Abstract
We prospectively examined associations between perceived imbalance and Parkinson’s disease (PD) risk in the Health Professional Follow-up Study (HPFS), and Nurses’ Health Study (NHS). We included 39,087 men and 82,299 women free of PD at baseline (1990) in the current analyses. We documented 449 incident PD cases during 12 years follow-up. Subjects who reported difficulty with balance before 1990 (baseline) were 1.8 more times likely to develop PD, relative to those who reported no balance difficulty (pooled multivariate RR=1.8; 95%CI: 1.3, 2.5; P<0.0001). When we further examined associations between perceived imbalance at baseline and PD onset during different time periods, we found a significant elevation of PD risk only during the first 4 years of follow-up. This result suggests that the imbalance may in some cases be an early sign of PD, and may represent the onset of motor symptoms although they have not been clinically recognized.
Keywords: Perceived imbalance, Parkinson’s disease, Prospective study
Balance impairment is common among Parkinson’s disease (PD) patients. Approximately 70% of PD patients fall at least once per year 1. In a recent longitudinal study, a feeling of imbalance was found to be associated with an increased risk of developing PD during 4–9 years follow-up 2 and may thus be a preclinical indicator of PD. Because PD may have a long preclinical stage 3, 4, it is interesting to examine how long preclinical imbalance may precede the diagnosis of PD in prospective studies with longer periods of follow-up. A better understanding of this and other preclinical signs may eventually lead to early identification of PD patients. We therefore prospectively examined associations between perceived imbalance and PD risk in the Health Professional Follow-up Study (HPFS), and Nurses’ Health Study (NHS) with 12 years follow-up.
Subjects and Methods
The NHS comprises 121,700 female registered nurses aged 30–55 years at the time of enrollment in 1976. The HPFS was established in 1986, when 51,529 male US health professionals aged 40–75 years completed a mailed questionnaire about their medical history and lifestyle. On the 1990 questionnaire, HPFS and NHS participants were asked whether they had difficulty with their balance. In the HPFS, the information of imbalance was updated every two year. In the NHS, it was collected again in 1996. We, therefore, used the 1990 as baseline for current study. We excluded participants with a diagnosis of PD at baseline or missing balance information, leaving us 39,087 men and 82,299 women in the current analyses. Information on potential confounders, including age, weight, height, smoking status, use of medicines, and history of chronic diseases, was collected via biennial questionnaires through the follow-up. Dietary intakes were assessed with a semi-quantitative food frequency questionnaire validated for use in this population. 5 Participants in the HPFS were asked to report the average time they spent per week on each of the following activities over the previous year: walking or hiking outdoors, jogging, running, bicycling, lap swimming, tennis, squash or racket ball, and calisthenics or rowing. For each activity, we asked the average time spent per week with 10 possible categories ranging from 0 to ≥11 hours. In addition, we asked the number of flights of stairs climbed per day with five categories ranging from ≤ 2 to >15. Similar questions were asked every 2 years during the follow-up. Based on these individual activities, we calculated total physical activity in metabolic equivalent tasks (METs) by multiplying the hours spent per week on each activity by its typical energy expenditure requirements. 6 A similar questionnaire has been used in the NHS cohort to assess physical activity. The validation studies indicated relatively good validity and reproducibility for the questionnaire for both cohorts. 7, 8
Assessment of PD has been described elsewhere 9. Briefly, we identified new PD cases by biennial self-reported questionnaires. We then asked the treating neurologists to complete a questionnaire to confirm the diagnosis of PD or to send a copy of the medical records. A case was confirmed if a diagnosis of PD was considered definite or probable by the treating neurologist or internist, or if the medical record included either a final diagnosis of PD made by a neurologist, or evidence of at least two of the three cardinal signs (rest tremor, rigidity, bradykinesia) in the absence of features suggesting other diagnoses. The review of medical records was conducted by the investigators, blind to the exposure status. Overall, the diagnosis was confirmed by the treating neurologist in >80% of the cases. We also requested the death certificates of the deceased study participants and identified PD diagnoses that were not reported in the regular follow-up (less than 2%).
We computed the person-time of follow-up for each participant from the return date of the baseline questionnaire (1990) to the date of the occurrence of the first symptoms of PD, the date of death, or the end of follow up (Jan 31st, 2002 for the HPFS and Jun 30th, 2002 for the NHS), whichever came first. Multivariate adjusted relative risks (RRs) of PD from each cohort were calculated using the Cox proportional hazard models and pooled by a random-effects model, weighted by the inverse of their variances. To explore the temporal relationship between imbalance and PD, we examined whether self-reported imbalance at baseline (1990) was associated with PD onset during different time period: 91–94, 95–98, and 99–02. . In secondary analyses, we examined the association between imbalance at different times during the follow-up and the risk of PD in the following 2-year period; information on imbalance was collected in 1992, 1994, 1996, 1998 and 2000 in the HPFS, and in 1990 and 1996 in the NHS. Statistical analyses were performed using SAS software (version 9.1, SAS Institute Inc., Cary, NC) and STATA (version 9.2, StatsCorp LP, College Station, TX).
Results and discussion
During 12 years follow-up, we documented 449 incident PD cases (242 men and 207 women). Subjects who reported difficulty with balance before 1990 were 1.8 more times likely to develop PD, relative to those who reported no balance difficulty (pooled multivariate RR=1.8; 95%CI: 1.3, 2.5; P<0.0001) (Table), after adjustment for age, smoking status, BMI, physical activity, caffeine intake and alcohol. The association was somewhat stronger in women (RR=2.1, P=0.0009) than in men (RR=1.5, P=0.09), but the difference between genders was not significant. This association did not change when we further adjusted the results for presence of stroke, cancer, hypertension, diabetes and heart diseases (myocardial infarction or angina pectoris) and was slightly stronger when we used updated information of imbalance ( pooled multivariate RR was 2.0; 95%CI: 1.5, 2.5; P<0.0001). When we further examined associations between perceived imbalance at baseline (1990) and PD onset during different time periods, we found a significant elevation of PD risk only during the first 4 years of follow-up (RR=2.2. 95%CI: 1.3, 3.7; P<0.001) (Figure).
Table.
Lifestyle characteristics and risk ratios (RRs) of Parkinson’s disease according to perceived imbalance in the HPFS and NHS (1990 for both)
| Self-reported difficulty with balance | ||||
|---|---|---|---|---|
| Men | Women | |||
| no | yes | no | yes | |
| n | 37567 | 1520 | 77695 | 4604 |
| # of PD cases | 222 | 19 | 183 | 24 |
| Age, y | 57.7 | 64.9 | 56.3 | 57.9 |
| Current smoking1, % | 7.2 | 9.6 | 16.7 | 16.3 |
| Past smokers1, % | 46.8 | 49.4 | 39.1 | 39.6 |
| BMI1, kg/m2 | 25.6 | 26.1 | 25.7 | 26.8 |
| Physical activity1, METs/wk | 37.8 | 33.3 | 15.8 | 13.5 |
| Alcohol intake1, g/d | 10.1 | 8.6 | 5.2 | 3.8 |
| Caffeine intake1, mg/d | 233 | 222 | 258 | 237 |
|
| ||||
| Age- and smoking- adjusted RR | 1 (ref.) | 1.6 (1.0, 2.6) | 1 (ref.) | 2.0(1.3, 3.1)* |
| Multiple adjusted RR2 | 1 (ref.) | 1.5 (0.9, 2.6) | 1 (ref.) | 2.1(1.3, 3.2)** |
| Pooled RR3 | 1 (ref.) | 1.8 (1.3, 2.5)*** | ||
Values were standardized to the age distribution of each cohort.
Adjusted for age (in months), smoking status (never smoker, former smoker, or current smoker: cigarettes/d, 1–14 or ≥ 15), BMI (<23, 23–24.9, 25–26.9, 27–29.9, or ≥30 kg/m2), physical activity (quintiles), caffeine intake (quintiles) and alcohol (none, 1–4.9, 5–9.9, 10–14.9, or ≥15 g/d for women; none, 1–9.9, 10–19.9, 20–29.9, or ≥30 g/d for men).
P<0.01,
P<0.001, and
P<0.0001, relative to subjects without perceived imbalance at baseline.
Based on multiple adjusted estimates, using the random-effects models.
Figure.
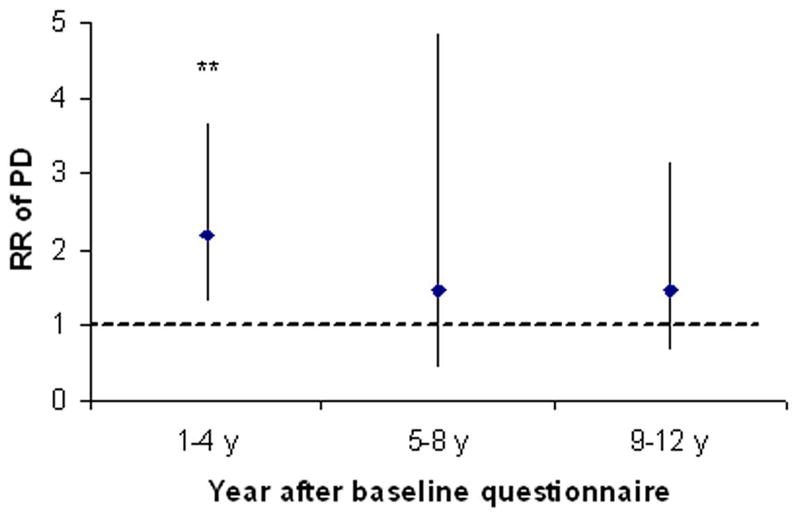
Relative risk (RR) and 95% confidence intervals of PD for subjects with perceived imbalance relative to those not, according to year of PD onset. Adjusted for age (months), smoking status (never smoker, former smoker, or current smoker: cigarettes/d, 1–14 or ≥ 15), BMI (<23, 23–24.9, 25–26.9, 27–29.9, or ≥30 kg/m2), physical activity (quintiles), caffeine intake (quintiles) and alcohol intake (none, 1–4.9, 5–9.9, 10–14.9, or ≥ 15 g/d for women; none, 1–9.9, 10–19.9, 20–29.9, or ≥30 g/d for men). **P<0.001, relative to subjects without perceived imbalance at baseline.
Our observations that self-reported feeling of imbalance was significantly associated with ~ 2-fold increased risk of PD are consistent with the results of previous studies 2, 10. The attenuation of the association after 4 year or longer suggests that imbalance is an early symptom of PD rather than a risk factor for developing PD. This estimation is consistent with the calculation that pre-symptomatic phase of PD from the onset of neuronal loss until the onset of classic PD symptoms was ~ 5 years based on postmortem neuronal counts in the substantia nigra of PD patients 4. Our findings indicate that perceived imbalance may represent the onset of motor symptoms although they have not been clinically recognized. The main limitation of the present study is that, the clinical diagnosis of PD is not perfect, and some degree of diagnostic error is thus likely. The inclusion of cases of atypical parkinsonism cannot be ruled out. We previously found that PD patients often began to lose weight at approximately 4 years before the diagnosis of PD 11. Previous investigations also showed that PD patients often had olfactory dysfunction, constipation, erectile dysfunction and fatigue many years before the disease diagnosis3, 12, 13. Better characterizations of these nonspecific early signs of PD will not only advance our knowledge about the pathogenesis of PD, but may also lead to early clinical diagnosis.
Acknowledgments
The study was supported by NIH/NINDS grant R01 NS048517, and in part by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences. None of the sponsors participated in the design and conduct of the study, collection, management, analysis, and interpretation of the data, or preparation, review, or approval of the manuscript.
References
- 1.Bohnen NI, Cham R. Postural control, gait, and dopamine functions in parkinsonian movement disorders. Clin Geriatr Med. 2006;22(4):797–812. vi. doi: 10.1016/j.cger.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 2.de Lau LM, Koudstaal PJ, Hofman A, Breteler MM. Serum uric acid levels and the risk of Parkinson disease. Ann Neurol. 2005;58(5):797–800. doi: 10.1002/ana.20663. [DOI] [PubMed] [Google Scholar]
- 3.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24(2):197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 4.Fearnley JM, Lees AJ. Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain. 1991;114 ( Pt 5):2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- 5.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135(10):1114–1126. 1127–1136. doi: 10.1093/oxfordjournals.aje.a116211. [DOI] [PubMed] [Google Scholar]
- 6.Ainsworth BE, Haskell WL, Leon AS, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25:71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Wolf AM, Hunter DJ, Colditz GA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol. 1994;23(5):991–999. doi: 10.1093/ije/23.5.991. [DOI] [PubMed] [Google Scholar]
- 8.Chasan-Taber S, Rimm EB, Stampfer MJ, et al. Reproducibility and validity of a self-administered physical activity questionnaire for male health professionals. Epidemiology. 1996;7(1):81–86. doi: 10.1097/00001648-199601000-00014. [DOI] [PubMed] [Google Scholar]
- 9.Ascherio A, Zhang SM, Hernan MA, et al. Prospective study of caffeine consumption and risk of Parkinson’s disease in men and women. Ann Neurol. 2001;50(1):56–63. doi: 10.1002/ana.1052. [DOI] [PubMed] [Google Scholar]
- 10.Gonera EG, van’t Hof M, Berger HJ, van Weel C, Horstink MW. Symptoms and duration of the prodromal phase in Parkinson’s disease. Mov Disord. 1997;12(6):871–876. doi: 10.1002/mds.870120607. [DOI] [PubMed] [Google Scholar]
- 11.Chen H, Zhang SM, Hernan MA, Willett WC, Ascherio A. Weight loss in Parkinson’s disease. Ann Neurol. 2003;53(5):676–679. doi: 10.1002/ana.10577. [DOI] [PubMed] [Google Scholar]
- 12.Abbott RD, Ross GW, White LR, et al. Midlife adiposity and the future risk of Parkinson’s disease. Neurology. 2002;59(7):1051–1057. doi: 10.1212/wnl.59.7.1051. [DOI] [PubMed] [Google Scholar]
- 13.Gao X, Chen H, Schwarzschild MA, et al. Erectile function and risk of Parkinson’s disease. Am J Epidemiol. 2007 Sep 17; doi: 10.1093/aje/kwm246. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]


