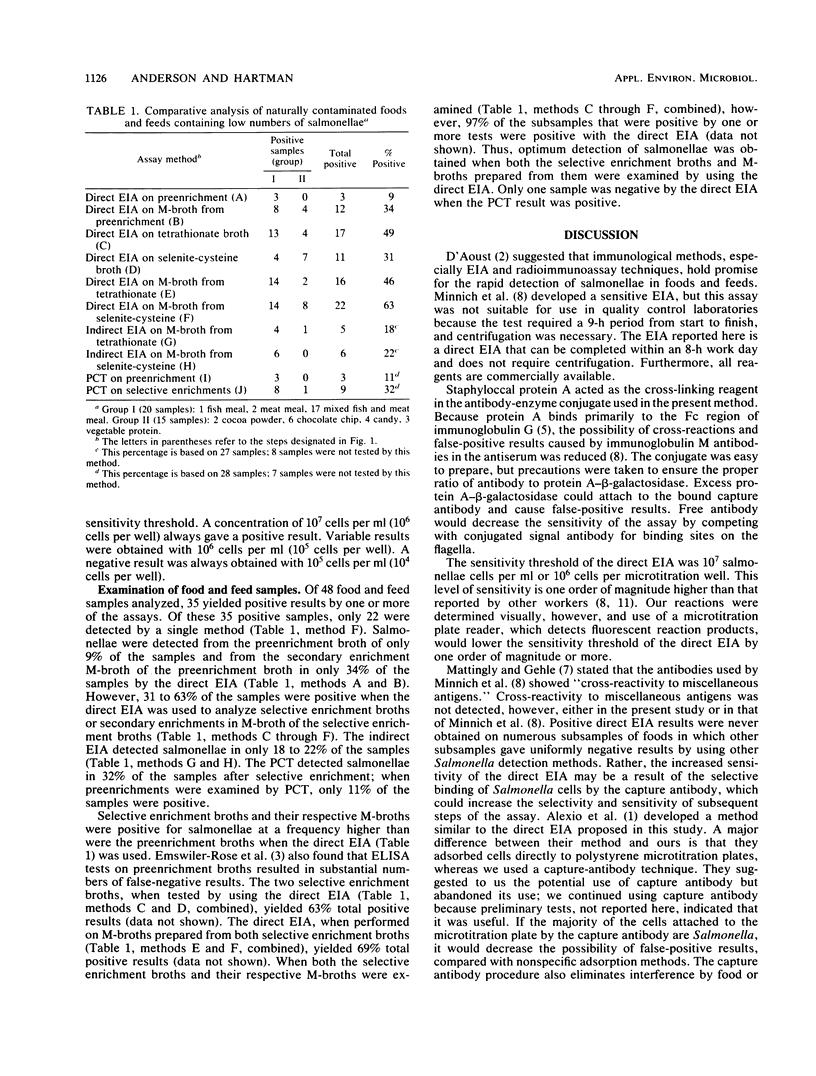
A direct enzyme immunoassay (EIA) with polyclonal antibodies was developed for detecting salmonellae in foods and feeds. Salmonella cells were attached firmly to the wells of polystyrene microtitration plates with a capture-antibody technique. Spicer-Edwards anti-H immunoglobulin G was bound to protein A-beta-D-galactosidase to serve as the signal; 4-methylumbelliferyl-beta-D-galactoside was used as the substrate. The sensitivity threshold was 10(7) cells per ml. Direct EIA, indirect EIA, and pure-culture techniques were compared by using 48 samples of naturally contaminated foods and feeds. The direct EIA was more sensitive than the indirect EIA or pure-culture technique. Food samples were analyzed within 3 working days, and 32 samples were tested simultaneously in a single 96-well microtitration plate. False-positive or false-negative results did not pose a problem. This direct EIA is sensitive, rapid, and amenable to automation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Goding J. W. Use of staphylococcal protein A as an immunological reagent. J Immunol Methods. 1978;20:241–253. doi: 10.1016/0022-1759(78)90259-4. [DOI] [PubMed] [Google Scholar]
- Krysinski E. P., Heimsch R. C. Use of enzyme-labeled antibodies to detect Salmonella in foods. Appl Environ Microbiol. 1977 Apr;33(4):947–954. doi: 10.1128/aem.33.4.947-954.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnich S. A., Hartman P. A., Heimsch R. C. Enzyme immunoassay for detection of Salmonellae in foods. Appl Environ Microbiol. 1982 Apr;43(4):877–893. doi: 10.1128/aem.43.4.877-893.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng L. K., Stiles M. E. Enterobacteriaceae in ground meats. Can J Microbiol. 1978 Dec;24(12):1574–1582. doi: 10.1139/m78-252. [DOI] [PubMed] [Google Scholar]
- Rigby C. E. Enzyme-linked immunosorbent assay for detection of Salmonella lipopolysaccharide in poultry specimens. Appl Environ Microbiol. 1984 Jun;47(6):1327–1330. doi: 10.1128/aem.47.6.1327-1330.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison B. J., Pretzman C. I., Mattingly J. A. Enzyme immunoassay in which a myeloma protein is used for detection of salmonellae. Appl Environ Microbiol. 1983 Jun;45(6):1816–1821. doi: 10.1128/aem.45.6.1816-1821.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperber W. H., Deibel R. H. Accelerated procedure for Salmonella detection in dried foods and feeds involving only borth cultures and serological reactions. Appl Microbiol. 1969 Apr;17(4):533–539. doi: 10.1128/am.17.4.533-539.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker J. W., Heusser C. H. Methods for binding cells to plastic: application to a solid-phase radioimmunoassay for cell-surface antigens. J Immunol Methods. 1979;26(1):87–95. doi: 10.1016/0022-1759(79)90044-9. [DOI] [PubMed] [Google Scholar]



