Abstract
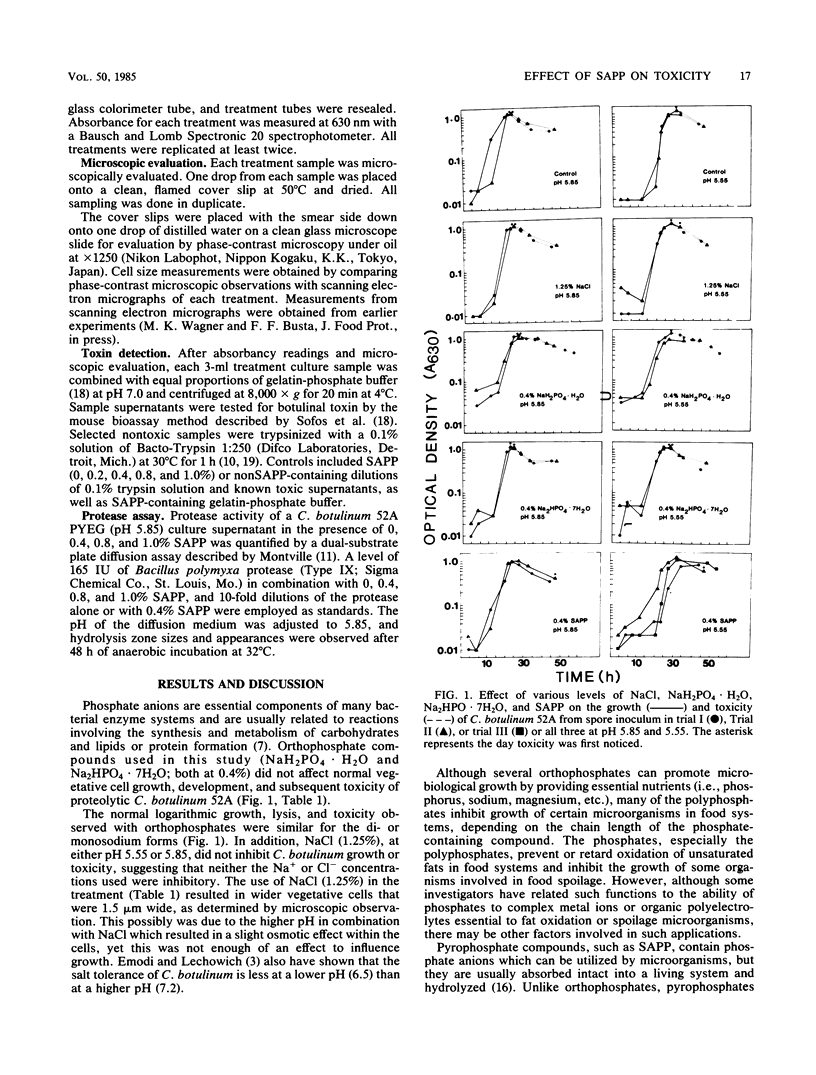
The effects of two pH levels (5.55 or 5.85) in combination with 0.4% sodium acid pyrophosphate (SAPP), NaH2PO4 X H2O, Na2HPO4 X 7H2O, or NaCl on the growth and toxicity of Clostridium botulinum 52A were studied. Absorbancy measurements at 630 nm, microscopic observations, and the mouse bioassay procedure were used to observe the effects. At pH 5.55 and 5.85 most control cultures exhibited toxicity when cell lysis began. Vegetative cell development was normal (4 micron long; 1 micron wide). SAPP-containing (0.4%) treatment cultures displayed similar growth and lysis but no or delayed (48 h) toxicity. Cells grown in the SAPP treatment culture were longer and wider (6 micron long; 1.5 micron wide) than in most other treatment cultures. Trypsinization of nontoxic supernatants from 0.4% SAPP resulted in toxicity. Addition of 0.4% SAPP to toxic C. botulinum supernatant delayed but did not prevent death of mice. The addition of various levels of SAPP to toxic supernatants resulted in a decrease in zone size with an increase in the level of SAPP (9 mm with 0.4% SAPP to 7 mm with 1.0% SAPP), using a dual substrate protease assay. A decrease in the zone size also occurred with the supernatant from cultures grown in the presence of SAPP and with Bacillus polymyxa protease dilutions containing 0.4% SAPP. Results suggest that the actual production or function of the protease responsible for toxin activation may have been inhibited by the presence of SAPP.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Christiansen L. N., Johnston R. W., Kautter D. A., Howard J. W., Aunan W. J. Effect of nitrite and nitrate on toxin production by Clostridium botulinum and on nitrosamine formation in perishable canned comminuted cured meat. Appl Microbiol. 1973 Mar;25(3):357–362. doi: 10.1128/am.25.3.357-362.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRECZ N., ANELLIS A., SCHNEIDER M. D. Procedure for cleaning of Clostridium botulinum spores. J Bacteriol. 1962 Sep;84:552–558. doi: 10.1128/jb.84.3.552-558.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M. Inorganic polyphosphates in biology: structure, metabolism, and function. Bacteriol Rev. 1966 Dec;30(4):772–794. doi: 10.1128/br.30.4.772-794.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATCHMAN B. J., FETTY W. O., BUSCH K. A. Effect of cell division inhibition on the phosphorus metabolism of growing cultures of Saccharomyces cerevisiae. J Bacteriol. 1959 Mar;77(3):331–338. doi: 10.1128/jb.77.3.331-338.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montville T. J. Dependence of Clostridium botulinum gas and protease production on culture conditions. Appl Environ Microbiol. 1983 Feb;45(2):571–575. doi: 10.1128/aem.45.2.571-575.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montville T. J. Dual-substrate plate diffusion assay for proteases. Appl Environ Microbiol. 1983 Jan;45(1):200–204. doi: 10.1128/aem.45.1.200-204.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogg J. E., Lee S. Y., Ogg B. J. A modified tube method for the cultivation and enumeration of anaerobic bacteria. Can J Microbiol. 1979 Sep;25(9):987–990. doi: 10.1139/m79-151. [DOI] [PubMed] [Google Scholar]
- Rowley D. B., Feeherry F. Conditions Affecting Germination of Clostridium botulinum 62A Spores in a Chemically Defined Medium. J Bacteriol. 1970 Dec;104(3):1151–1157. doi: 10.1128/jb.104.3.1151-1157.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHERBAUM O. H. Synchronous division of microorganisms. Annu Rev Microbiol. 1960;14:283–310. doi: 10.1146/annurev.mi.14.100160.001435. [DOI] [PubMed] [Google Scholar]
- SCHREIER K., NOLLER H. G. Stoffwechselversuche mit verschiedenen markierten Polyphosphaten. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1955;227(3):199–209. [PubMed] [Google Scholar]
- Seward R. A., Deibel R. H., Lindsay R. C. Effects of potassium sorbate and other antibotulinal agents on germination and outgrowth of Clostridium botulinum type E spores in microcultures. Appl Environ Microbiol. 1982 Nov;44(5):1212–1221. doi: 10.1128/aem.44.5.1212-1221.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VISHNIAC W. The antagonism of sodium tripolyphosphate and adenosine triphosphate in yeast. Arch Biochem. 1950 Apr;26(2):167–172. [PubMed] [Google Scholar]



