Abstract
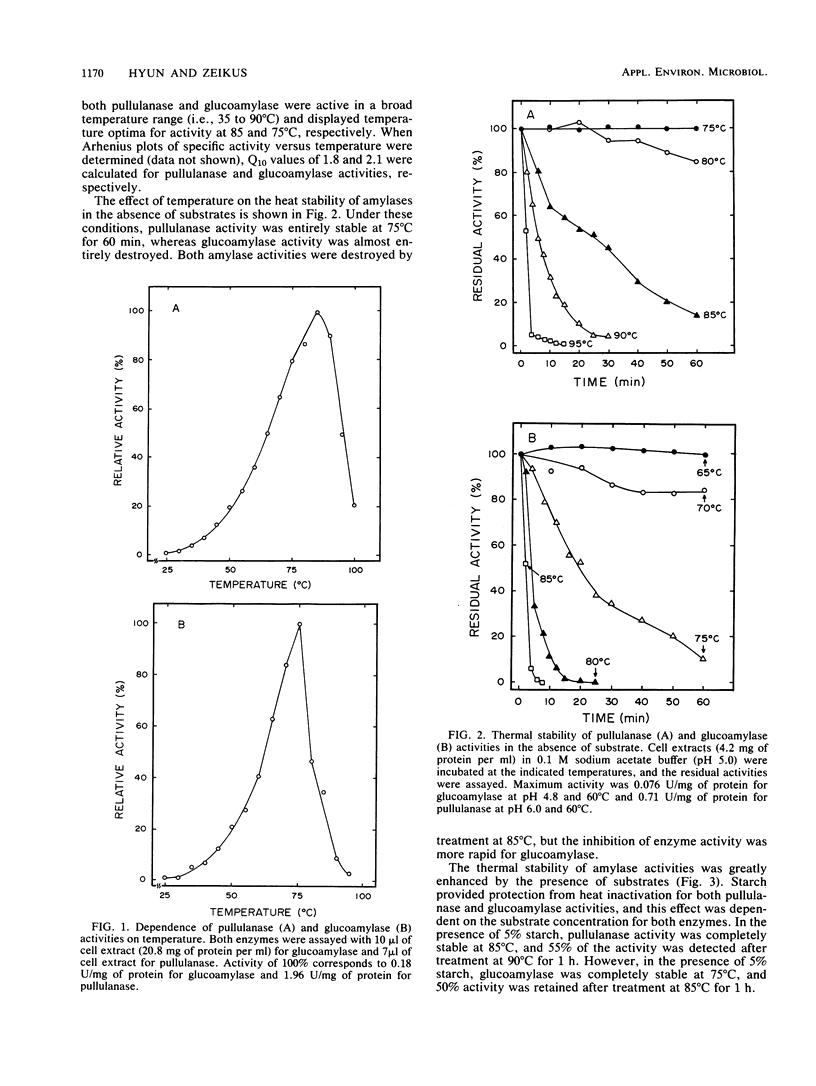
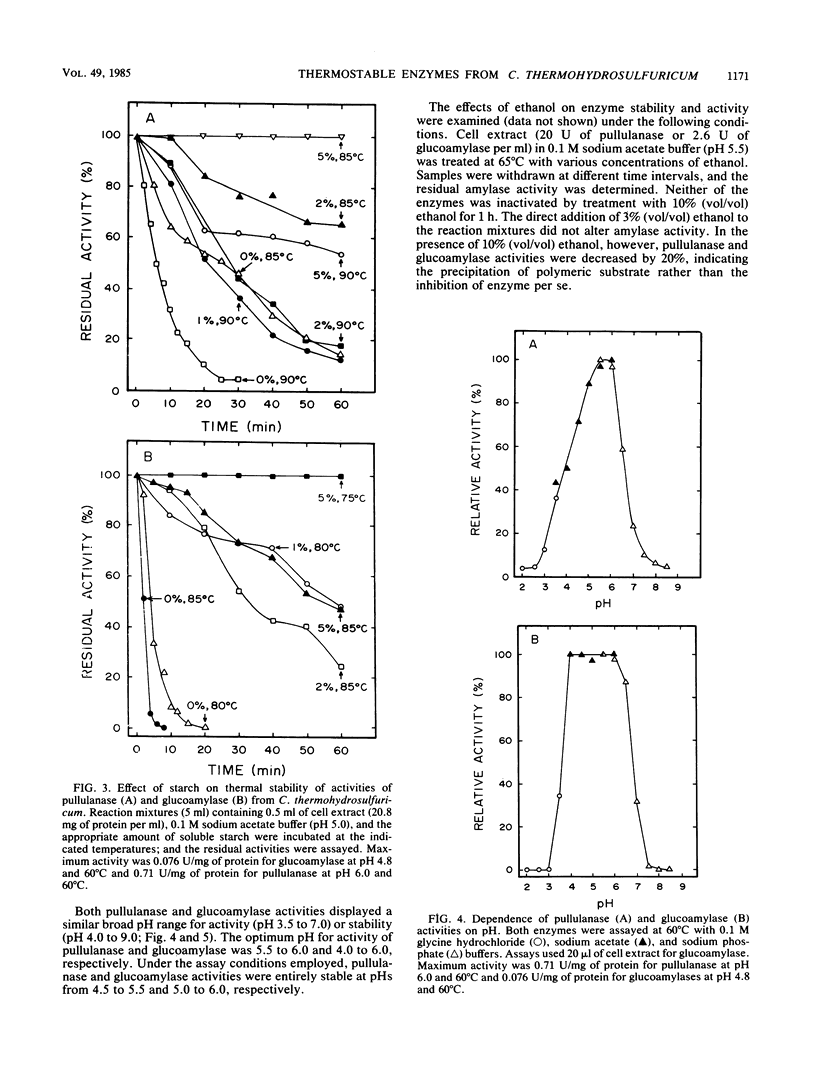
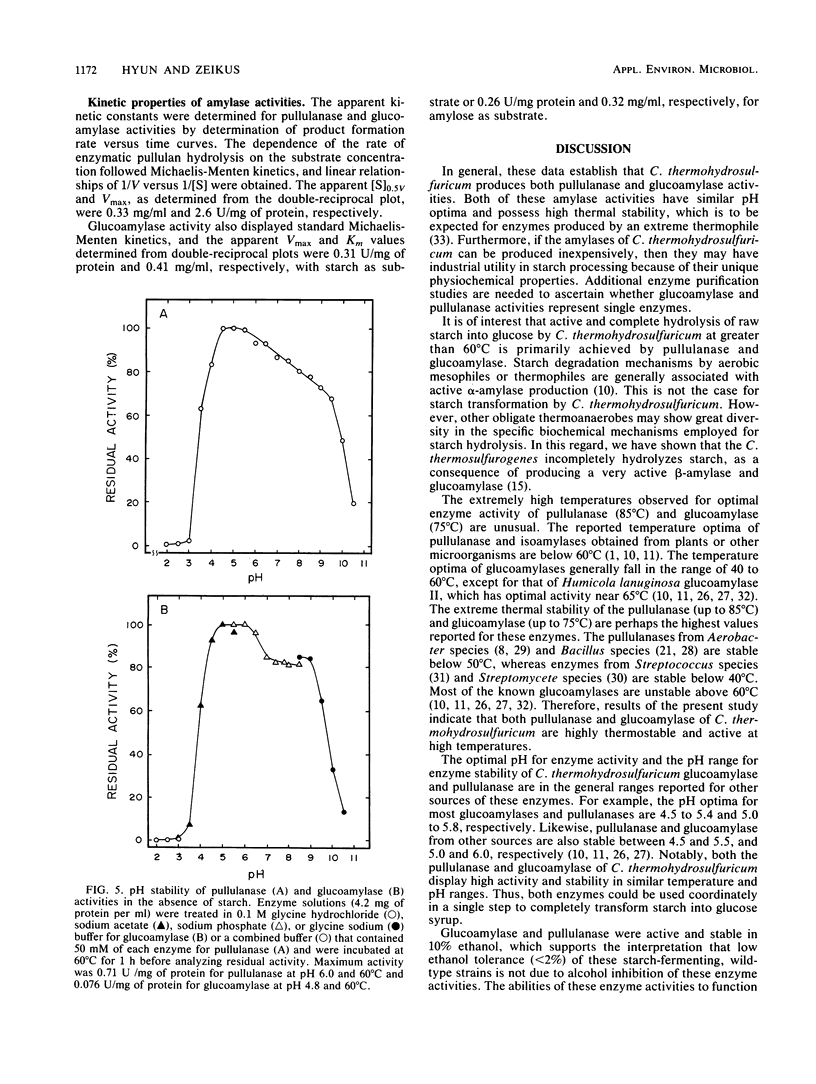
Cell extracts of Clostridium thermohydrosulfuricum, an anaerobic bacterium which ferments starch into ethanol at 65°C, contained both pullulanase and glucoamylase activities. The general physiochemical and catalytic properties of these enzyme activities were compared. Pullulanase and glucoamylase activities were stable and optimally active at 85 and 75°C, respectively. The pH optima for activity and pH stability ranges were, respectively, 5.5 to 6 and 4.5 to 5.5 for pullulanase and 4 to 6 and 5 to 6 for glucoamylase. The apparent [S]0.5v and Vmax for pullulanase activity on pullulan were 0.33 mg/ml and 2.6 U/mg of protein. The apparent [S]0.5v and Vmax for glucoamylase activity on starch were of 0.41 mg/ml and 0.31 U/mg of protein. These enzymes were active and stable in the presence of air or 10% (vol/vol) ethanol. These enzyme activities allowed the organism to actively degrade raw starch into glucose in the absence of significant α-amylase activity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Eisele B., Rasched I. R., Wallenfels K. Molecular characterization of pullulanase from Aerobacter aerogenes. Eur J Biochem. 1972 Mar 15;26(1):62–67. doi: 10.1111/j.1432-1033.1972.tb01739.x. [DOI] [PubMed] [Google Scholar]
- Hyun H. H., Zeikus J. G. General Biochemical Characterization of Thermostable Extracellular beta-Amylase from Clostridium thermosulfurogenes. Appl Environ Microbiol. 1985 May;49(5):1162–1167. doi: 10.1128/aem.49.5.1162-1167.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun H. H., Zeikus J. G., Longin R., Millet J., Ryter A. Ultrastructure and extreme heat resistance of spores from thermophilic Clostridium species. J Bacteriol. 1983 Dec;156(3):1332–1337. doi: 10.1128/jb.156.3.1332-1337.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun H. H., Zeikus J. G. Simultaneous and Enhanced Production of Thermostable Amylases and Ethanol from Starch by Cocultures of Clostridium thermosulfurogenes and Clostridium thermohydrosulfuricum. Appl Environ Microbiol. 1985 May;49(5):1174–1181. doi: 10.1128/aem.49.5.1174-1181.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nakamura N., Watanabe K., Horikoshi K. Purification and some properties of alkaline pullulanase from a strain of bacillus no. 202-1, an alkalophilic microorganism. Biochim Biophys Acta. 1975 Jul 27;397(1):188–193. doi: 10.1016/0005-2744(75)90192-8. [DOI] [PubMed] [Google Scholar]
- Ng T. K., Ben-Bassat A., Zeikus J. G. Ethanol Production by Thermophilic Bacteria: Fermentation of Cellulosic Substrates by Cocultures of Clostridium thermocellum and Clostridium thermohydrosulfuricum. Appl Environ Microbiol. 1981 Jun;41(6):1337–1343. doi: 10.1128/aem.41.6.1337-1343.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Inokuchi N., Irie M. Purification and characterization of a glucoamylase from Aspergillus saitoi. J Biochem. 1981 Jan;89(1):125–134. doi: 10.1093/oxfordjournals.jbchem.a133172. [DOI] [PubMed] [Google Scholar]
- Walker G. J. Metabolism of the reserve polysaccharide of Streptococcus mitis. Some properties of a pullulanase. Biochem J. 1968 Jun;108(1):33–40. doi: 10.1042/bj1080033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. J., Ingledew W. M. Isolation and characterization of Schwanniomyces alluvius amylolytic enzymes. Appl Environ Microbiol. 1982 Aug;44(2):301–307. doi: 10.1128/aem.44.2.301-307.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G., Ben-Bassat A., Hegge P. W. Microbiology of methanogenesis in thermal, volcanic environments. J Bacteriol. 1980 Jul;143(1):432–440. doi: 10.1128/jb.143.1.432-440.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]



