Abstract
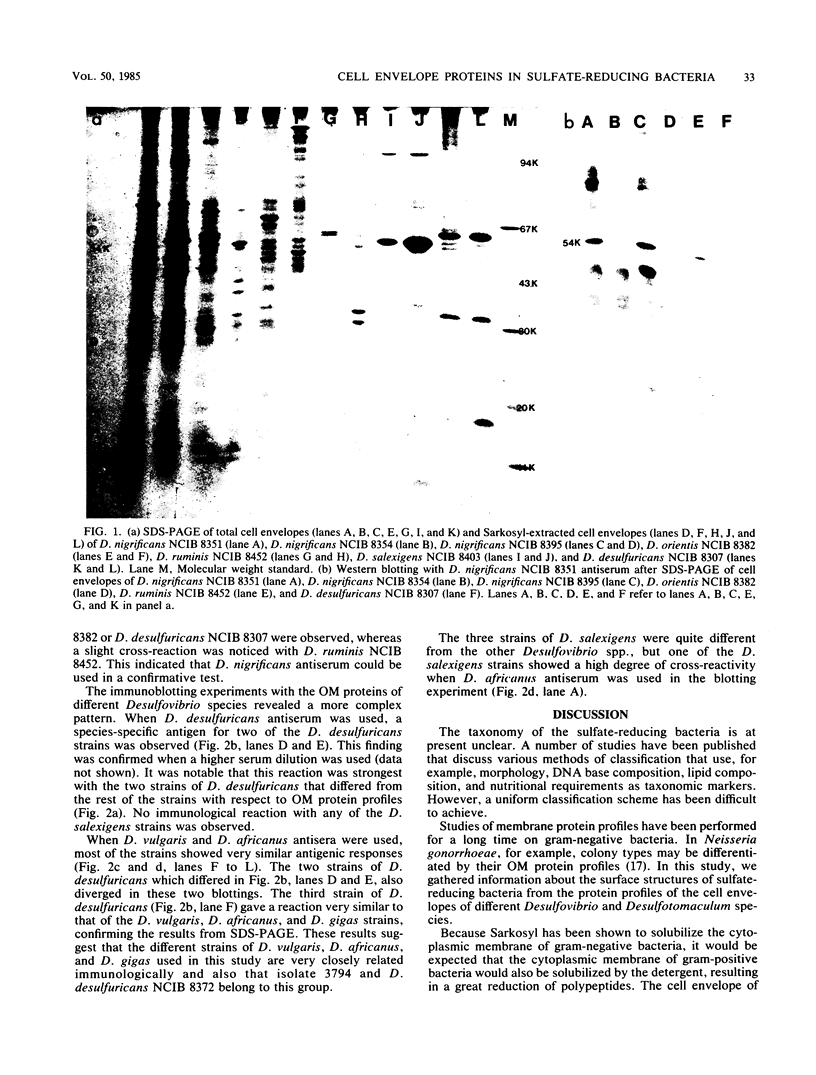
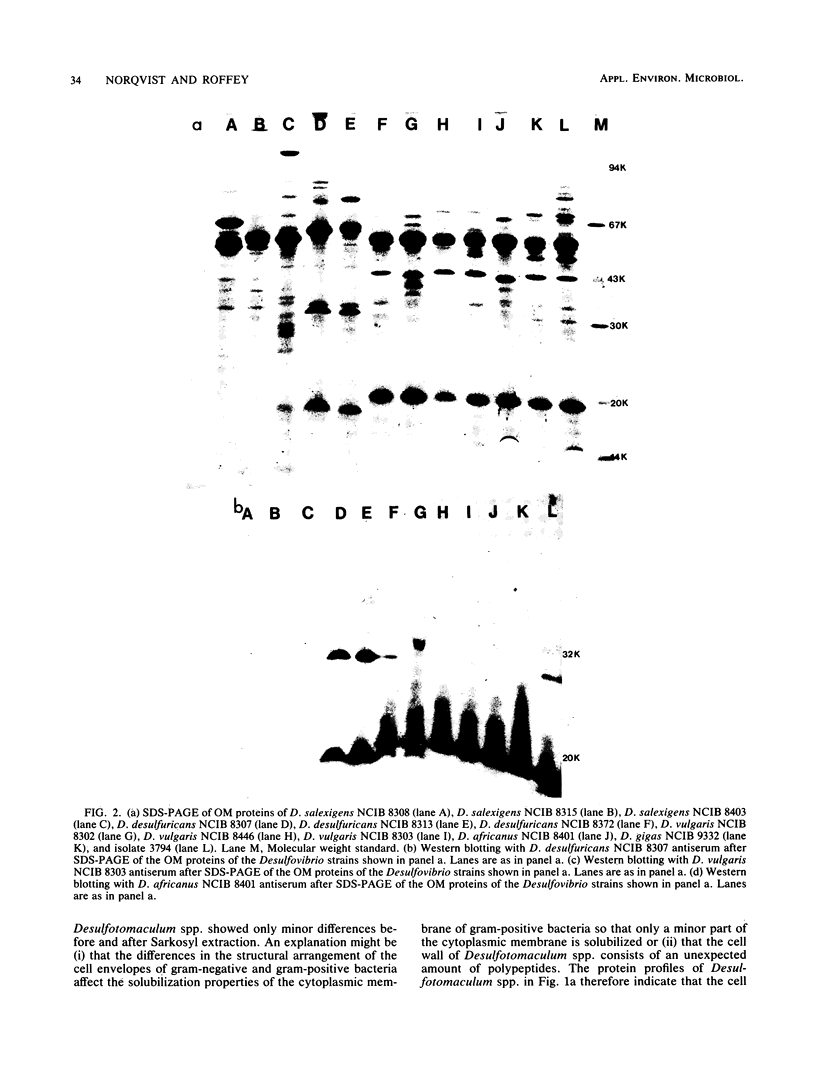
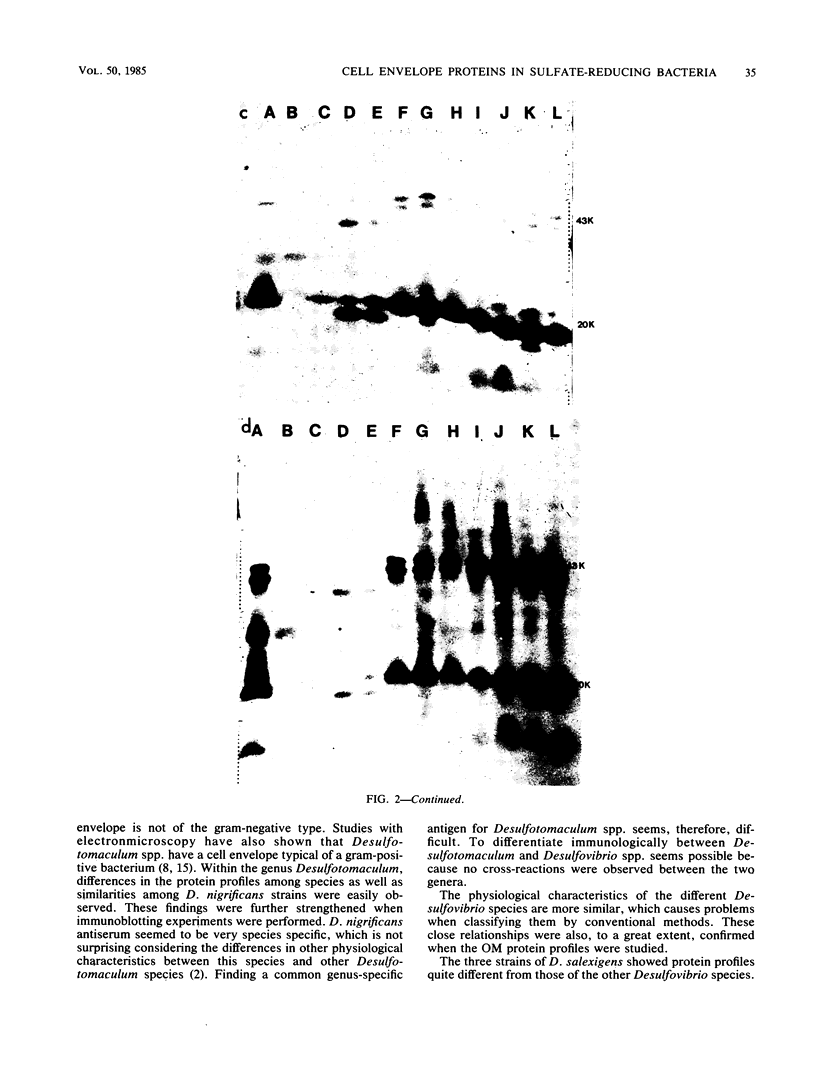
The envelope proteins of 5 strains of the genus Desulfotomaculum and 12 strains of the genus Desulfovibrio were studied by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting. The Desulfovibrio strains exhibited a typical gram-negative cell envelope, whereas the cell envelope of Desulfotomaculum strains appeared to be gram-positive. A close relationship between strains of Desulfotomaculum nigrificans was observed. A comparison between different species of Desulfotomaculum revealed some degree of similarity between Desulfotomaculum nigrificans and Desulfotomaculum ruminis, whereas Desulfotomaculum orientis seemed unique. The strains of Desulfovibrio salexigens were quite different from the strains of the other species of Desulfovibrio. In two of the strains of Desulfovibrio desulfuricans, a species-specific antigen was observed. The strains of Desulfovibrio vulgaris, Desulfovibrio africanus, and Desulfovibrio gigas and one strain of Desulfovibrio desulfuricans exhibited a similar outer membrane protein profile and also showed very similar antigenic reactions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdollahi H., Nedwell D. B. Serological characteristics within the genus Desulfovibrio. Antonie Van Leeuwenhoek. 1980;46(1):73–83. doi: 10.1007/BF00422231. [DOI] [PubMed] [Google Scholar]
- Campbell L. L., Postgate J. R. Classification of the spore-forming sulfate-reducing bacteria. Bacteriol Rev. 1965 Sep;29(3):359–363. doi: 10.1128/br.29.3.359-363.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Petris S. Ultrastructure of the cell wall of Escherichia coli and chemical nature of its constituent layers. J Ultrastruct Res. 1967 Jul;19(1):45–83. doi: 10.1016/s0022-5320(67)80059-5. [DOI] [PubMed] [Google Scholar]
- DiRienzo J. M., Nakamura K., Inouye M. The outer membrane proteins of Gram-negative bacteria: biosynthesis, assembly, and functions. Annu Rev Biochem. 1978;47:481–532. doi: 10.1146/annurev.bi.47.070178.002405. [DOI] [PubMed] [Google Scholar]
- Filip C., Fletcher G., Wulff J. L., Earhart C. F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973 Sep;115(3):717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Postgate J. R., Campbell L. L. Classification of Desulfovibrio species, the nonsporulating sulfate-reducing bacteria. Bacteriol Rev. 1966 Dec;30(4):732–738. doi: 10.1128/br.30.4.732-738.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoberth S. A new strain of Desulfovibrio gigas isolated from a sewage plant. Arch Mikrobiol. 1973;92(4):365–368. doi: 10.1007/BF00409290. [DOI] [PubMed] [Google Scholar]
- Sefer M., Pozsgi N. Etude sérologique des bactéries sulfatoréductrices (genre Desulfovibrio) isolées en Roumanie. Arch Roum Pathol Exp Microbiol. 1968 Dec;27(4):867–873. [PubMed] [Google Scholar]
- Shockman G. D., Barrett J. F. Structure, function, and assembly of cell walls of gram-positive bacteria. Annu Rev Microbiol. 1983;37:501–527. doi: 10.1146/annurev.mi.37.100183.002441. [DOI] [PubMed] [Google Scholar]
- Skyring G. W., Jones H. E., Goodchild D. The taxonomy of some new isolates of dissimilatory sulfate-reducing bacteria. Can J Microbiol. 1977 Oct;23(10):1415–1425. doi: 10.1139/m77-210. [DOI] [PubMed] [Google Scholar]
- Sleytr U., Adam H., Klaushofer H. Die Feinstruktur der Zellwand und Cytoplasmamembran von Clostridium nigrificans, dargestellt mit Hilfe der Gefrierätz- und Ultradünnschnittechnik. Arch Mikrobiol. 1969;66(1):40–58. [PubMed] [Google Scholar]
- Swanson J., Mayer L. W., Tam M. R. Antigenicity of Neisseria gonorrhoeae outer membrane protein(s) III detected by immunoprecipitation and Western blot transfer with a monoclonal antibody. Infect Immun. 1982 Nov;38(2):668–672. doi: 10.1128/iai.38.2.668-672.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XIV. Cell wall protein differences among color/opacity colony variants of Neisseria gonorrhoeae. Infect Immun. 1978 Jul;21(1):292–302. doi: 10.1128/iai.21.1.292-302.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trüper H. G., Kelleher J. J., Jannasch H. W. Isolation and characterization of sulfate-reducing bacteria from various marine environments. Arch Mikrobiol. 1969;65(3):208–217. doi: 10.1007/BF00407104. [DOI] [PubMed] [Google Scholar]