Abstract
Several studies have demonstrated that the mammalian retina contains an autonomous circadian clock. Dopaminergic and other inner retinal neurons express many of the clock genes, whereas some of these genes seem to be absent from the photoreceptors. This observation has led to the suggestion that in mammalian retina the circadian pacemaker driving retinal rhythms is located in the inner nuclear layer. However, other evidence points to the photoreceptor layer as the site of the mammalian retinal clock. The goal of the present study was to demonstrate the presence of a functional circadian clock in photoreceptors. First, using laser capture microdissection and reverse transcriptase-polymerase chain reaction, we investigated which of the clock genes are expressed in rat photoreceptors. We then prepared photoreceptor layer cultures from the retina to test whether these isolated cultures were viable and could drive circadian rhythms. Our data indicated that Per1, Per3, Cry1, Cry2, Clock, Bmal1, Rev-erbα, and Rora RNAs were present in the photoreceptors, whereas we were unable to amplify mRNA for Per2 and Npas2. Photoreceptor layers obtained from Period1-luciferase rats expressed a robust circadian rhythm in bioluminescence and melatonin synthesis. These results demonstrate that mammalian photoreceptors contain the circadian pacemaker driving rhythmic melatonin synthesis.
Keywords: melatonin, circadian rhythms, clock genes, retina, rat
Several studies have demonstrated that the mammalian retina contains an intrinsic circadian clock that controls melatonin synthesis and many other retinal functions (1, 2). Cultured mammalian retina shows a clear circadian rhythm in melatonin release (3-5), and some of the retinal circadian rhythms may persist in animals in which the suprachiasmatic nuclei of the hypothalamus have been lesioned (6, 7).
The regulation of melatonin levels primarily depends on arylalkylamine N-acetyltransferase (Aanat) transcription and enzyme activity. Several studies have focused on the mechanisms that regulate this enzyme. Retinal Aanat mRNA levels show marked day-night and circadian variations in rat and in mouse (8, 9), and the mRNA for the enzyme has been localized to the photoreceptor layer (PRL; 10, 11). Moreover, Aanat transcription in the photoreceptors is under direct control of the circadian clock via the action of the CLOCK: BMAL1 complex on the E-box contained in the proximal promoter of the gene (12, 13).
Melatonin and dopamine (DA) play opposing roles in the regulation of retinal adaptive physiology. DA functions as a humoral signal for light, producing light-adaptive physiology. Melatonin, on the other hand, has dark-adaptive effects. In many species, the synthesis and release of both melatonin and dopamine are under circadian control, with melatonin released at night and dopamine during the daytime. Thus, the melatonin-secreting photoreceptors and dopamine-secreting amacrine and interplexiform cells form a cellular feedback loop functioning to regulate circadian retinal physiology (1, 2).
Recent studies have suggested that DA is the key player in the control of retinal circadian rhythmicity since dopaminergic neurons express several clock genes (14, 15). Furthermore, a recent study (16) has reported that individual horizontal, bipolar, amacrine, and ganglion cells, but not photoreceptors, express six of the core clock genes. These findings support the hypothesis that dopaminergic amacrine cells may contain an autonomous circadian clock that drives DA release and thus retinal rhythmicity. However, it must be noted that retinal DA content and metabolism are circadian in mice that synthesize melatonin but not in mice that are genetically incapable of synthesizing melatonin (17, 18), and daily injections of melatonin induce circadian rhythms of DA in retinas of mice that are unable to synthesize melatonin (18). Furthermore, removal of the rhythmic dopaminergic signal did not abolish the in vivo circadian rhythm of Aanat mRNA in photoreceptors of the rat retina (19). In the present study, we demonstrate that the isolated mammalian PRL contains an autonomous circadian oscillator, capable of driving Period1 (Per1) gene expression and melatonin synthesis in the absence of inner retina input.
MATERIALS AND METHODS
Animals and Tissue Collection
Wistar rats (Jackson laboratories, Bar Harbor, ME, USA) and Per1-luciferase (Per1-luc) rats (gift of Dr. Menaker, University of Virginia) were raised from birth at Morehouse School of Medicine in a 12 h light–12 h dark (LD) cycle of illumination with light (250 Lux) on from Zeitgeber Time (ZT) 0–12. Food and water were available ad libitum. All procedures were approved by the Morehouse School of Medicine Institutional Animal Care and Use Committee.
Laser capture microdissection of photoreceptor cells
Whole eyes were embedded in OCT (Tissue-Tek, Torrance, CA, USA), frozen on dry ice, and stored at −80°C. Frozen tissues were cut at 8 μm thickness and mounted on uncharged glass slides (VWR Scientific, West Chester, PA, USA). The frozen sections were thawed for 30 s and fixed in 75% ethanol for 30 s followed by a wash in RNase-free water for 30 s. The sections were stained with HistoGene (Arcturus Engineering, Mountain View, CA, USA) staining solutions for 15 s followed by a wash with RNase-free water for 30 s. The sections were dehydrated in graded ethanol solutions (75%, 30 s; 95%, 30 s; 100%, 30 s) and cleared in xylene (5 min). After being air dried for 30 min, the slides were kept in a vacuum desiccator for a minimum of 2 h. Laser capture was performed by lifting the outer nuclear layer and inner segments of photoreceptor cells onto HS-CapSure noncontact laser capture microdissection film using a PixCell IIe laser capture microdissection (LCM) system (Arcturus Engineering). Total RNA was extracted from the captured cells using the PicoPure RNA Isolation Kit (Arcturus Engineering). Samples were then reverse transcribed and subjected to reverse transciptase-polymerase chain reaction (RT-PCR) analysis. Details about the primers used are reported by Kamphuis et al. (20).
Preparation of the PRLs
Adult rats received a 5 μl (200 nmol) intraocular injection of kainic acid (KA; Sigma, St. Louis, MO, USA) in sterile saline (pH 7.4) with a Hamilton microsyringe. Animals were euthanized 48 h after the injection. Eyeballs were immediately removed, and eyecups were prepared. The eyecups were filled with a solution containing distilled water and 1% Triton X-100, after 1 min this solution was removed from the eyecup and replaced with distilled water, and then after 1 min the water was removed and replaced with Dulbecco's modified Eagle's medium (DMEM) and placed in a refrigerator at 4°C for 30–40 min. This treatment allows the separation and removal of the remaining damaged inner retina from the photoreceptors (21).
To verify the purity of our preparation, PRLs (n=4) were subjected to molecular analysis using common markers for inner retinal neurons and ganglion cells. Samples were reverse transcribed and subjected to RT-PCR using the following primers: tyrosine hydroxylase (Th; Genbank accession number M10244) forward 5′-cagggctgctgtcttcctac-3′ and reverse 5′-gggctgtccagtacgtcaat-3′ (247 bp product); glutamic acid decarboxylase (Gad65; Genbank accession number M72422) forward 5′-cgcactgccaaacaactcta-3′ and reverse 5′-caggggcgatctcataggta-3′ (153 bp product); rhodopsin (Rho, Genbank accession number NM033441) forward 5′-gcagtgttcatgtgggattg-3′ and reverse 5′ctgccttctgagtggtagcc-3′ (191 bp product); short wavelength opsin (SWL or blue cone, Genbank accession number AF051163) forward 5′-gtaccacattgctcccgtct-3′ and reverse 5′-agacctgctacagagcccaa-3′ (282 bp product); and Thy-1 (Genbank accession number X03150) forward 5′-gagggcgactacatgtgtga-3′ and reverse 5′-aggaaggagagggaaagcag-3′ (164 bp product).
Bioluminescence recording
Per1-luc rats were anesthetized with CO2 and decapitated. The PRL (prepared as described above) or the whole retinas were explanted from the eyeball and cultured in a 35 mm petri dish with 1.2 ml culture medium [serum-free, low sodium bicarbonate, no phenol red, and DMEM (D2902–10×1L, Sigma)] supplemented with 10 mM HEPES (pH 7.2), B27 (2%; 17504–044, Life Technologies, Inc., Gaithersburg, MD, USA), and 0.1 mM luciferin (beetle luciferin, potassium salt, Molecular Imaging Products) and antibiotics (25 U/ml penicillin and 25 μg/ml streptomycin). No change of medium occurred during the whole duration of the experiment. Bioluminescence in the PRLs was measured with Lumicycle (Actimetrics, Evanston, IL, USA). The device was maintained in a light-tight incubator at 36°C and interfaced to a PC for continuous data acquisition (22, 23).
Data analysis
Lumicycle files were exported for analysis using Origin software. Rhythms were plotted following a standard detrending procedure whereby the 24 h running mean was subtracted from the raw data to remove baseline drift that can mask the circadian rhythm. The data were then smoothed with a 2 h running mean. Daily peaks were identified by the software using a local maximum (22).
Culture of PRLs and melatonin measurement
PRLs were gently removed from the eyecup and placed in Transwell culture system and cultured with DMEM plus antibiotics (10 U/ml penicillin and 10 μg/ml streptomycin). The PRLs were individually cultured in an incubator (95% O2 and 5% CO2) at 36°C for 5 days in constant darkness. To increase melatonin production, 10 μM of 5-HT (Sigma) were added to the medium. PRLs were prepared in the morning and placed in the incubators around 1:00–2:00 pm; just before 7:00 pm the culturing medium was changed and the PRLs were placed in complete darkness for the following 5 days. On the day of the sampling (i.e., on the 1st, 3rd, and 5th days of culture), the medium was replaced every 12 h starting at circadian time (CT)0 (i.e., the time of the expected light onset). The sampling of the medium was carried out under red dim light (<3 lux, lower wavelength cutoff at 640 nm). Melatonin levels were measured by radio-immunoassay as described previously (3-5).
RESULTS
Expression of clock genes in the photoreceptors
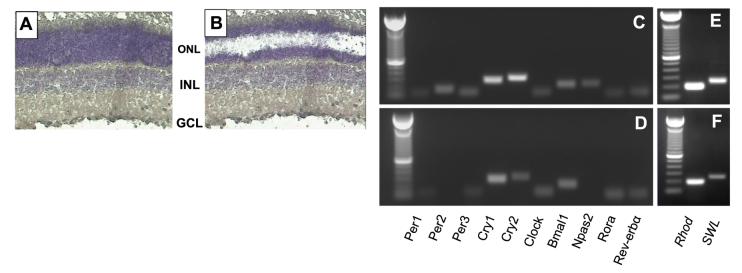
To resolve this important issue, we collected photoreceptor cells from rat retina using laser capture microdissection to investigate the expression of Per1, Period2 (Per2), Period3 (Per3), Cryptochrome1 (Cry1), Cryptochrome2 (Cry2), Clock, Bmal1, Npas2, and Rev-erbα (Rora), by RT-PCR. Transcripts for all these genes were amplified from the entire retina (Fig. 1C), whereas in the photoreceptors we amplified mRNAs for Per1, Per3, Cry1, Cry2, Clock, Bmal1, Rev-erbα, and Rora but not for Per2 and Npas2 (Fig. 1D). Such a result indicates that the key components of the molecular clock are present in the photoreceptor cells. Specific photoreceptor markers for such as Rho and SWL opsin mRNAs were also amplified from these samples (Fig. 1E, F).
Figure 1.
LCM of individual cell layers of rat retina. Photomicrographs of a retinal section before (A) and after photoreceptors (B) have been captured using LCM. Agarose gel electrophoresis of amplicons generated after 35 (40 for LCM samples) amplification cycles for the 10 clock genes investigated in whole retina (C) and in photoreceptor cells (D). Rhodopsin and blue cone opsin were also amplified in both samples (E, F). RT-PCR products are of expected length. Similar results have been also obtained in samples obtained at different time points. ONL = outer nuclear layer; INL = inner nuclear layer; GCL = ganglion cell layer.
PRLs
Figure 2 shows photomicrographs of cross sections obtained from an untreated retina (A) and from a retina treated with KA and Triton (C). The histological analysis indicated that the KA and Triton treatment causes lysis of nearly all inner retinal cells and completely removes the ganglion cell layer. As reported by Cahill and Besharse (21), photoreceptor cells were not damaged by this treatment. Figure 2B, D shows the molecular analysis of the PRLs using common markers for photoreceptors, inner retinal neurons and ganglion cells such as Rho, Th, Gad65, and Thy-1 mRNA. All these mRNAs were amplified in whole retina (Fig. 2B), whereas only Rho mRNA was amplified in the PRLs (Fig. 2D).
Figure 2.
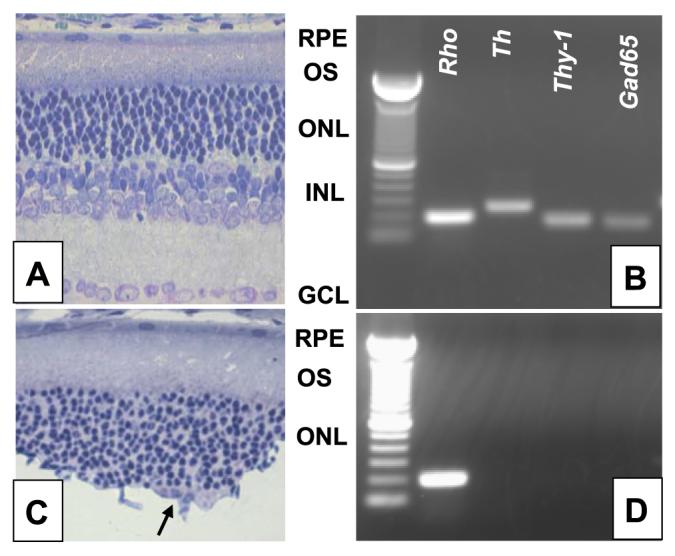
Preparation of PRLs in eyecup after lesion of inner retina. Photomicrographs of a control (A) and of an eyecup (C) treated with our protocol. Entire ganglion cells layer and inner nuclear layer are removed by our experimental procedure. However, a few inner pyknotic nuclear neurons may be still present in some retinal sections (arrow). Agarose gel electrophoresis of RT-PCR generated amplicons, generated after 35 cycles for Rhodopsin, Th, Thy-1, and Gad65 in intact retina (B) and photoreceptors (D). No mRNA for inner retinal neurons and ganglion cells were amplified in the PRL. Products are of predicted sizes (Rho: 191 bp; Th: 247 bp, Thy-1:164 bp, Gad65: 153). A similar result has been obtained at least in 4 independent samples for each group. RPE = retinal pigmented ephitelium; OS = outer segment.
Per1-luc rhythms in the intact retina and in PRLs
We then investigated if these PRLs were viable and could drive circadian rhythms. We cultured intact retinas and PRLs from Per1-luc rats at 5 wk of age to measure bioluminescence rhythms in vitro. As shown in Fig. 3, intact retinas were mostly arrhythmic (n=16, top panel) or showed a low amplitude circadian rhythm (n=4, t=22.3±0.63 se, middle panel). In contrast, all the PRLs tested (n=5) expressed a robust circadian rhythm (n=5, τ=23.0±0.47 se, bottom panel). Interestingly, we also observed that the peak phase of the Per1-luc rhythm in the PRLs (CT=9.1±021) was significantly advanced (t test, P<0.001) with respect to what we observed in the few rhythmic retinas (CT=16.6±1.43).
Figure 3.
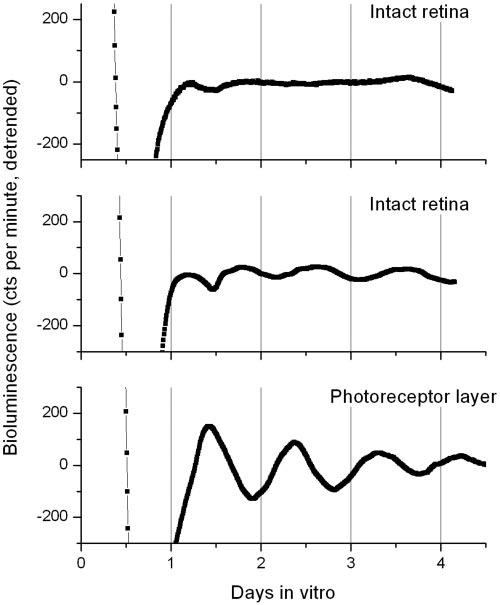
PRLs or intact retinas harvested from Per1-luciferase rats were placed in 35 mm dishes containing 1.2 ml DMEM media with luciferin and sealed with vacuum grease. Medium was not changed for duration of entire experiment. Dishes were recorded using Lumicycle. Raw bioluminescence data were detrended and smoothed (2 h) to facilitate period and phase calculations. A clear rhythm was observed in all (n=5) PRL tested (bottom panel, χ2 P<0.05), whereas vast majority (12 out of 16) intact retinas were mostly arrhythymic.
Melatonin synthesis in PRLs
Previous studies have shown that melatonin synthesis is rhythmic in cultured mammalian retina (3-5) and in PRLs obtained from Xenopus laevis (21). Therefore, we decided to investigate whether mammalian PRLs were capable of rhythmic melatonin synthesis when cultured in constant darkness. As shown in Fig. 4, PRLs synthesized melatonin in a rhythmic fashion for 5 days. As expected, melatonin levels were significantly higher during the subjective night than during the day (t-tests, P<0.01 in all cases). Such a result directly demonstrates that the circadian pacemaker located in the photoreceptors is responsible for driving the circadian rhythm in melatonin synthesis.
Figure 4.
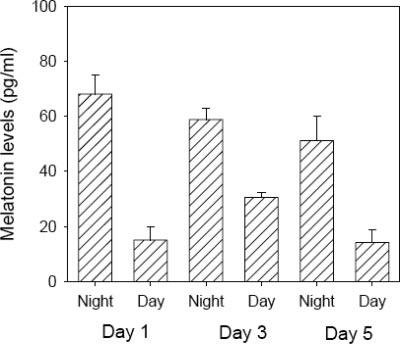
Melatonin levels in PRLs (n=6) showed a significant variation between subjective day and subjective night (t-tests, P<0.01 in all cases). In this experiment, medium was replaced every 12 h.
DISCUSSION
The presence of a circadian clock within the mammalian retina has been reported by a series of in vivo and in vitro studies (3-7, 9, 16). In nonmammalian vertebrates, several studies have established that a circadian clock system is present in the photoreceptors (21, 24). In mammals, although a few studies have provided compelling evidence suggesting that mammalian photoreceptors contain a circadian clock (9, 12-13, 19), a direct demonstration of a circadian clock in mammalian photoreceptors is missing. The data reported here constitute the first direct demonstration that mammalian photoreceptors contain the circadian clock that drives rhythmic melatonin synthesis.
A few studies have investigated the distribution of clock genes in the rat and mouse retina. In the rat, with the use of in situ hybridization Per1 transcripts were detected (but with a low level) in the photoreceptors, whereas Per2 transcripts were present in the inner nuclear layer and ganglion cell layer (25) but not in PRL. Our data agree well with these results, since we detected Per1 mRNA, but not Per2, in the photoreceptors (Fig. 1D).
Early studies in the mouse reported that Per1, Bmal1, and Clock transcripts were present in the photoreceptors (26), whereas Cry1 and Cry2 transcripts were not detectable in the outer nuclear layer of mice (27). More recently, a series of contradictory results have been reported with respect to Per1 and Cry2 distribution in the retina. Witkovsky et al., (15) and Garcia-Fernandez et al., (28) reported that Per1 mRNA and protein are absent from the photoreceptors, whereas Yujnovsky et al., (29) and Dinet et al., (30) observed Per1 transcripts and immunoreactivity in the photoreceptors. Recently, CRY2 immunoreactivity in the photoreceptors has been also reported (30).
In the last few years, several studies have shown that LCM is a powerful and very reliable method to investigate the presence of specific mRNA in retinal layers and/or cells (11, 31-32). Therefore, we decided to use this technique to investigate which of the clock genes are expressed in the photoreceptor cells to provide some definitive answers about which of the clock genes are expressed in the photoreceptors. Our data indicate that the transcripts for all the clock genes, except for Per2 and Npas2, are present in the photoreceptors, thus indicating that most of the molecular components of the circadian clock are present in these cells.
Previous studies have reported that intravitreal injection of KA causes excitotoxic cell death of several classes of neurons in the inner retina but spares photoreceptors (33). However, it is worthwhile to mention that retinal ganglion cells are less sensitive to KA treatment since ∼50% of the retinal ganglion cells survive KA treatment (33, 34). In our new preparation, we have combined this experimental approach with the technique developed by Cahill and Besharse (21) to obtain PRLs. Indeed, our histological and molecular analysis indicated that the PRLs obtained with this new procedure are not likely to contain viable ganglion cells and/or inner retinal neurons (Fig. 2C, D).
A series of recent studies have shown that a circadian clock is also present in the inner retina. Circadian rhythms in the dopaminergic and melatonergic systems are present in rats with dystrophic retina (5, 35), and mouse inner retinal neurons coexpress mRNA for several clock genes (15-16). Finally, a very recent study (16) has reported a circadian rhythm in the retina of mPer2::LUC expression in animals with almost no photoreceptors. These results, together with the data reported here, establish the presence of at least two different circadian clock systems in the mammalian retina. The presence of multiple circadian clocks within the vertebrate retina has also been observed in the chicken (36).
An intriguing aspect of our data is the observation that intact retinas show a very low amplitude rhythm or no rhythm at all in Per1-luc bioluminescence. These data are consistent with what has been reported in Per2::Luc mice (16). Conversely, a clear circadian rhythm in cultured mice and rat retina is present in melatonin levels (5). Such a discrepancy can be easily explained by the fact that Per1 and Per2 transcripts are expressed in more than one retinal layer and therefore it is likely that the Per1-luc, or the mPer2::LUC, bioluminescence levels represent the expression of the multiple clocks that are present in the retina (5, 16). This is supported by our results with Per1-luc PRL in which we observed that the phase of the PRL rhythms was significantly different from the phase of the few rhythmic whole retinas (Fig. 3). Further support is provided by recently published data (19) in which a significant change in the phase of Per1 mRNA was observed once the inner retina has been damaged. Such a problem is not present when the output measured is represented by melatonin, since this hormone, under normal conditions, is almost exclusively synthesized in the photoreceptors (11).
Since Aanat and Per1 transcription in rat photoreceptors is under the control of the circadian clock via the action of the CLOCK:BMAL1 complex (13), it is likely that changes in the circadian control of Per1 transcription will be also observed in Aanat regulation. Indeed, a previous study (19) reported that phase of the circadian rhythm in Aanat mRNA is advanced (∼6-h) in rats in which the inner retina has been removed. This observation suggests that although PRLs contain the circadian pacemaker driving the melatonin synthesis, some interaction must exist between the photoreceptor and the inner retinal clocks and this relationship seems to be important to set the phase of the clock in the photoreceptors (5, 19).
Melatonin and DA play opposing roles in the regulation of retinal adaptive physiology (1-2). Melatonin inhibits the release of DA through an action on melatonin receptors (37-40), and dopamine inhibits the synthesis and release of melatonin from photoreceptor cells by acting on D2-like dopamine receptors (41-44). Thus, the melatonin-secreting photoreceptors and DA-secreting inner retinal neurons form a cellular feedback loop functioning to regulate circadian retinal physiology. Additional experimental evidence suggests that melatonin is the key player in the generation of the circadian rhythms in the dopaminergic system since retinal dopamine levels show a circadian rhythm in mice that synthesizes melatonin (17) but not in mouse strains that are genetically unable to synthesize melatonin (17, 18). Furthermore, dopamine rhythmicity in goldfish retina is abolished in retinas cultured in the presence of melatonin or of the melatonin antagonist luzindole (40). Hence, these results indicate that rhythmic melatonin is required for the circadian rhythm in dopamine. Our results (Fig. 4) demonstrate that in the rat rhythmic melatonin synthesis persists in cultured PRLs and therefore it must be concluded that clock driving melatonin synthesis is located within the photoreceptors.
In conclusion, our study provides clear and compelling evidence demonstrating that the mammalian photoreceptor contains a circadian clock controlling melatonin synthesis. This preparation can provide a unique model in which the mechanisms that generate the circadian oscillation, the pathways of entrainment, and the mechanisms by which the clock controls circadian outputs all within a single preparation can be studied.
Acknowledgments
This study was supported by National Institutes of Health Grant NS-43459 and the NASA Cooperative Agreement NCC 9–58 with the National Space Biomedical Research Institute to G. Tosini, Grant NS 34194 to C. Fukuhara, and Grant CA116261 to A. J. Davidson. We also thank the Keck Genomic Center at Morehouse School of Medicine for the use of their facilities.
REFERENCES
- 1.Green CB, Besharse JC. Retinal circadian clocks and control of retinal physiology. J. Biol. Rhythms. 2004;19:91–102. doi: 10.1177/0748730404263002. [DOI] [PubMed] [Google Scholar]
- 2.Iuvone PM, Tosini G, Haque R, Klein DC, Chaurasia SS. Circadian clocks, clock-controlled genes and melatonin biosynthesis in the retina. Prog. Retin. Eye Res. 2005;24:433–456. doi: 10.1016/j.preteyeres.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Tosini G, Menaker M. Circadian rhythms in cultured mammalian retina. Science. 1996;272:419–421. doi: 10.1126/science.272.5260.419. [DOI] [PubMed] [Google Scholar]
- 4.Tosini G, Menaker M. The clock in the mouse retina: melatonin synthesis and photoreceptor degeneration. Brain Res. 1998;789:221–228. doi: 10.1016/s0006-8993(97)01446-7. [DOI] [PubMed] [Google Scholar]
- 5.Sakamoto K, Liu C, Tosini G. Circadian Rhythms in the retina of rats with photoreceptor degeneration. J. Neurochemistry. 2004;90:1019–1024. doi: 10.1111/j.1471-4159.2004.02571.x. [DOI] [PubMed] [Google Scholar]
- 6.Terman JS, Remé CE, Wirz-Justice A. Rod outer segment disk shedding in rats with lesions of the suprachiasmatic nucleus. Brain Res. 1993;605:256–264. doi: 10.1016/0006-8993(93)91748-h. [DOI] [PubMed] [Google Scholar]
- 7.Sakamoto K, Oishi K, Shiraishi M, Hamano S, Otsuka H, Miyake Y, Ishida N. Two circadian oscillatory mechanisms in the mammalian retina. Neuroreport. 2000;18:3995–3997. doi: 10.1097/00001756-200012180-00018. [DOI] [PubMed] [Google Scholar]
- 8.Sakamoto K, Ishida N. Circadian expression of serotonin N-acetyltransferase mRNA in the rat retina. Neurosci. Lett. 1998;245:113–116. doi: 10.1016/s0304-3940(98)00189-x. [DOI] [PubMed] [Google Scholar]
- 9.Fukuhara C, Liu C, Ivanova TN, Chan GC-K, Storm DR, Iuvone PM, Tosini G. Gating of the cAMP signaling cascade and melatonin synthesis by the circadian clock in mammalian retina. J. Neurosci. 2004;24:1803–1811. doi: 10.1523/JNEUROSCI.4988-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niki T, Hamada T, Ohtomi M, Sakamoto K, Suzuki S, Kako K, Hosoya Y, Horikawa K, Ishida N. The localization of the site of arylalkylamine N-acetyltransferase circadian expression in the photoreceptor cells of mammalian retina. Biochem. Biophy. Res. Comm. 1998;248:115–120. doi: 10.1006/bbrc.1998.8916. [DOI] [PubMed] [Google Scholar]
- 11.Liu C, Fukuhara C, Wessel JH, Iuvone PM, Tosini G. Localization of Aanat mRNA in the rat retina by fluorescence in situ hybridization and laser capture microdissection. Cell Tissue Res. 2004;315:197–201. doi: 10.1007/s00441-003-0822-1. [DOI] [PubMed] [Google Scholar]
- 12.Chen W, Baler R. The rat arylalkylamine N-acetyltransferase E-box: different use in a master vs. slave oscillator. Mol. Brain Res. 2000;81:43–50. doi: 10.1016/s0169-328x(00)00160-1. [DOI] [PubMed] [Google Scholar]
- 13.Tosini G, Fukuhara C. The mammalian retina as a clock. Cell Tissue Res. 2002;309:119–126. doi: 10.1007/s00441-002-0578-z. [DOI] [PubMed] [Google Scholar]
- 14.Gustincich S, Contini M, Gariboldi M, Puopolo M, Kadota K, Bono H, LeMieux J, Walsh P, Carninci P, Hayashizaki Y, et al. Gene discovery in genetically labeled single dopaminergic neurons of the retina. Proc. Natl. Acad. Sci. U. S. A. 2004;101:5069–5074. doi: 10.1073/pnas.0400913101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Witkovsky P, Veseinberger E, LeSauter J, Yan L, Johnson M, Zhang DQ, McMahon DG, Silver R. Cellular location and circadian rhythm of expression of the biological clock gene Period 1 in the mouse retina. J. Neurosci. 2003;23:7670–7676. doi: 10.1523/JNEUROSCI.23-20-07670.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruan G-X, Zhang D-Q, Zhou T, Yamazaki S, McMahon DG. Circadian organization of the mammalian retina. Proc. Natl. Acad. Sci. U. S. A. 2006;103:9703–9708. doi: 10.1073/pnas.0601940103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nir I, Haque R, Iuvone PM. Diurnal metabolism of dopamine in the mouse retina. Brain Res. 2000;870:118–125. doi: 10.1016/s0006-8993(00)02409-4. [DOI] [PubMed] [Google Scholar]
- 18.Doyle SE, Grace MS, McIvor W, Menaker M. Circadian rhythms of dopamine in mouse retina: the role of melatonin. Visual Neurosci. 2002;19:593–601. doi: 10.1017/s0952523802195058. [DOI] [PubMed] [Google Scholar]
- 19.Sakamoto K, Liu C, Kasamatsu M, Iuvone PM, Tosini G. Intraocular injection of kainic acid does not abolish the circadian rhythm of Aanat mRNA in the rat photoreceptors. Molecular Vision. 2006;12:117–124. [PubMed] [Google Scholar]
- 20.Kamphuis W, Cailotto C, Dijk F, Bergen A, Buijs RM. Circadian expression of clock genes and clock-controlled genes in the rat retina. Biochem. Biophys. Res. Commun. 2005;330:18–26. doi: 10.1016/j.bbrc.2005.02.118. [DOI] [PubMed] [Google Scholar]
- 21.Cahill GM, Besharse JC. Circadian clock functions localized in Xenopus retinal photoreceptors. Neuron. 1993;10:573–577. doi: 10.1016/0896-6273(93)90160-s. [DOI] [PubMed] [Google Scholar]
- 22.Davidson AJ, Straume M, Block GD, Menaker M. Daily timed meals dissociate circadian rhythms in hepatoma and healthy host liver. Int. J. Cancer. 2006;118:1623–1627. doi: 10.1002/ijc.21591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chansard M, Molyneux P, Nomura K, Harrington ME, Fukuhara C. c-Jun N-terminal kinase inhibitor SP600125 modulates the period of mammalian circadian rhythms. Neuroscience. 2007;145:812–823. doi: 10.1016/j.neuroscience.2006.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pierce ME, Sheshberadan H, Zhang Z, Fox LE, Apple-bury ML, Takahashi JS. Circadian regulation of iodopsin gene expression in embryonic photoreceptors in retinal cell culture. Neuron. 1993;10:579–584. doi: 10.1016/0896-6273(93)90161-j. [DOI] [PubMed] [Google Scholar]
- 25.Namihira M, Honma S, Abe H, Masubuchi S, Ikeda M, Honma K-I. Circadian pattern, light responsiveness and localization of rPer1 and r Per2 gene expression in the rat retina. Neuroreport. 2001;12:471–475. doi: 10.1097/00001756-200103050-00010. [DOI] [PubMed] [Google Scholar]
- 26.Gekakis N, Staknis D, Nguyen HB, Davis FC, Wilsbacher LD, King DP, Takahashi JS, Weitz CJ. Role of the CLOCK protein in the mammalian circadian mechanism. Science. 1998;280:1564–1569. doi: 10.1126/science.280.5369.1564. [DOI] [PubMed] [Google Scholar]
- 27.Miyamoto Y, Sancar A. Vitamin B2-based blue-light photoreceptors in the retynohypothalamic tract as photoactive pigments for setting the circadian clock in mammals. Proc. Natl. Acad. Sci. U. S. A. 1998;95:6097–6102. doi: 10.1073/pnas.95.11.6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia-Fernandez JM, Alvarez-Lopez C, Cernuda-Cernuda R. Cytoplasmic localization of mPER1 clock protein isoforms in the mouse retina. Neuroscience Lett. 2007;419:55–58. doi: 10.1016/j.neulet.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 29.Yujnovsky I, Hirayama J, Doi M, Borrelli E, Sassone-Corsi P. Signaling mediated by the dopamine D2 receptor potentiates circadian regulation by CLOCK:BMAL1. Proc. Natl. Acad. Sci. U. S. A. 2006;103:6386–6391. doi: 10.1073/pnas.0510691103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dinet V, Ansari N, Torres-Farfan C, Korf H-W. Clock gene expression in the retina of melatonin-proficient (C3H) and melatonin-deficient (C57BL) mice. J. Pineal. Res. 2007;42:83–91. doi: 10.1111/j.1600-079X.2006.00387.x. [DOI] [PubMed] [Google Scholar]
- 31.Chaurasia SS, Rollag MD, Jiang G, Hayes WP, Hague R, Natesan A, Zatz M, Tosini G, Liu C, Korf HW, Iuvone PM, Provencio I. Molecular cloning, localization and circadian expression of chicken melanopsin (Opn4): differential regulation of expression in pineal and retinal cell types. J. Neurochem. 2005;92:158–170. doi: 10.1111/j.1471-4159.2004.02874.x. [DOI] [PubMed] [Google Scholar]
- 32.Chaurasia SS, Rollag MD, Jiang G, Haynes WP, Haque R, Natesan A, Zatz M, Tosini G, Liu C, Korf KW, et al. Molecular cloning, localization and circadian expression of melanopsini (Opn4): Differential regulation of expression in pineal and retinal cell types. J. Neurochemistry. 2005;92:158–170. doi: 10.1111/j.1471-4159.2004.02874.x. [DOI] [PubMed] [Google Scholar]
- 33.Chidlow G, Osborne NN. Rat retinal ganglion cell loss caused by kainate, NMDA and ischemia correlates with a reduction in mRNA and protein of Thy-1 and neurofilament light. Brain Res. 2003;963:298–306. doi: 10.1016/s0006-8993(02)04052-0. [DOI] [PubMed] [Google Scholar]
- 34.Sakamoto K, Liu C, Pozdeyev NV, Iuvone PM, Tosini G. Dopamine regulates melanopsin expression in intrinsically photosensitive retinal ganglion cells. Eur. J. Neuroscience. 2005;22:3536–3544. doi: 10.1111/j.1460-9568.2005.04512.x. [DOI] [PubMed] [Google Scholar]
- 35.Doyle SE, McIvor WE, Menaker M. Circadian rhythmicity in dopamine content of mammalian retina: role of the photoreceptors. J. Neurochem. 2002;83:211–219. doi: 10.1046/j.1471-4159.2002.01149.x. [DOI] [PubMed] [Google Scholar]
- 36.Garbarino-Pico E, Carpentieri AR, Contin MA, Sarmiento MI, Brocco MA, Panzetta P, Rosenstein RE, Caputto BL, Guido ME. Retinal ganglion cells are autonomous circadian oscillators synthesizing N-acetylserotonin during the day. J. Biol. Chem. 2004;279:51172–51181. doi: 10.1074/jbc.M309248200. [DOI] [PubMed] [Google Scholar]
- 37.Dubocovich ML. Melatonin is a potent modulator of dopamine release in the retina. Nature. 1983;306:782–786. doi: 10.1038/306782a0. [DOI] [PubMed] [Google Scholar]
- 38.Dubocovich ML, Massana MI, Stanca I, Sauri DM. Melatonin receptor antagonists that differenciate between the human Mel1a and Mel1b recombinant subtypes are used to assess the pharmacological profile of the rabbit retina ML1 presynaptic heteroreceptors. Naunyn-Schmiedeberg's Arch. Pharmacol. 1998;355:365–375. doi: 10.1007/pl00004956. [DOI] [PubMed] [Google Scholar]
- 39.Boatright JH, Rubim NM, Iuvone PM. Regulation of endogenous dopamine release in amphibian retina by melatonin: the role of GABA. Vis. Neurosci. 1994;11:1013–1018. doi: 10.1017/s0952523800003941. [DOI] [PubMed] [Google Scholar]
- 40.Ribelayga C, Wang Y, Mangel SC. A circadian clock in the fish retina regulates dopamine release via activation of melatonin receptors. J. Physiol. 2004;554:467–482. doi: 10.1113/jphysiol.2003.053710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iuvone PM, Besharse JC. Dopamine receptor-mediated inhibition of serotonin N-acetyltransferase activity in retina. Brain Res. 1986;369:168–176. doi: 10.1016/0006-8993(86)90525-1. [DOI] [PubMed] [Google Scholar]
- 42.Zawilska JB, Iuvone PM. Melatonin synthesis in chicken retina: effect of kainic acid-induced lesions on the diurnal rhythm and D2-dopamine receptor-mediated regulation of serotonin N-acetyltransferase activity. Neurosci. Lett. 1992;135:71–74. doi: 10.1016/0304-3940(92)90138-w. [DOI] [PubMed] [Google Scholar]
- 43.Nguyen-Legros J, Chanut E, Versaux-Botteri C, Simon A, Trouvin JH. Dopamine inhibits melatonin synthesis in photorecptor cells through a D2-like receptor subtype in the rat retina: biochemical and histochemical evidence. J. Neurochem. 1996;67:2514–2520. doi: 10.1046/j.1471-4159.1996.67062514.x. [DOI] [PubMed] [Google Scholar]
- 44.Tosini G, Dirden JC. Dopamine inhibits melatonin release in the mammalian retina: in vitro evidence. Neurosci. Lett. 2000;286:119–122. doi: 10.1016/s0304-3940(00)01117-4. [DOI] [PubMed] [Google Scholar]