Abstract
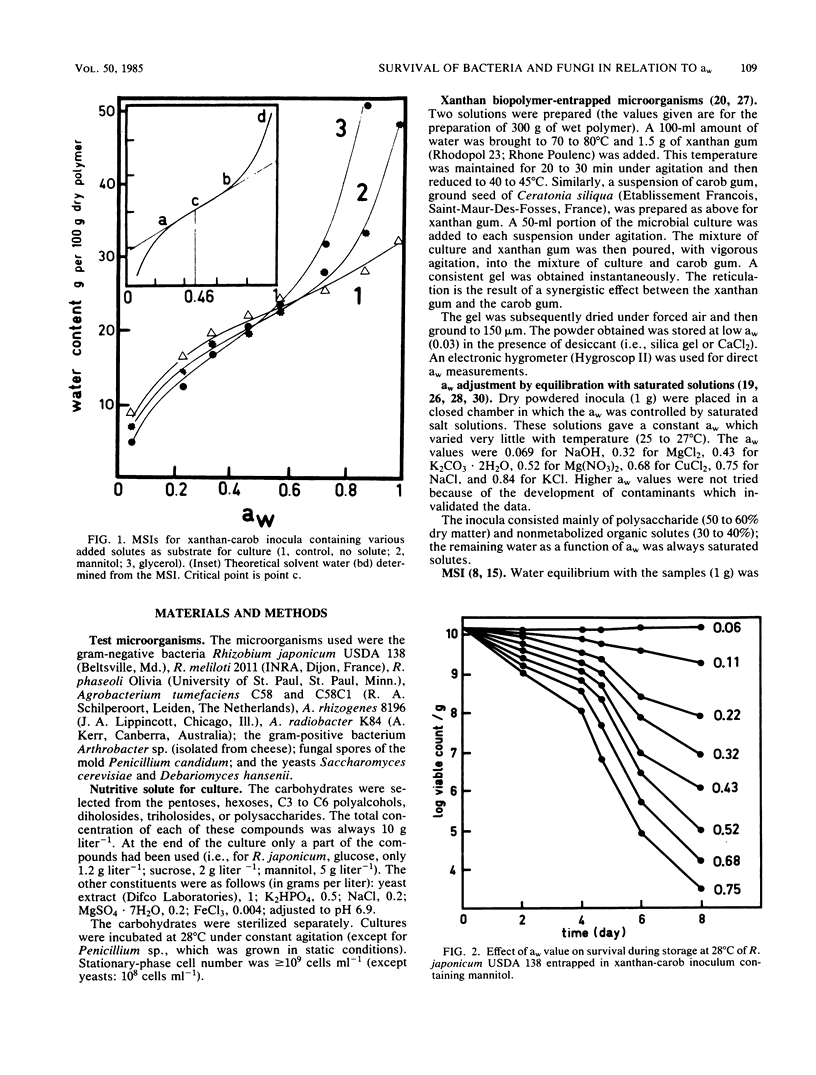
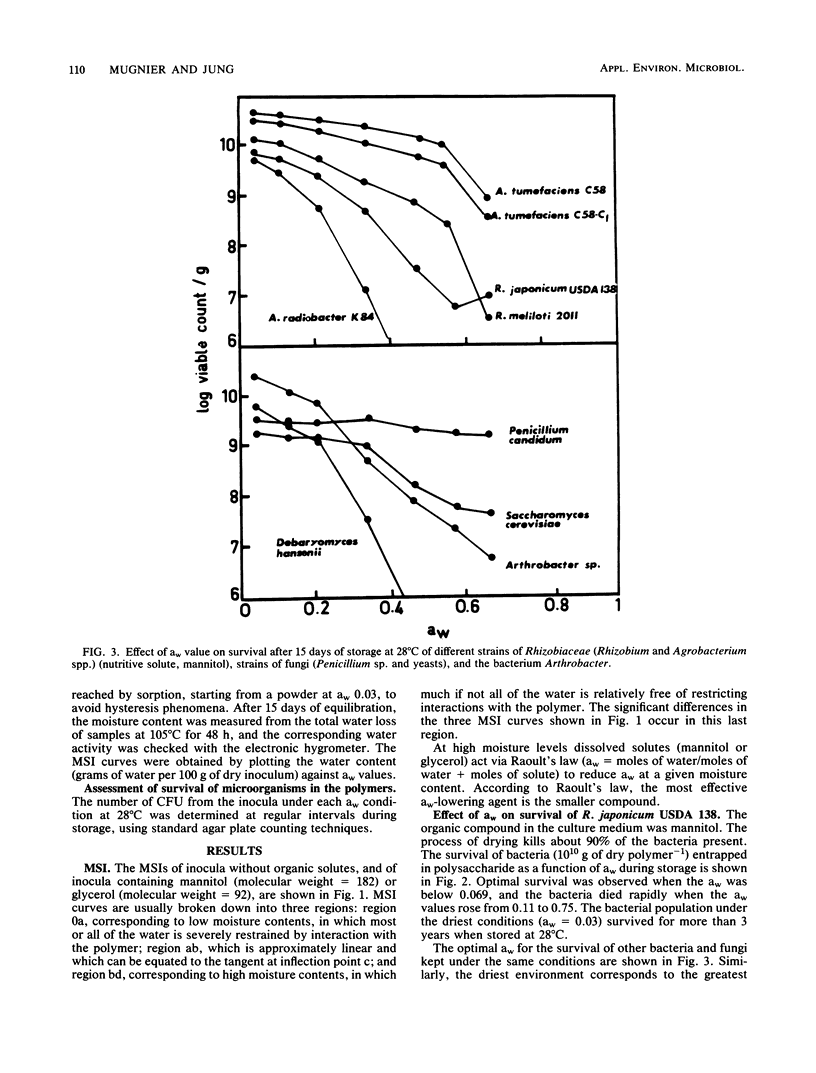
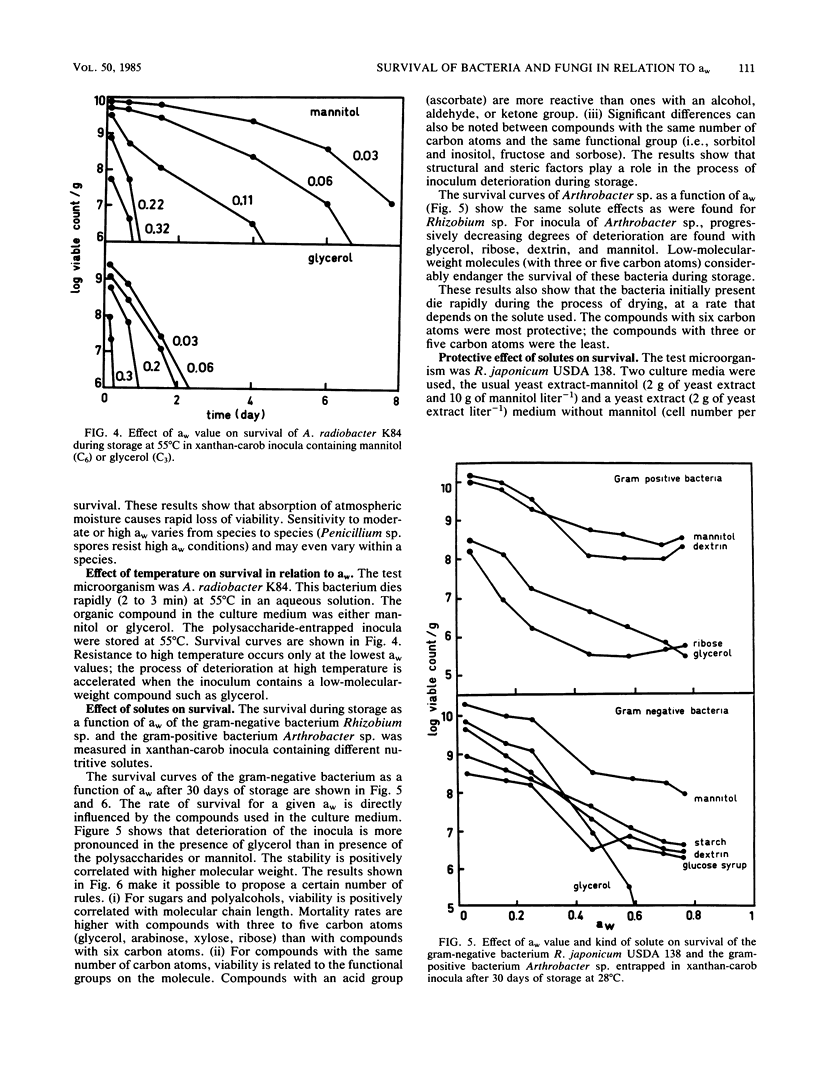
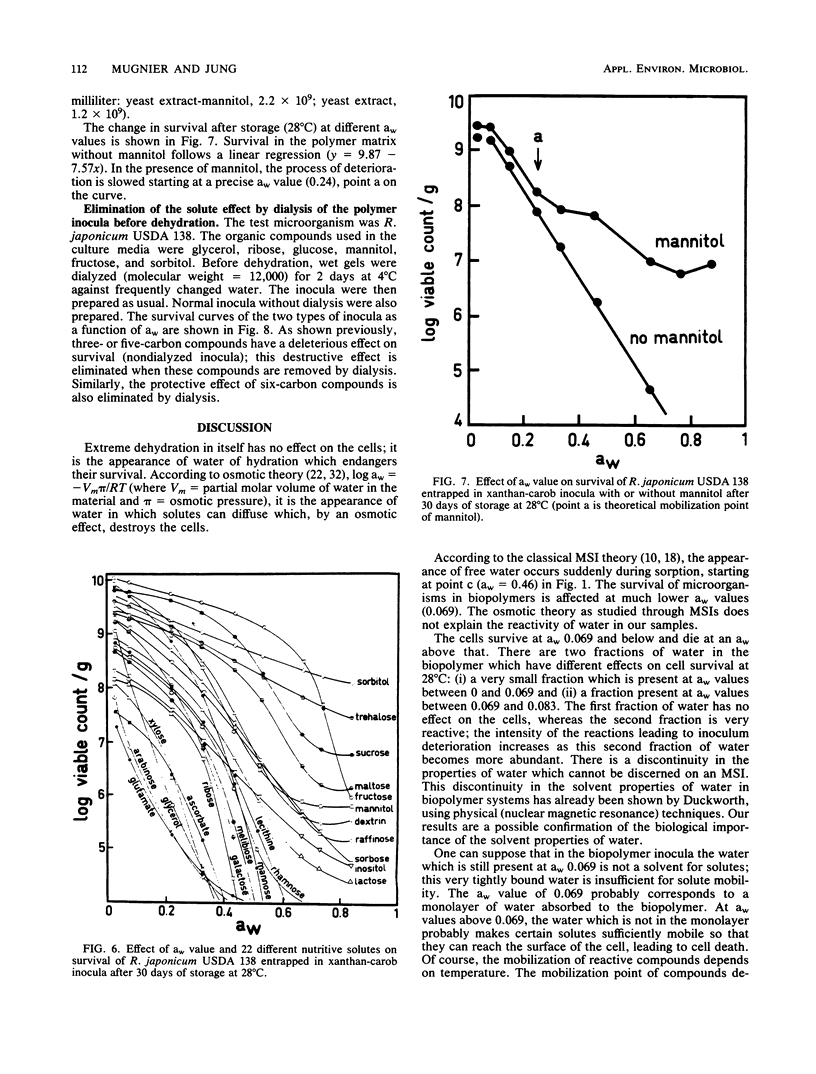
Survival of bacteria (Rhizobium, Agrobacterium, and Arthrobacter spp.), fungal spores (Penicillium sp.), and yeasts (Saccharomyces sp.) was studied in relation to water activity (aw) and the presence of nutritive solutes. The cells were entrapped in polysaccharide gels, as is done to immobilize cells or enzymes, and then dehydrated. The number of living cells (1010 g of dry polymer−1) remained constant for periods of storage of >3 years at 28°C when the inocula were kept at an aw of <0.069. At aw values between 0.069 and 0.83 the number of survivors diminished more and more rapidly as the aw was raised. For a given aw and organism, there were large differences in survival rate as a function of the nutritive solutes used to culture the microorganisms. Low-molecular-weight compounds (with three or five carbon atoms) had a deleterious effect on survival, whereas compounds of higher molecular weight (C6 to C12) had a protecting effect. Thus, the aw alone was not a sufficient explanation for the deterioration of the inocula. Survival seemed to be more directly related to some properties of the water in the biopolymer. New concepts such as the discontinuity of properties of water and the point of mobilization of solutes, already proposed by Duckworth and Kelly (J. Food Technol. 8:105-113, 1973) and Seow (J. Sci. Food Agric., 26:535-536, 1975), have been taken into consideration to explain the interactions of water with the biopolymer and their specific effects on the microorganisms.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown A. D. Compatible solutes and extreme water stress in eukaryotic micro-organisms. Adv Microb Physiol. 1978;17:181–242. doi: 10.1016/s0065-2911(08)60058-2. [DOI] [PubMed] [Google Scholar]
- Brown A. D. Microbial water stress. Bacteriol Rev. 1976 Dec;40(4):803–846. doi: 10.1128/br.40.4.803-846.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corry J. E. The effect of sugars and polysols on the heat resistance of salmonellae. J Appl Bacteriol. 1974 Mar;37(1):31–43. doi: 10.1111/j.1365-2672.1974.tb00412.x. [DOI] [PubMed] [Google Scholar]
- Dommergues Y. R., Diem H. G., Divies C. Polyacrylamide-entrapped Rhizobium as an inoculant for legumes. Appl Environ Microbiol. 1979 Apr;37(4):779–781. doi: 10.1128/aem.37.4.779-781.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUILBOT A., LINDENBERG A. B. [Non-solving and absorbed water in yeast cells]. Biochim Biophys Acta. 1960 Apr 22;39:389–397. doi: 10.1016/0006-3002(60)90190-6. [DOI] [PubMed] [Google Scholar]
- Gould G. W., Measures J. C. Water relations in single cells. Philos Trans R Soc Lond B Biol Sci. 1977 Mar 29;278(959):151–166. doi: 10.1098/rstb.1977.0035. [DOI] [PubMed] [Google Scholar]
- SCOTT W. J. The effect of residual water on the survival of dried bacteria during storage. J Gen Microbiol. 1958 Dec;19(3):624–633. doi: 10.1099/00221287-19-3-624. [DOI] [PubMed] [Google Scholar]
- SCOTT W. J. Water relations of Staphylococcus aureus at 30 degrees C. Aust J Biol Sci. 1953 Nov;6(4):549–564. [PubMed] [Google Scholar]
- Tait M. J., Franks F. Water in biological systems. Nature. 1971 Mar 12;230(5289):91–94. doi: 10.1038/230091a0. [DOI] [PubMed] [Google Scholar]



