Abstract
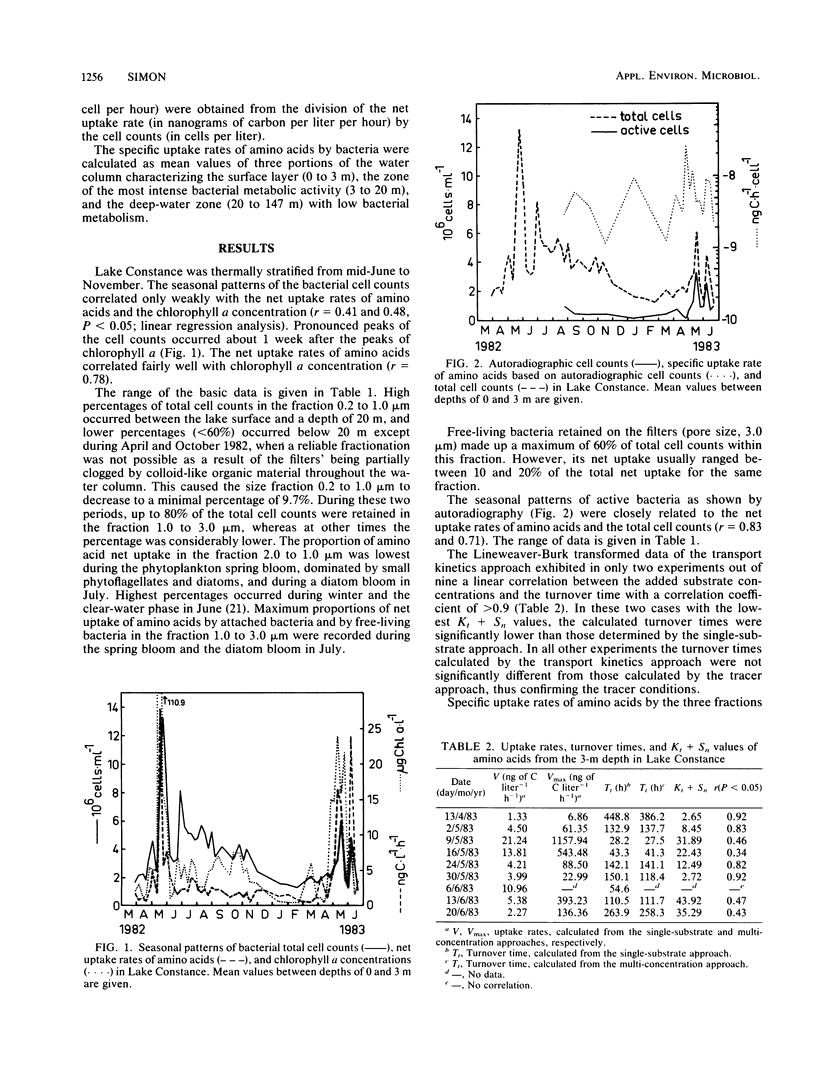
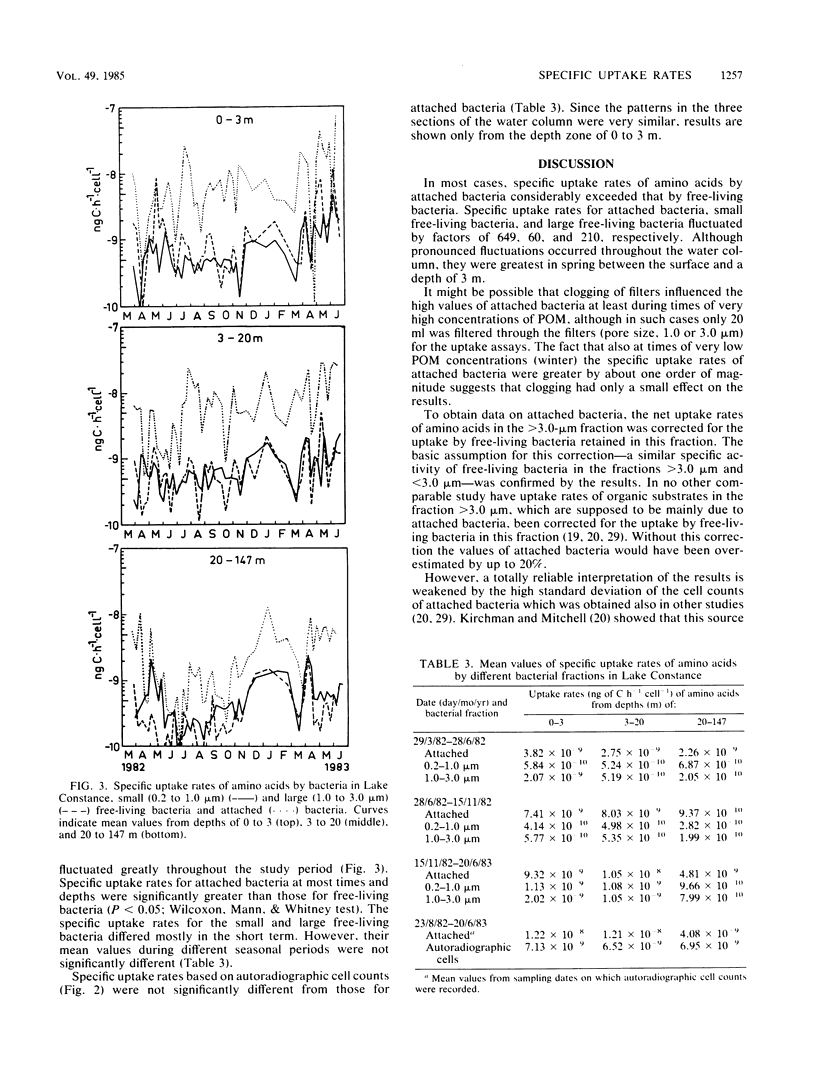
Seasonal and spatial patterns of specific uptake rates of amino acids by bacteria in Lake Constance were studied. The total bacterial population was divided into small (0.2- to 1.0-micron) and large (1.0- to 3.0-micron) free-living bacteria and attached bacteria by fractionated filtration. Data for attached bacteria, received by retention on 3.0-micron-pore Nuclepore filters, were corrected for free-living bacteria in this fraction. Specific uptake rates based on autoradiography were also recorded. Specific uptake rates for attached bacteria ranged from 9.41 X 10(-11) to 6.11 X 10(-8) ng of C h-1 cell-1 and were therefore significantly greater than those for free-living bacteria during most time periods. However, they were not significantly different from those for cells proven to be active by autoradiography. Specific uptake rates for small free-living bacteria ranged between 7.68 X 10(-11) and 4.60 X 10(-9) ng of C h-1 cell-1. They were nearly in the same range of those for large free-living bacteria (5.10 X 10(-11) to 1.07 X 10(-8) ng of C h-1 cell-1), although both fractions exhibited pronounced differences in their seasonal and vertical distributions.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell C. R., Albright L. J. Attached and free-floating bacteria in a diverse selection of water bodies. Appl Environ Microbiol. 1982 Jun;43(6):1227–1237. doi: 10.1128/aem.43.6.1227-1237.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright J. J., Fletcher M. Amino Acid assimilation and electron transport system activity in attached and free-living marine bacteria. Appl Environ Microbiol. 1983 Mar;45(3):818–825. doi: 10.1128/aem.45.3.818-825.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey R. W., Young L. Y. Enumeration of particle-bound and unattached respiring bacteria in the salt marsh environment. Appl Environ Microbiol. 1980 Jul;40(1):156–160. doi: 10.1128/aem.40.1.156-160.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchman D., Mitchell R. Contribution of particle-bound bacteria to total microheterotrophic activity in five ponds and two marshes. Appl Environ Microbiol. 1982 Jan;43(1):200–209. doi: 10.1128/aem.43.1.200-209.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbo A. V., Ferguson R. L., Rublee P. A. Size of suspended bacterial cells and association of heterotrophic activity with size fractions of particles in estuarine and coastal waters. Appl Environ Microbiol. 1984 Jul;48(1):157–164. doi: 10.1128/aem.48.1.157-164.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon A. J., Torbati D. Effects of hyperbaric oxygen on heart, brain, and lung functions in rat. Undersea Biomed Res. 1982 Sep;9(3):263–275. [PubMed] [Google Scholar]
- Torrella F., Morita R. Y. Microcultural study of bacterial size changes and microcolony and ultramicrocolony formation by heterotrophic bacteria in seawater. Appl Environ Microbiol. 1981 Feb;41(2):518–527. doi: 10.1128/aem.41.2.518-527.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright R. T. Measurement and significance of specific activity in the heterotrophic bacteria of natural waters. Appl Environ Microbiol. 1978 Aug;36(2):297–305. doi: 10.1128/aem.36.2.297-305.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]



