Abstract
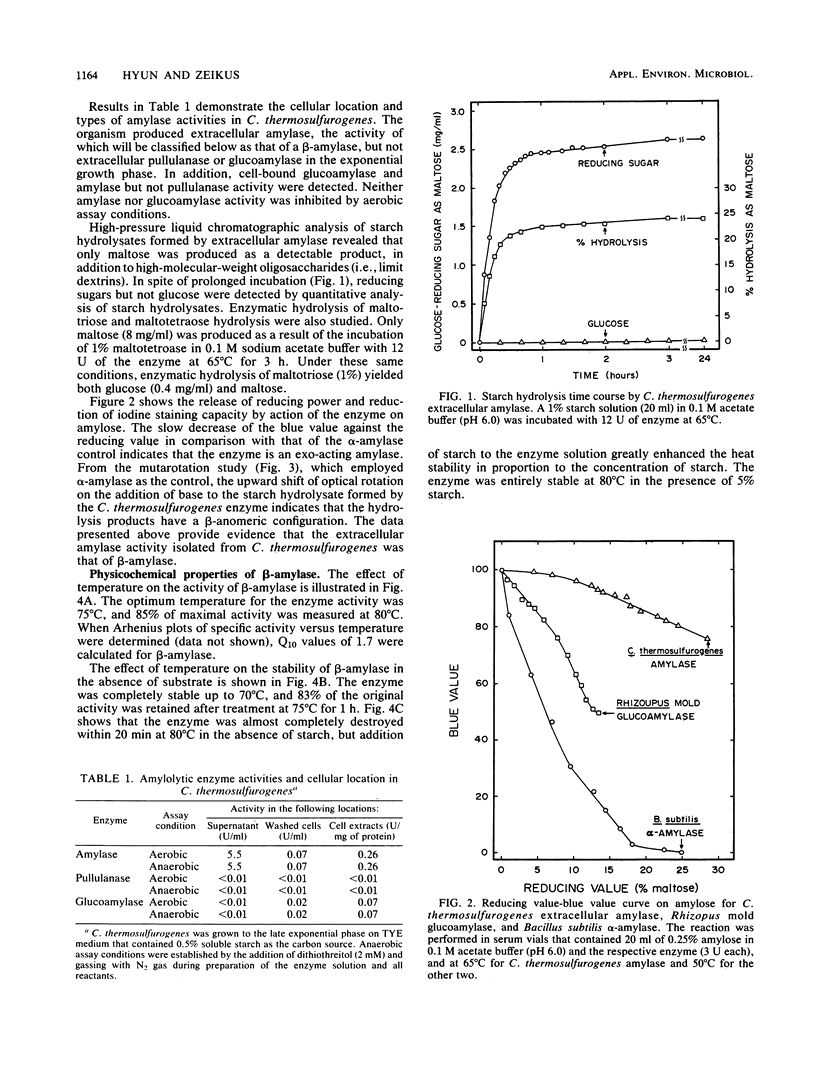
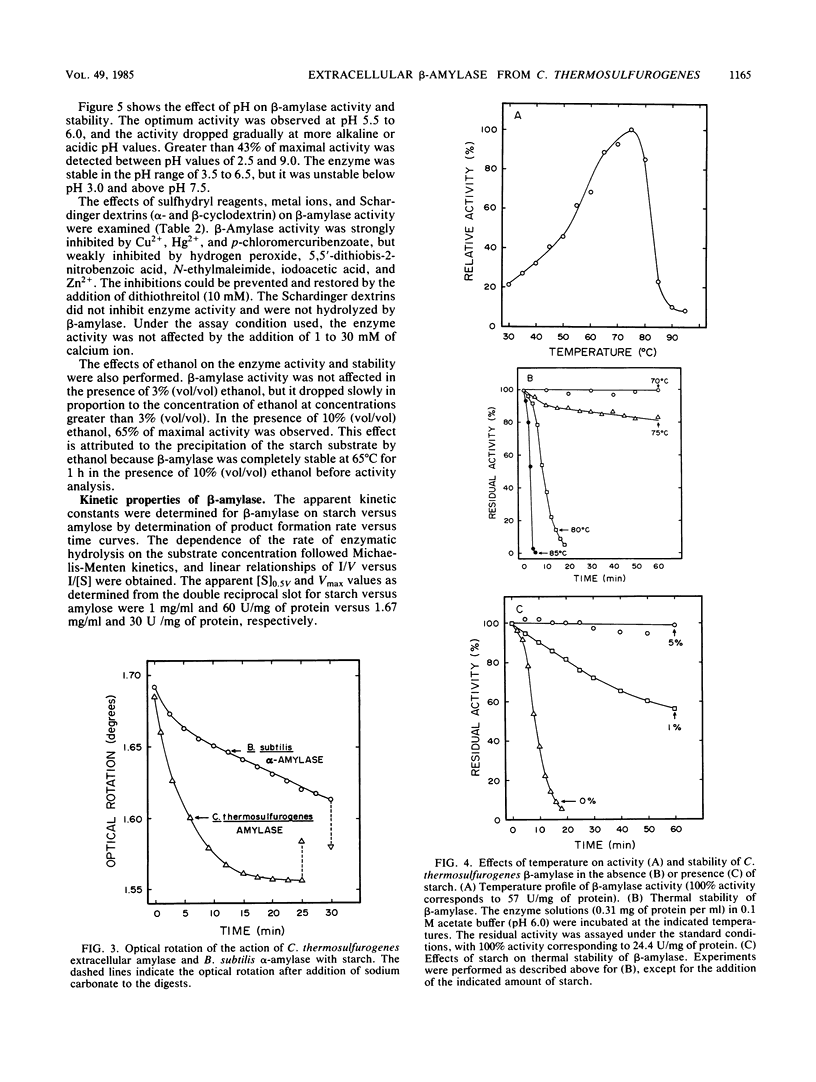
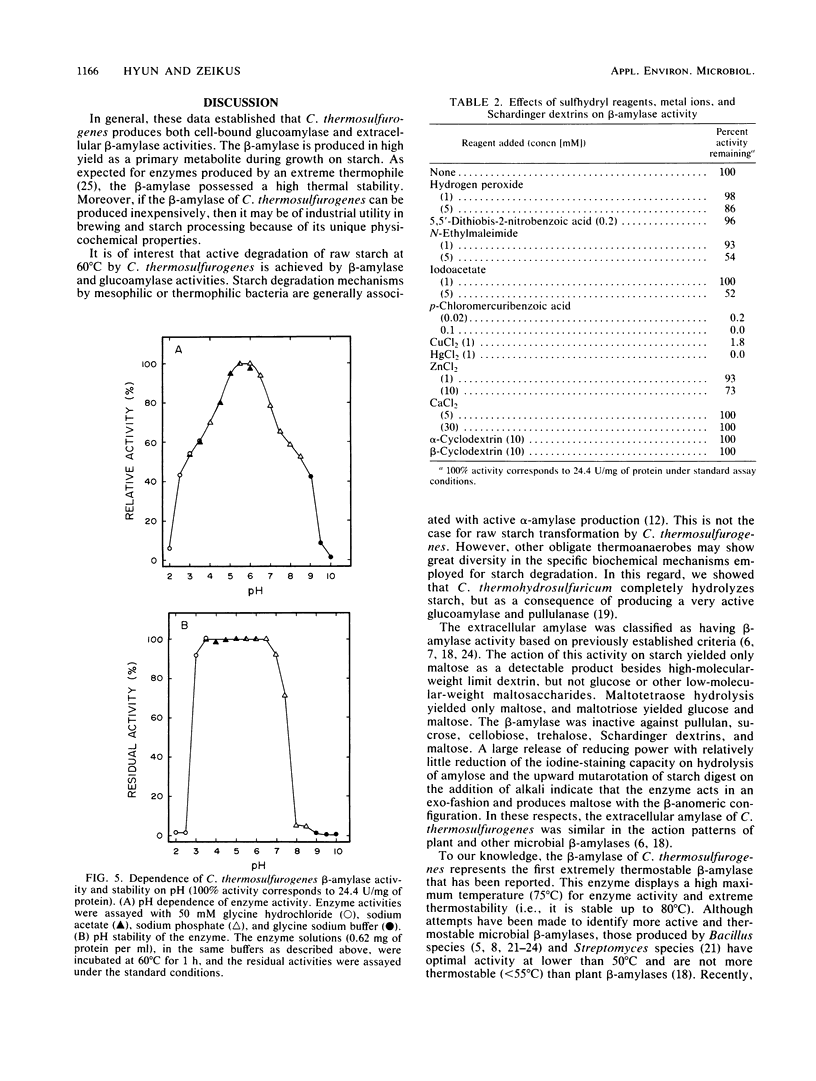
Clostridium thermosulfurogenes, an anaerobic bacterium which ferments starch into ethanol at 62°C, produced an active extracellular amylase and contained intracellular glucoamylase but not pullulanase activity. The extracellular amylase was purified 2.4-fold, and its general physicochemical and catalytic properties were examined. The extracellular amylase was characterized as a β-amylase (1,4-α-d-glucan maltohydrolase) based on demonstration of exocleavage activity and the production of maltose with a β-anomeric configuration from starch. The β-amylase activity was stable and optimally active at 80 and 75°C, respectively. The pH optimum for activity and the pH stability range was 5.5 to 6 and 3.5 to 6.5, respectively. The apparent [S]0.5V and Vmax for β-amylase activity on starch was 1 mg/ml and 60 U/mg of protein. Similar to described β-amylase, the enzyme was inhibited by p-chloromercuribenzoate, Cu2+, and Hg2+; however, α- and β-cyclodextrins were not competitive inhibitors. The β-amylase was active and stable in the presence of air or 10% (vol/vol) ethanol. The β-amylase and glucoamylase activities enabled the organism to actively ferment raw starch in the absence of significant pullulanase or α-amylase activity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hyun H. H., Zeikus J. G. General Biochemical Characterization of Thermostable Pullulanase and Glucoamylase from Clostridium thermohydrosulfuricum. Appl Environ Microbiol. 1985 May;49(5):1168–1173. doi: 10.1128/aem.49.5.1168-1173.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun H. H., Zeikus J. G., Longin R., Millet J., Ryter A. Ultrastructure and extreme heat resistance of spores from thermophilic Clostridium species. J Bacteriol. 1983 Dec;156(3):1332–1337. doi: 10.1128/jb.156.3.1332-1337.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun H. H., Zeikus J. G. Simultaneous and Enhanced Production of Thermostable Amylases and Ethanol from Starch by Cocultures of Clostridium thermosulfurogenes and Clostridium thermohydrosulfuricum. Appl Environ Microbiol. 1985 May;49(5):1174–1181. doi: 10.1128/aem.49.5.1174-1181.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lamed R. J., Zeikus J. G. Novel NADP-linked alcohol--aldehyde/ketone oxidoreductase in thermophilic ethanologenic bacteria. Biochem J. 1981 Apr 1;195(1):183–190. doi: 10.1042/bj1950183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng T. K., Zeikus J. G. Comparison of Extracellular Cellulase Activities of Clostridium thermocellum LQRI and Trichoderma reesei QM9414. Appl Environ Microbiol. 1981 Aug;42(2):231–240. doi: 10.1128/aem.42.2.231-240.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng T. K., Zeikus J. G. Purification and characterization of an endoglucanase (1,4-beta-D-glucan glucanohydrolase) from Clostridium thermocellum. Biochem J. 1981 Nov 1;199(2):341–350. doi: 10.1042/bj1990341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obi S. K., Odibo F. J. Partial Purification and Characterization of a Thermostable Actinomycete beta-Amylase. Appl Environ Microbiol. 1984 Mar;47(3):571–575. doi: 10.1128/aem.47.3.571-575.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G., Ben-Bassat A., Hegge P. W. Microbiology of methanogenesis in thermal, volcanic environments. J Bacteriol. 1980 Jul;143(1):432–440. doi: 10.1128/jb.143.1.432-440.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]



