Abstract
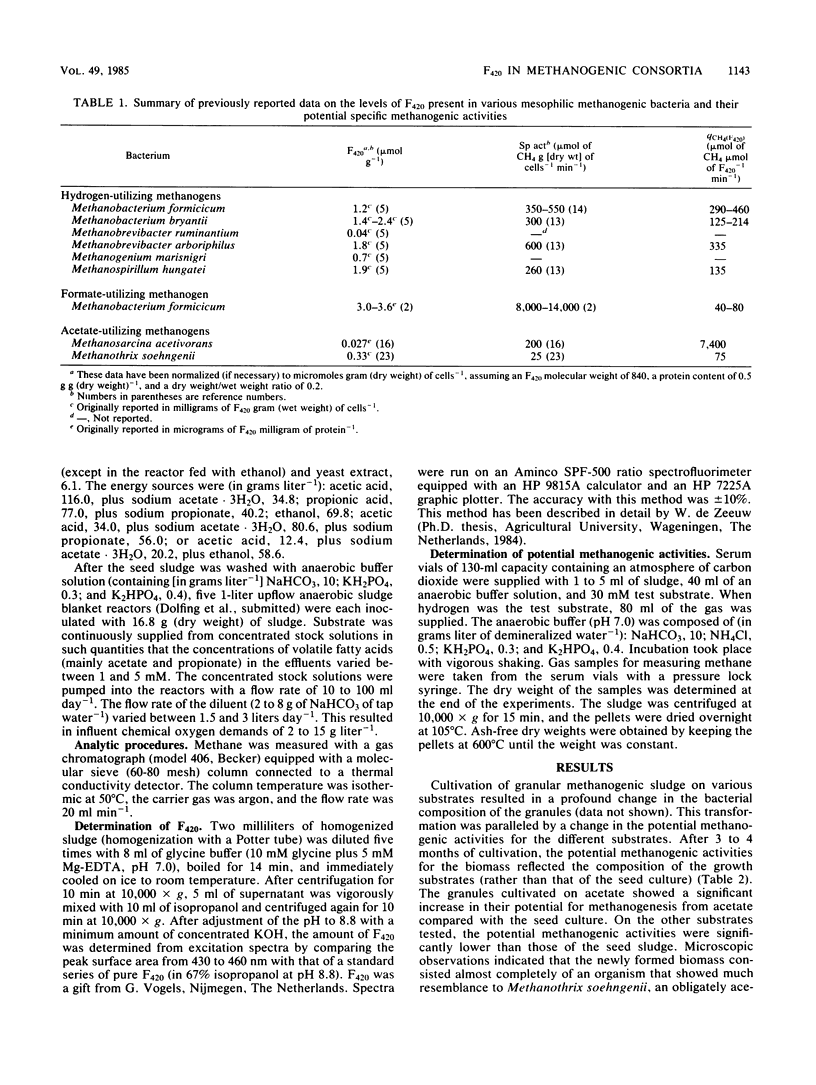
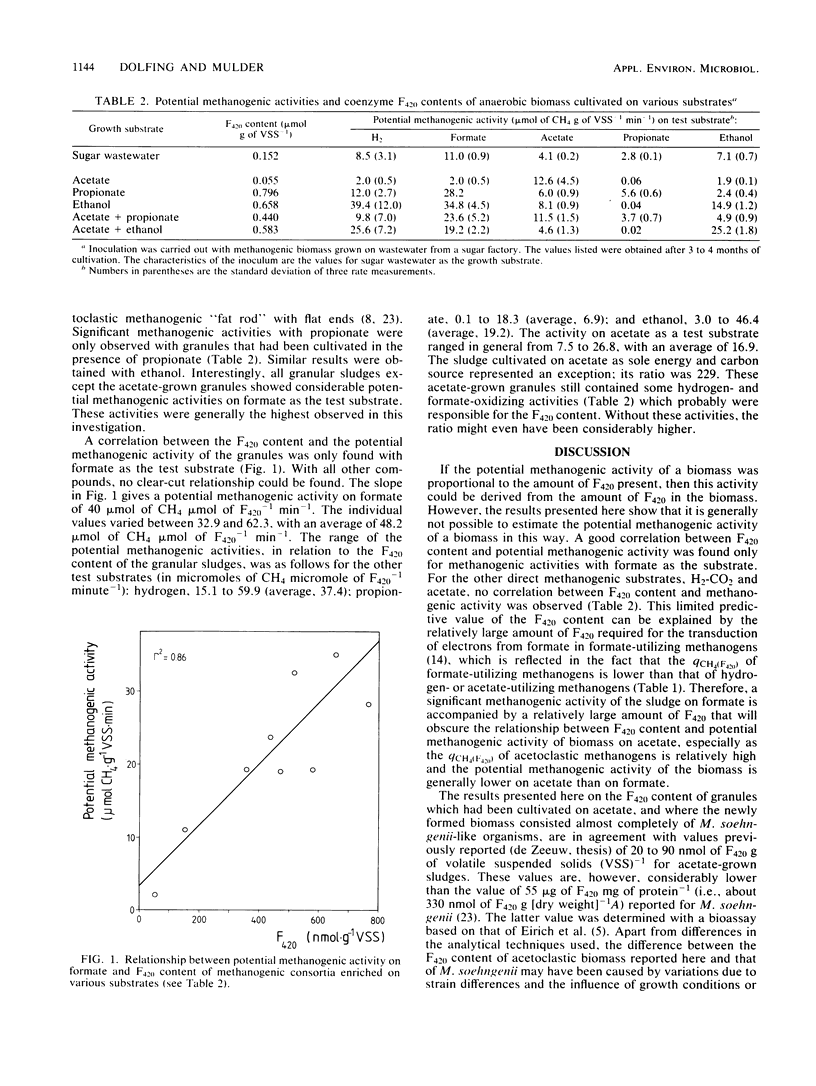
The coenzyme F420 content of granular sludge grown on various substrates and substrate combinations was measured, and the potential of the sludge to form methane (maximum specific methane production rate) from hydrogen, formate, acetate, propionate, and ethanol was determined. The F420 content varied between 55 nmol g of volatile suspended solids (VSS)−1 for sludge grown on acetate and 796 nmol g of VSS−1 for sludge grown on propionate. The best correlation was found between the F420 content and the potential activity for methane formation from formate; almost no correlation, however, was found with acetate as the test substrate. The ratio between the potential methanogenic activities (qch4) of sludges grown on various substrates and their F420 content was in general highest for formate (48.2 μmol of CH4 μmol of F420−1 min−1) and lowest for propionate (6.9 μmol of CH4 μmol of F420−1 min−1) as test substrates. However, acetate-grown granular sludge with acetate as test substrate showed the highest ratio, namely, 229 μmol of CH4 μmol of F420−1 min−1. The data presented indicate that the F420 content of methanogenic consortia can be misleading for the assessment of their potential acetoclastic methanogenic activity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cheeseman P., Toms-Wood A., Wolfe R. S. Isolation and properties of a fluorescent compound, factor 420 , from Methanobacterium strain M.o.H. J Bacteriol. 1972 Oct;112(1):527–531. doi: 10.1128/jb.112.1.527-531.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eirich L. D., Vogels G. D., Wolfe R. S. Distribution of coenzyme F420 and properties of its hydrolytic fragments. J Bacteriol. 1979 Oct;140(1):20–27. doi: 10.1128/jb.140.1.20-27.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eirich L. D., Vogels G. D., Wolfe R. S. Proposed structure for coenzyme F420 from Methanobacterium. Biochemistry. 1978 Oct 31;17(22):4583–4593. doi: 10.1021/bi00615a002. [DOI] [PubMed] [Google Scholar]
- Horridge G. A., Tamm S. L. Critical point drying for scanning electron microscopic sthdy of ciliary motion. Science. 1969 Feb 21;163(3869):817–818. doi: 10.1126/science.163.3869.817. [DOI] [PubMed] [Google Scholar]
- Jones J. B., Stadtman T. C. Reconstitution of a formate-NADP+ oxidoreductase from formate dehydrogenase and a 5-deazaflavin-linked NADP+ reductase isolated from Methanococcus vannielii. J Biol Chem. 1980 Feb 10;255(3):1049–1053. [PubMed] [Google Scholar]
- Lovley D. R., Klug M. J. Intermediary metabolism of organic matter in the sediments of a eutrophic lake. Appl Environ Microbiol. 1982 Mar;43(3):552–560. doi: 10.1128/aem.43.3.552-560.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer N. L., Ferry J. G. Metabolism of formate in Methanobacterium formicicum. J Bacteriol. 1980 Jun;142(3):800–807. doi: 10.1128/jb.142.3.800-807.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. H., Mah R. A. Kinetics of acetate metabolism during sludge digestion. Appl Microbiol. 1966 May;14(3):368–371. doi: 10.1128/am.14.3.368-371.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowers K. R., Baron S. F., Ferry J. G. Methanosarcina acetivorans sp. nov., an Acetotrophic Methane-Producing Bacterium Isolated from Marine Sediments. Appl Environ Microbiol. 1984 May;47(5):971–978. doi: 10.1128/aem.47.5.971-978.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng S. F., Wolfe R. S., Bryant M. P. Factor 420-dependent pyridine nucleotide-linked hydrogenase system of Methanobacterium ruminantium. J Bacteriol. 1975 Jan;121(1):184–191. doi: 10.1128/jb.121.1.184-191.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzing S. F., Bryant M. P., Wolfe R. S. Factor 420-dependent pyridine nucleotide-linked formate metabolism of Methanobacterium ruminantium. J Bacteriol. 1975 Jan;121(1):192–196. doi: 10.1128/jb.121.1.192-196.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Beelen P., Geerts W. J., Pol A., Vogels G. D. Quantification of coenzymes and related compounds from methanogenic bacteria by high-performance liquid chromatography. Anal Biochem. 1983 Jun;131(2):285–290. doi: 10.1016/0003-2697(83)90171-9. [DOI] [PubMed] [Google Scholar]
- Zehnder A. J., Huser B. A., Brock T. D., Wuhrmann K. Characterization of an acetate-decarboxylating, non-hydrogen-oxidizing methane bacterium. Arch Microbiol. 1980 Jan;124(1):1–11. doi: 10.1007/BF00407022. [DOI] [PubMed] [Google Scholar]
- Zeikus J. G., Fuchs G., Kenealy W., Thauer R. K. Oxidoreductases involved in cell carbon synthesis of Methanobacterium thermoautotrophicum. J Bacteriol. 1977 Nov;132(2):604–613. doi: 10.1128/jb.132.2.604-613.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]



