Abstract
A cross-sectional survey was made in 56 exceptionally healthy males, ranging in age from 20 to 84 years. Measurements were made of selected steroidal components and peptidic hormones in blood serum, and cognitive and physical tests were performed. Of those blood serum variables that gave highly significant negative correlations with age (r > −0.6), bioavailable testosterone (BT), dehydroepiandrosterone sulfate (DHEAS), and the ratio of insulin-like growth factor 1 (IGF-1) to growth hormone (GH) showed a stepwise pattern of age-related changes most closely resembling those of the age steps themselves. Of these, BT correlated best with significantly age-correlated cognitive and physical measures. Because DHEAS correlated well with BT and considerably less well than BT with the cognitive and physical measures, it seems likely that BT and/or substances to which BT gives rise in tissues play a more direct role in whatever processes are rate-limiting in the functions measured and that DHEAS relates more indirectly to these functions. The high correlation of IGF-1/GH with age, its relatively low correlation with BT, and the patterns of correlations of IGF-1/GH and BT with significantly age-correlated cognitive and physical measures suggest that the GH–IGF-1 axis and BT play independent roles in affecting these functions. Serial determinations made after oral ingestion of pregnenolone and data from the literature suggest there is interdependence of steroid metabolic systems with those operational in control of interrelations in the GH–IGF-1 axis. Longitudinal concurrent measurements of serum levels of BT, DHEAS, and IGF-1/GH together with detailed studies of their correlations with age-correlated functional measures may be useful in detecting early age-related dysregulations and may be helpful in devising ameliorative approaches.
Keywords: pregnenolone sulfate, learning, memory, vestibular function
It is difficult to view problems of human aging at all levels, from molecular-genetic to social. The loci of command-control within the different levels must be identified and the nature of their hierarchical nesting elucidated (1). Herein, we undertake a “broad brush stroke,” impressionistic approach in an effort to identify for further in-depth study some of the key rate-limiting variables in the human male aging progression when it is uncomplicated by chronic and acute disease processes.
It seemed reasonable to begin by looking among serum-reflected aspects of the neuroendocrine machinery, components of which modulate homeostatic and integrative systems of the body. Degenerative processes associated with aging become evident among these variables toward the close of the reproductive period in the human. The steroidal hormones of the neuroendocrine system play multifactorial roles, acting as pleiotropic facilitators of coordinative processes that enable neural, endocrine, and metabolic systems, separately and together, to cycle freely through their operational modes in solving problems of survival and reproduction and in achieving repair and rebalancing when malfunctioning occurs (see refs. 1–8).
Measurements of serum levels of a number of potential steroidal and peptidic neuroendocrine aging markers in 56 exceptionally healthy males ranging in age from 20 to 84 years were combined with concomitant cognitive and physical tests that may be affected in aging. Of the chemical variables observed, serum levels of bioavailable testosterone (BT), dehydroepiandrosterone sulfate (DHEAS), and the ratio of insulin-like growth factor 1 (IGF-1) to growth hormone (GH) as an interdigitated group were the most importantly related to aspects of functional decrements with aging. Details of these findings and their potential significance and utility are dealt with herein.
MATERIALS AND METHODS
Subjects.
Fifty-six healthy male subjects, ages 21–84, were recruited at the St. Louis Veterans Administration Medical Center. Written informed consent was obtained. With the exception of a few instances of minor arthritis, none showed evidence of acute or chronic disease when the study was performed. Preprandial blood samples were drawn in the morning, and light breakfasts were eaten before cognitive and physical tests were administered.
Serum samples were obtained from two healthy adult males ages 45 and 46 years, respectively, at the Harbor–University of California at Los Angeles Medical Center in Los Angeles before and after preprandial oral ingestion of a capsule containing 175 mg of pregnenolone (P). Postingestion blood samples were taken at 1, 2, 4, 8, and 24 hr.
Serum Determinations.
Measurements were made of levels of the following: cholesterol, P, pregnenolone sulfate (PS), 17α-OH P, dehydroepiandrosterone (DHEA), DHEAS, androstenedione (OH), total testosterone (total T), BT, estradiol, progesterone (PROG), 17α-OH progesterone (17α-OH PROG), aldosterone, 1,25(OH) vitamin D, 25(OH) vitamin D, parathyroid hormone, osteocalcin, luteinizing hormone, GH, and IGF-1. Although 24-hr sampling is preferable for the measurements of GH because of its episodic secretion, it was possible for us to perform measurements only on single samples. State-of-the-art assay procedures were used under conditions meeting requirements for reproducibility and accuracy. With the exception of BT, the methods will not be discussed, but details will be furnished on request.
Total T was measured by double-antibody radioimmunoassay. BT was estimated using precipitation of T bound to globulins with 50% ammonium sulfate after equilibration with [3H]T, as described previously (9). The results are calculated as follows: % nonglobulin bound T (%BT) = supernatant T/total T × 100; BT (ng/ml) = total T (ng/ml) × %BT.
Detailed results will be given in the text only for those substances whose values correlated significantly with age and at least one of the significantly age-correlated cognitive and physical variables cited below.
Cognitive and Physical Measures.
Only those measures will be mentioned that correlated significantly with age (P < 0.01). The Rey Visual Design Learning Test (10–12) is a penta-trial test of nonverbal learning and memory in which 15 geometric patterns on separate cards are presented to the subject, who is requested to present all remembered designs. In the Rey Auditory Verbal Test (10–12), which measures verbal learning and memory, 15 nouns are read aloud at 1-sec intervals for three consecutive trials. Each trial is followed by a free-recall test. Animal naming (13, 14) is a 60-sec test that measures production by the subject of individual animal names. The Folstein Mini-Mental Status Examination (15) is a comprehensive screening test for cognitive dysfunction.
Physical Tests.
Waist/hip ratio was calculated from direct measurements. Balance, a reflection of vestibular compensation and functionality of the vestibules receptor complex, was measured with a stop watch as time of retention of balance after requesting the subject to stand on one leg with eyes closed. Handgrip strength was estimated with a Jamar dynamometer by selecting the best of three attempts.
Statistical Analysis.
Statistical analysis was carried out by the statview II program on the Macintosh II using the χ2 test and one-way ANOVA. Differences at the 95% level by posthoc analysis by the rigorous Sheffe F test were considered to be significant. The relationship among the various parameters were assessed by simple or multiple regression analysis.
RESULTS
Relations of Serum Steroids, IGF-1, and GH to Chronological and Functional Aging.
From among all of the serum variables measured, only those that showed a high negative correlation with age, r > −0.6, were selected for further consideration. With the exception of PS, the steroids meeting this criterion were the androgen-related substances listed in Table 1 in decreasing order of r. Values of all of the above showed invariantly progressive mean decrements with median age of cohort (Table 2). None of the peptidic substances studied attained either of the above criteria, but the ratio IGF-1/GH met both requirements (Tables 1 and 2). Only BT, DHEAS, and IGF-1/GH showed stepwise patterns of age-related changes closely resembling that of the age steps themselves (Fig. 1).
Table 1.
Correlations of serum components with age and with levels of BT, DHEAS, and IGH/GH
| Variable | Age
|
BT
|
DHEAS
|
IGF/GH
|
||||
|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | |
| BT | −0.77 | 0.0001 | — | — | 0.68 | 0.0001 | 0.38 | 0.0043 |
| DHEAS | −0.76 | 0.0001 | 0.68 | 0.0001 | — | — | 0.51 | 0.0001 |
| DHEA | −0.73 | 0.0001 | 0.60 | 0.0001 | 0.82 | 0.0001 | 0.48 | 0.0002 |
| AD | −0.72 | 0.0001 | 0.68 | 0.0001 | 0.76 | 0.0001 | 0.44 | 0.0007 |
| IGF-1/GH | −0.65 | 0.0001 | 0.38 | 0.0043 | 0.51 | 0.0001 | — | — |
| Total T | −0.62 | 0.0001 | 0.71 | 0.0001 | 0.67 | 0.0001 | 0.22 | 0.1114 |
| PS | −0.60 | 0.0001 | 0.44 | 0.0007 | 0.69 | 0.0001 | 0.41 | 0.0007 |
The r and P values (in parentheses) for correlation with age of some other chemical variables not listed above were as follows: cholesterol, 0.52 (0.0001); IGF-1, −0.50 (0.0001); 17α-OH PROG, −0.48 (0.0002); 17α-OH P, −0.44 (0.0006); P, −0.40 (0.0022); luteinizing hormone, 0.39 (0.0031); GH, 0.24 (0.073); cortisol, −0.23 (0.1114); estradiol, 0.08 (0.5774); PROG, 0.08 (0.5817); and aldosterone, 0.06 (0.6478).
Table 2.
Stepwise age-related changes of several steroids and IGF-1/GH
| Variable | Age ranges, years
|
ANOVA P | ||||
|---|---|---|---|---|---|---|
| 20–29 (16) | 30–49 (10) | 50–59 (12) | 60–69 (10) | 70–84 (8) | ||
| BT | 1.90 ± 0.20 | 1.44 ± 0.17* | 0.87 ± 0.10* | 0.56 ± 0.06* | 0.45 ± 0.10* | 0.0001 |
| DHEAS | 3,828 ± 317 | 2,821 ± 472* | 1,712 ± 246* | 1,123 ± 186* | 870 ± 245* | 0.0001 |
| DHEA | 3.36 ± 0.37 | 2.25 ± 0.58* | 0.93 ± 0.26* | 0.66 ± 0.25* | 0.05 ± 0.02* | 0.0001 |
| Androstenedione | 2.09 ± 0.11 | 1.86 ± 0.17 | 1.52 ± 0.13* | 1.33 ± 0.10* | 0.96 ± 0.10* | 0.0001 |
| Total T | 6.17 ± 0.37 | 5.86 ± 0.73 | 4.89 ± 0.15* | 4.36 ± 0.27* | 3.18 ± 0.27* | 0.0001 |
| PS | 57.5 ± 5.8 | 50.3 ± 6.1 | 43.0 ± 4.8* | 25.3 ± 4.8* | 22.6 ± 4.6* | 0.0001 |
| GH | 0.40 ± 0.02 | 0.66 ± 0.15 | 1.74 ± 0.71 | 1.44 ± 0.58 | 0.74 ± 0.08 | 0.10 |
| IGF-1 | 325 ± 19 | 256 ± 21* | 260 ± 34* | 207 ± 20* | 203 ± 27* | 0.0035 |
| IGF-1/GH | 869 ± 88 | 502 ± 78* | 357 ± 67* | 331 ± 84* | 295 ± 53* | 0.0001 |
Significance of difference between individual mean value and the mean value for the 20–29-yr group is P < 0.05; means ± SEM, ng/ml. Number in parentheses = number of individuals.
Figure 1.
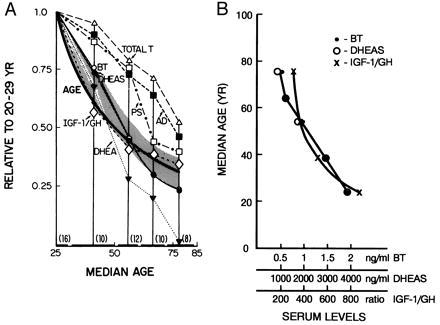
Stepwise age-related changes. (A) The age curve was obtained by plotting the median age of a particular cohort (Table 2) vs. median age of the 20–29-yr cohort/median age of the particular cohort. The curves for the biochemical variables were obtained by plotting the median age of a particular cohort vs. the mean value (ng/ml) for the variable in the cohort/the mean value for the 20–29-yr cohort. (B) Plot of the actual serum values of BT, DHEAS, and IGF-1/GH as a function of median age of the cohort.
Table 3 shows the r values of age, DHEAS, BT, and IGF-1/GH with the significantly age-correlated cognitive and physical measures as well as those of age, DHEAS, and IGF-1/GH with BT. The correlations of age with the functional variables in all, but one, instance were higher than those of any of the biochemical variables. BT was significantly correlated with seven of nine cognitive and behavioral measures, and DHEAS, which correlated highly with BT, with only three of nine. Values for DHEA, androstenedione, and PS also are shown in Table 3.
Table 3.
Correlation coefficients of age and several serum components with levels of BT and with significantly age-correlated cognitive and physical measures
| Variable | BT | Waist/hip ratio | Physical measures
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cognitive measures
|
Balance
|
Grip
|
||||||||
| RVDLT | RAVLT | Animal naming | Folstein | Eyes closed right | Eyes closed left | Right hand | Left hand | |||
| Age | −0.77 | −0.71 | −0.56 | −0.56 | −0.37 | −0.65 | −0.45 | −0.56 | −0.50 | −0.44 |
| BT | — | 0.53 | 0.52 | 0.45 | 0.34 | 0.46 | 0.45 | 0.45 | 0.44 | 0.33 |
| DHEAS | 0.68 | 0.49 | 0.47 | 0.33 | 0.34 | 0.39 | 0.10 | 0.25 | 0.31 | 0.25 |
| IGF-1/GH | 0.38 | 0.47 | 0.43 | 0.30 | 0.20 | 0.19 | 0.42 | 0.41 | 0.32 | 0.11 |
| DHEA | 0.60 | 0.47 | 0.41 | 0.28 | 0.26 | 0.39 | 0.11 | 0.17 | 0.37 | 0.18 |
| AD | 0.68 | 0.44 | 0.42 | 0.24 | 0.30 | 0.37 | 0.13 | 0.24 | 0.36 | 0.34 |
| PS | 0.44 | 0.34 | 0.37 | 0.19 | 0.35 | 0.37 | 0.05 | 0.03 | 0.30 | 0.28 |
The cognitive and physical measures are described briefly under Materials and Methods and references are given therein. Bold type, P < 0.01. AD, androstenedione; RVDLT, Rey Visual Design Learning Test; RAVLT, Rey Auditory Verbal Test.
Because DHEAS correlated well with BT, but less well than BT with age and less well overall than BT with the functional measures, it seems likely that T derived from BT and/or substances to which it gives rise in tissues plays a more direct role in whatever processes may be rate-limiting in the functions measured and that DHEAS relates more indirectly to these functions. The high negative correlation of IGF-1/GH with age (r = −0.65, Table 1), its relatively low correlation with BT (r = 0.38, Table 1), and a comparison of patterns of correlation with functional measures (particularly see r values in Table 3 for waist/hip ratio and balance) suggest that the GH–IGF-1 axis and BT may play independent roles in these functions.
Oral Ingestion of P Results in Increases in Steroid and IGF-1/GH Levels in Serum.
Data obtained in two healthy male subjects during the first 8 hr after ingestion of 175 mg of P (Fig. 2) are consistent with the idea that oral P could be a precursor of glucocorticoids, mineralocorticoids, and sex steroids produced, as depicted in Fig. 2. PS showed the greatest relative postingestion increase of any of the steroids (Figs. 2 and 3), increasing more than P. PS levels remained elevated at 24 hr, at which time P values had returned to control levels or below. Orally ingested P probably largely is converted to PS in the intestine, which is rich in steroid-conjugating enzyme activity, the PS being absorbed rapidly into the blood (17). Various tissues, blood cells (18), and blood lipoprotein complexes (19, 20) may take up PS and use it either as such or, after uptake convert it to P by widely occurring, tissue-specific, steroid sulfate-splitting enzymes (21–23). A small amount of P enters the blood stream. The prolonged elevation of serum PS by comparison with P is attributable to the fact that the latter is cleared approximately three times more quickly than PS (24). Elevations over control values were noted after ingestion of P in both subjects at all or most of the time intervals up to 8 hr in contents of P, PS, DHEA, total estrogens, 17α-OH PROG, and PROG.
Figure 2.
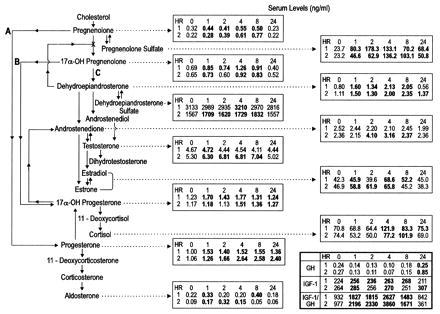
Effect of ingestion of P on blood serum levels of steroids, IGF-1, and GH. All values in bold type are higher than the control values. The biosynthesis of steroid hormones begins with cholesterol, from which the sex steroids (e.g., T), glucocorticoids (e.g., cortisol), and mineralocorticosteroids (e.g., aldosterone) all derive. P is believed to be a major precursor for the steroid hormones. It is formed from cholesterol in mitochondria of tissues that produce steroid hormones (consult ref. 16 for discussion of biochemical details).
Figure 3.
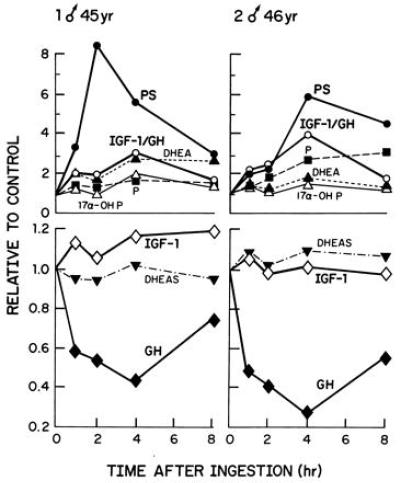
Changes in serum levels of several pertinent variables at intervals over an 8-hr period after preprandial oral ingestion of 175 mg of P by two healthy males. The values are expressed as ratios of the values at various times after ingestion of P to those observed before ingestion. The 24-hr values are not shown on the plot but are recorded in Fig. 2.
The relative decreases in levels of GH reflected in inverse fashion the relative increases in PS in both individuals studied. Of the substances measured other than PS, the IGF-1/GH ratio was elevated over the 8-hr period in a pattern most closely resembling that of PS (Fig. 3). In subject 1 the IGF-1 levels were elevated for at least 8 hr but not at 24 hr; the IGF-1/GH levels were elevated at 1, 4, and 24 hr in subject 2. The elevations in IGF-1/GH largely are attributable to decreased levels of GH after PS ingestion. Inferences cannot be made at present as to whether or not a particular steroid or combination of steroids elevated after P administration or feedback inhibition by elevation of IGF-1 might be causally related to the decrements in GH.
DISCUSSION
About BT.
In the human male, aging changes most easily observed are those associated with decrements in androgenic functionality. The quintessential endogenous androgenic substances are T and some of its metabolites and derivatives, the most obvious functions of which are the maintenance of secondary male sex characteristics. BT, which represents T bound to nonglobulin serum constituents, is believed to furnish the best estimate of tissue exposure to androgens. T bound to high-affinity T-binding globulin (TeBG), a major binding entity, or to other globulins is not readily available for intracellular transport (9, 25–27).
The current survey identified the BT fraction of the total serum T as the variable that correlated best among those measured with chronological age and with age-related cognitive and physical deficits. The BT fraction consists largely (approximately 98%) of T bound to albumin. Albumin has a low affinity for T, but because of its high concentration in serum, it binds most of the T not bound to the TeBG. BT accounts for most of the serum T that is readily available to tissues. The low affinity of albumin for T and its high concentration in blood currently is believed to insure that the T carried by albumin is the fraction of serum T that is most rapidly delivered and released to tissues (but see below).
Total T fell with age, but the rate of decrement was less than that of BT; whereas the rate of decrease in BT paralleled almost exactly that of DHEAS, even though the concentrations of DHEAS were three orders of magnitude higher than those of BT (Fig. 1). The fall in total T with age (Tables 1 and 2) probably reflects an age-related decrease in enzymatic capacity to form DHEA and DHEAS, the precursors of T. Cytochrome P450c17 is the enzyme that catalyzes both the 17α hydroxylation of P and PS and the subsequent scission of the side chains of 17α-OH P and 17α-OH PS (17,20 lyase activity) to form DHEA or DHEAS. Both activities of this enzyme are required for formation of the sex-related steroids beginning with DHEA and DHEAS (see Fig. 2). The two activities of cytochrome P450c17 decrease differentially with age, the 17,20 lyase activity of the enzyme declining at a greater rate than the 17α hydroxylase activity. This decrease in 17,20 lyase activity restricts the metabolic flow downline from 17α-OH P to DHEA and DHEAS and to the T formed therefrom. The molecular mechanisms by which the differential decrease of 17,20 lyase activity might occur have been elucidated elegantly (28).
The availability of serum DHEAS appears to be limiting in determining the level of total serum T. The rate of increase of total serum T with increase in DHEAS declined with increasing level of DHEAS, approaching asymptotically a maximum somewhat above 4,000 ng/ml of DHEAS (A in Fig. 4). This decrement may be attributable to inhibition by DHEA and DHEAS of the conversion of P to 17α-OH P. For example, there were reductions in 17α-OH P and 17α-OH PROG levels after greatly increasing serum contents of DHEA and DHEAS in men 75–83 yr by single oral doses of DHEA (400 mg) or by high chronic oral doses of DHEA (1,200 and 2,400 mg/day). With the latter doses, T levels actually were reduced below the low control levels already seen in these individuals (ref. 29 and E.R. and L. J. Fitten, unpublished work). The values obtained by subtracting BT from total T (B in Fig. 3), presumably reflecting the amount of T bound to TeBG, plateaued at a level of DHEAS of approximately 2,000 ng/ml, possibly indicating saturation by T of binding sites on TeBG and decreased production of TeBG at higher levels of serum T.
Figure 4.
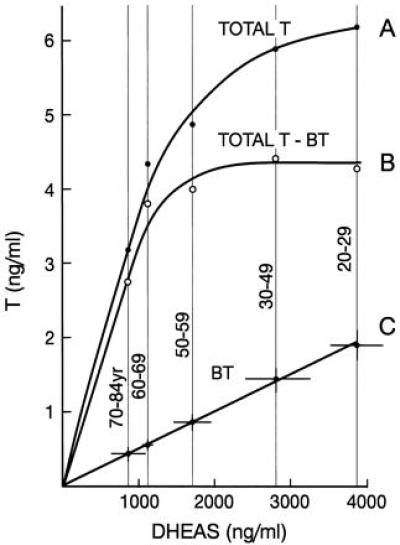
Serum levels of T as a function of levels of DHEAS. In C, the SEM for the mean values is shown by the lines through the data points.
To what might be attributed the yoking to each other of the age-related declines of BT and DHEAS so that the molar ratios of DHEAS to BT remained essentially constant at 1577 at all levels of DHEAS observed (C in Fig. 4)? In the youngest cohort (20–29 yr), the mean molar concentrations of DHEAS and of the T contained in the BT fraction were 1 × 10−5 M and 6.6 × 10−9 M, respectively. The molar concentration of albumin, approximately 6.3 × 10−4 M, is independent of age in healthy individuals. DHEAS and T both can bind to albumin, DHEAS with considerably greater affinity than T because of its anionic nature. DHEAS can form a mass-action driven complex with albumin, the quantity of complex decreasing with decreasing DHEAS content. The albumin molecule has great flexibility and exists in different configurations when bound to various ligands, there being a mutuality of interaction so that the configurations of both albumin and ligands in the complexes may be different from those in the unbound states (30–35). We posit that DHEAS at 10−5 M concentration or less forms a 1:1 complex with albumin (32) that has a greater affinity for T than unbound albumin has for T. A molecule of T may bind to the DHEAS-albumin complex to form a ternary 1:1:1 complex, with T and DHEAS binding to separate sites on the same albumin molecule. Upon contact with a cell membrane, surface-induced configurational changes in the complex could be initiated that release the T from the complex to bind to specific cellular receptors, e.g., androgen receptors, initiating cascades of subsequent reactions characteristic of the particular cells to which the binding takes place, the DHEAS and albumin being released simultaneously to the surrounding medium. This proposal is compatible with the linear relationship shown between DHEAS and BT (C in Fig. 4) and can be tested experimentally with tools at hand. It has not escaped our attention that the above, if true, would have wide ramifications.
In summary, we suggest that DHEAS not only is a reservoir of precursor for T but also that it is a facilitator of the binding of T to albumin, thereby helping target T to specific receptors for rapid action at the cellular level.
About the IGF-1/GH Ratio.
Serum levels of IGF-1 were measured because previously it had been shown that administration of DHEA not only raised serum levels of DHEA and DHEAS but also increased IGF-1 and decreased IGF-1 binding protein-1, thereby possibly increasing the bioavailability of IGF-1 to target tissues (36). IGF-1 is a pleiotropic regulator of anabolic aspects of cellular metabolism. GH was measured because its effects on growth, differentiation, and metabolism largely are exerted by IGF-1, the production of which GH stimulates.
The actions of IGF-1 are exerted locally or more widely in the secreted form. Serum levels of IGF-1 largely are determined by secretion from the liver, a major site of its production. Once formed, IGF-1 may engage with GH in mutually interactive fashion through paracrine, autocrine, and endocrine mechanisms, IGF-1 decreasing the release of GH from the pituitary and GH inhibiting some of the actions of IGF-1, the ratio of the two in serum at any particular time reflecting the state of a complex yin-yang relationship.
The serum content of IGF-1 fell significantly with age, and the GH level showed statistically insignificant increases. IGF-1/GH correlated much better with age than did IGF-1 or GH alone (Table 1) and conformed more closely to the pattern of age-related stepwise decrements in BT and DHEAS than did any of the other steroidal or peptidic substances measured (Fig. 1). However, the r values of IGF-1/GH with BT (0.38) and DHEAS (0.51) were lower than that of BT with DHEAS (0.68). Age-related decrement in IGF-1/GH may be related to a progressive decrease in production of hypothalamic thyrotropin-releasing hormone and the consequential dysregulation of the hypothalamo-pituitary-thyroid axis (37–39).
With regard to the significantly age-correlated cognitive and physical measures (Table 3), the pattern of correlation shown by IGF-1/GH differed from those of DHEAS and the metabolically closely related DHEA, androstenedione, and PS in that IGF-1/GH, like BT, was significantly negatively correlated with balance measures, whereas the r values of the others were lower and not significant. The ability to remain standing on one leg with both eyes closed is a dynamic measure of the capability of vestibular function, in which cerebellar and oculomotor inputs to the vestibular receptor complex are of great importance. Removal of visual inputs to the vestibular receptor complex by closing eyes requires considerable neural compensation to take place to maintain normally adaptive function (40). The decrease with aging in achieving this compensation correlates significantly with the decrease in serum levels of BT and with IGF-1/GH; but the relatively poor correlation of BT and IGF-1/GH with each other suggests that they probably are influencing different aspects of vestibular function (Table 3). IGF-1 plays an important role in cerebellar function, being released onto cerebellar Purkinje cells by the climbing fiber afferents arising from the inferior olivary complex (41, 42).
Decreases in IGF-1/GH and BT correlated well with decrements in visual and auditory learning (Table 3), but data is not available to determine whether or not BT and IGF-1/GH have similar effects on the same brain structures. By its action on the cerebellum, IGF-1 plays an essential role in the learning of the conditioned eye-blink reflex in the rat (43). To our knowledge, T has not been tested in this paradigm. PS, which on post-training injection into the amygdala was shown to exert remarkable effects on retention of footshock active avoidance training in mice (44), had no effect on the learning and retention of the eye blink reflex in rabbits when injected into the nucleus interpositus, a key site for the latter kind of learning (R. F. Thompson, personal communication).
What Is To Be Done?
Eliminating many of the variables measured originally, it is possible to focus on measurement of age-related changes of BT, DHEAS, and IGF-1/GH, while adding to the latter group measurement of thyroid-related variables during states of rest and functional challenge (45–47). The functional variables that can be assessed quantitatively and with which the biochemical data probably would be correlated best are those related to learning and memory and to vestibular function.
Decreased performance with age in memory tests largely can be attributed to an increasingly fragile working memory that falters or fails when its span is exceeded. Batteries of well designed cognitive tests, available in equivalent forms that are matched as to relative difficulty, retest reliability, and factorial composition can be used in suitably designed longitudinal tests with cohorts of different ages (e.g., see ref. 48). From results of the present study, it was deduced that BT and IGF-1/GH levels are related to different functional aspects of vestibular compensation. Application of an available battery of elegant quantitative tools that dynamically evaluate inner ear, brain stem, and cerebellar function and the competence of vestibular-visual interactions could help identify early age-related functional deficits that correlate with early changes in the biochemical and cognitive variables (e.g., see ref. 49).
With the knowledge at hand, would it be possible to return reduced serum levels of BT, IGF-1/GH, and DHEAS in older individuals to those found in normal young adults? Ingestion of moderate amounts of DHEA daily normalized reduced DHEA and DHEAS levels in males, but levels of total T were not changed (36); nor was total T increased by daily feeding of 525 mg of P over a 3-month period (E.R. and B.L. Miller, unpublished work). High oral doses of DHEA actually reduce serum T levels (29). Intramuscular administration of T increases total serum T and BT (50) and intramuscular IGF-1 mRNA concentrations (51). Chronic feeding of DHEA increases levels of IGF-1 and does not affect GH, thereby increasing IGF-1/GH (36). From the above, it may be anticipated that coadministration of DHEA and T in appropriate small amounts might make it possible to raise serum BT, IGF-1/GH, and DHEAS to normal young adult levels in individuals with age-related reduced levels. Were this found to be the case, it would be of great interest to determine whether or not decrements in some of the age-related cognitive and physical measures would be reversed as well.
Acknowledgments
The work of the St. Louis group was supported by the Medical Research Service of the Department of Veterans Affairs and that of E.R. and W.J.R. was supported by institutional funds from the Beckman Research Institute of the City of Hope.
ABBREVIATIONS
- T
testosterone
- BT
bioavailable testosterone
- DHEA
dehydroepiandrosterone
- DHEAS
dehydroepiandrosterone sulfate
- P
pregnenolone
- PS
pregnenolone sulfate
- 17α-OH P
17α-OH pregnenolone
- PROG
progesterone
- 17α-OH PROG
17α-OH progesterone
- TeBG
high-affinity testosterone-binding globulin
- IGF-1
insulin-like growth factor 1
- GH
growth hormone
- r
correlation coefficient
References
- 1.Roberts E. Neurochem Res. 1991;16:409–421. doi: 10.1007/BF00966104. [DOI] [PubMed] [Google Scholar]
- 2.Parker L N. Adrenal Androgens in Clinical Medicine. New York: Academic; 1989. [Google Scholar]
- 3.Roberts E. Prog Brain Res. 1990;86:339–355. doi: 10.1016/s0079-6123(08)63190-8. [DOI] [PubMed] [Google Scholar]
- 4.Kalimi M, Regelson W, editors. The Biological Role of Dehydroepiandrosterone (DHEA) Berlin: de Gruyter; 1990. [Google Scholar]
- 5.Bellino F L, Daynes R A, Hornsby P J, Lavrin D H, Nestler J E, editors. Dehydroepiandrosterone (DHEA) and Aging. New York: New York Academy of Sciences; 1995. [Google Scholar]
- 6.Roberts E. Biochem Pharmacol. 1995;49:1–16. doi: 10.1016/0006-2952(94)00258-n. [DOI] [PubMed] [Google Scholar]
- 7.Guth L, Zhang Z, Roberts E. Proc Natl Acad Sci USA. 1994;91:12308–12312. doi: 10.1073/pnas.91.25.12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baulieu E-E. J Clin Endocrinol Metab. 1996;81:3147–3151. doi: 10.1210/jcem.81.9.8784058. [DOI] [PubMed] [Google Scholar]
- 9.Korenman S G, Morley J E, Mooradian A D, Davis S S, Kaiser F E, Silver A J, Viosca S P, Garza D. J Clin Endocrinol Metab. 1990;71:963–969. doi: 10.1210/jcem-71-4-963. [DOI] [PubMed] [Google Scholar]
- 10.Mungas D. J Consult Clin Psychol. 1983;51:848–855. doi: 10.1037//0022-006x.51.6.848. [DOI] [PubMed] [Google Scholar]
- 11.Rey A. L’Examen Clinique en Psychologie. Paris: Press Universitaire de France; 1964. [Google Scholar]
- 12.Ryan J J, Rosenberg S J, Mittenberg W. Int J Clin Neuropsychol. 1984;6:239–241. [Google Scholar]
- 13.Goodglass H, Kaplan E. The Assessment of Aphasia and Related Disorders. 2nd Ed. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- 14.Wilson R S, Kaszniak A W, Fox J H. Cortex. 1981;17:41–48. doi: 10.1016/s0010-9452(81)80005-6. [DOI] [PubMed] [Google Scholar]
- 15.Folstein M F, Folstein S E, McHugh P R. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 16.Miller W L. Endocr Rev. 1988;9:295–318. doi: 10.1210/edrv-9-3-295. [DOI] [PubMed] [Google Scholar]
- 17.Bostrom H, Bromster D, Nordenstam H, Wengle B. Scand J Gastroenterol. 1968;3:369–375. doi: 10.3109/00365526809180131. [DOI] [PubMed] [Google Scholar]
- 18.Milewich L, Whisenant M G. J Clin Endocrinol Metab. 1982;54:969–974. doi: 10.1210/jcem-54-5-969. [DOI] [PubMed] [Google Scholar]
- 19.Leszczynski D E, Schafer R M. Biochim Biophys Acta. 1991;1083:18–28. doi: 10.1016/0005-2760(91)90120-7. [DOI] [PubMed] [Google Scholar]
- 20.Belanger B, Roy R, Belanger A. Steroids. 1992;57:430–436. doi: 10.1016/0039-128x(92)90096-r. [DOI] [PubMed] [Google Scholar]
- 21.Hobkirk R. Can J Biochem Cell Biol. 1985;63:1127–1144. doi: 10.1139/o85-141. [DOI] [PubMed] [Google Scholar]
- 22.Munroe D G, Chang P L. Am J Hum Genet. 1987;40:102–114. [PMC free article] [PubMed] [Google Scholar]
- 23.Van Eldere J, Robben J, DePauw G, Merckx R, Eyssen H. Appl Environ Microbiol. 1988;54:2112–2117. doi: 10.1128/aem.54.8.2112-2117.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang D Y, Bulbrook R D, Ellis F, Coombs M M. J Endocrinol. 1967;39:395–403. doi: 10.1677/joe.0.0390395. [DOI] [PubMed] [Google Scholar]
- 25.Nankin H R, Calkins J H. J Clin Endocrinol Metab. 1986;63:1418–1420. doi: 10.1210/jcem-63-6-1418. [DOI] [PubMed] [Google Scholar]
- 26.Manni A, Pardridge W M, Cefalu W, Nisula B C, Bardin C W, Santner S J, Santen R J. J Clin Endocrinol Metab. 1985;61:705–710. doi: 10.1210/jcem-61-4-705. [DOI] [PubMed] [Google Scholar]
- 27.Vermeulen A, Verdonck L, Van der Straeten M, Orie N. J Clin Endocrinol. 1969;29:1470–1480. [Google Scholar]
- 28.Miller W L, Auchus R J, Geller D H. Steroids. 1997;62:133–142. doi: 10.1016/s0039-128x(96)00172-9. [DOI] [PubMed] [Google Scholar]
- 29.Roberts E, Fitten L J. In: The Biological Role of Dehydroepiandrosterone (DHEA) Kalimi M, Regelson W, editors. Berlin: de Gruyter; 1990. pp. 43–63. [Google Scholar]
- 30.Peters T., Jr . All About Albumin. San Diego: Academic; 1996. [Google Scholar]
- 31.Wang D Y, Bulbrook R D. J Endocrinol. 1967;39:405–413. doi: 10.1677/joe.0.0390405. [DOI] [PubMed] [Google Scholar]
- 32.Romeu A M, Martino E E, Stoppani A O M. Biochim Biophys Acta. 1975;409:376–386. doi: 10.1016/0005-2760(75)90033-8. [DOI] [PubMed] [Google Scholar]
- 33.Romeu A M, Martino E E, Stoppani A O M. Biochim Biophys Acta. 1976;439:175–193. doi: 10.1016/0005-2795(76)90174-4. [DOI] [PubMed] [Google Scholar]
- 34.Cekan S Z, Xing S, Ritzén M. Experientia. 1984;40:949–951. doi: 10.1007/BF01946453. [DOI] [PubMed] [Google Scholar]
- 35.Fischer M J E, Bos O J M, van der Linden R F, Wilting J, Janssen L H M. Biochem Pharmacol. 1993;45:2411–2416. doi: 10.1016/0006-2952(93)90221-h. [DOI] [PubMed] [Google Scholar]
- 36.Morales A J, Nolan J J, Nelson J C, Yen S S C. J Clin Endocrinol Metab. 1994;78:1360–1367. doi: 10.1210/jcem.78.6.7515387. [DOI] [PubMed] [Google Scholar]
- 37.Cacicedo L, de los Frailes M T, Lorenzo M J, Franco F S. Ann NY Acad Sci. 1993;692:287–290. doi: 10.1111/j.1749-6632.1993.tb26236.x. [DOI] [PubMed] [Google Scholar]
- 38.Gershengorn M C. Annu Rev Physiol. 1986;48:515–526. doi: 10.1146/annurev.ph.48.030186.002503. [DOI] [PubMed] [Google Scholar]
- 39.Le Dafniet M, Le Dafniet M, Garnier P, Bression D, Brandi A M, Racadot J, Peillon F. Horm Metab Res. 1985;17:476–479. doi: 10.1055/s-2007-1013580. [DOI] [PubMed] [Google Scholar]
- 40.Wilson V J, Jones G M. Mammalian Vestibular Physiology. New York: Plenum; 1979. [Google Scholar]
- 41.Nieto-Bona M P, Garcia-Segura L M, Torres-Aleman I. J Neurosci Res. 1993;36:520–527. doi: 10.1002/jnr.490360504. [DOI] [PubMed] [Google Scholar]
- 42.Torres-Aleman I, Pons S, Garcia-Segura L M. Brain Res. 1991;564:348–351. doi: 10.1016/0006-8993(91)91476-h. [DOI] [PubMed] [Google Scholar]
- 43.Castro-Alamancos M A, Torres-Aleman I. Proc Natl Acad Sci USA. 1994;91:10203–10207. doi: 10.1073/pnas.91.21.10203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Flood J F, Morley J E, Roberts E. Proc Natl Acad Sci USA. 1995;92:10806–10810. doi: 10.1073/pnas.92.23.10806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lewis G F, Alessi C A, Imperial J G, Refetoff S. J Clin Endocrinol Metab. 1991;73:843–849. doi: 10.1210/jcem-73-4-843. [DOI] [PubMed] [Google Scholar]
- 46.Nishikawa M, Inada M, Naito K, Ishii H, Tanaka K, Mashio Y, Imura H. J Clin Endocrinol Metab. 1981;52:517–522. doi: 10.1210/jcem-52-3-517. [DOI] [PubMed] [Google Scholar]
- 47.Garnier P E, Roger M, Chaussain J L, Canlorbe P, Job J C. Acta Endocrinol. 1983;103:433–440. doi: 10.1530/acta.0.1030433. [DOI] [PubMed] [Google Scholar]
- 48.Teng E L, Wimer C, Roberts E, Damasio A R, Eslinger P J, Folstein M F, Tune L E, Whitehouse P J, Bardolph E L, Chui H C, Henderson V W. J Clin Exp Neuropsychol. 1989;11:899–912. doi: 10.1080/01688638908400943. [DOI] [PubMed] [Google Scholar]
- 49.Parker S W. Clin Electroenceph. 1993;24:151–159. doi: 10.1177/155005949302400405. [DOI] [PubMed] [Google Scholar]
- 50.Morley J E, Perry H M, III, Kaiser F E, Kraenzle D, Jensen J, Houston K, Mattammal M, Perry H M., Jr J Am Geriatr Soc. 1993;41:149–152. doi: 10.1111/j.1532-5415.1993.tb02049.x. [DOI] [PubMed] [Google Scholar]
- 51.Urban R J, Bodenburg Y H, Gilkison C, Foxworth J, Coggan A R, Wolfe R R, Ferrando A. Am J Physiol. 1995;269:E820–E826. doi: 10.1152/ajpendo.1995.269.5.E820. [DOI] [PubMed] [Google Scholar]


