Abstract
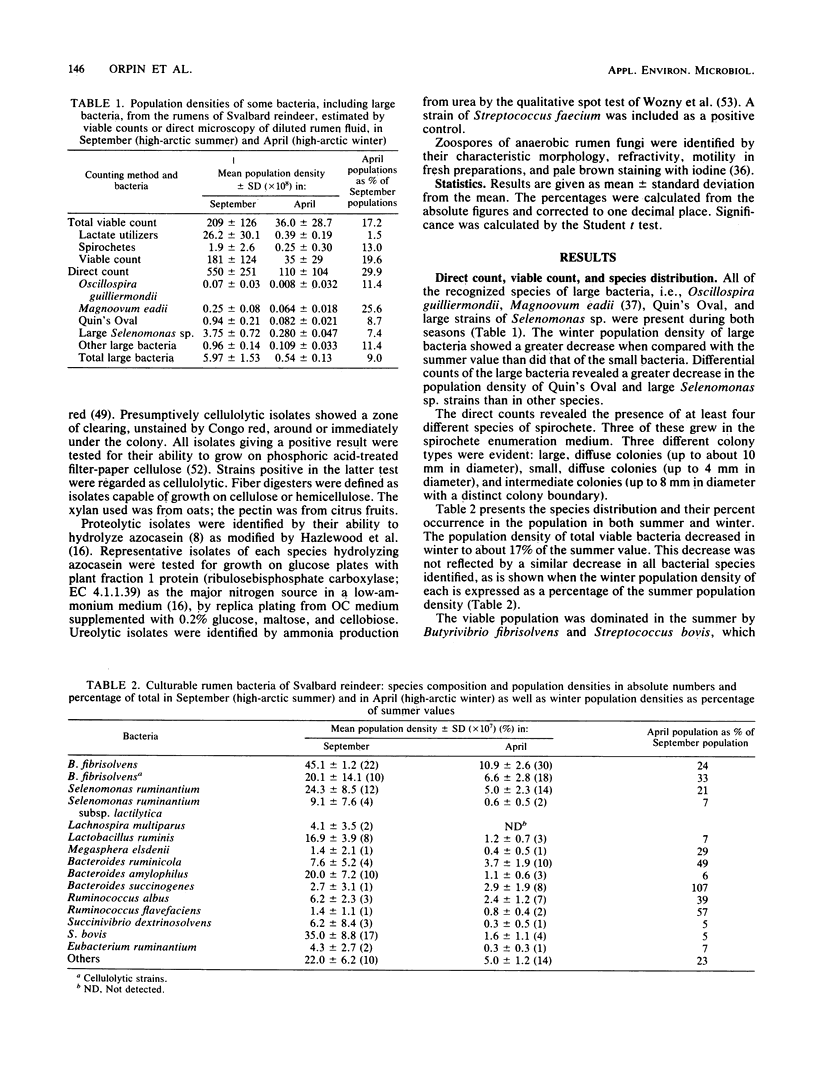
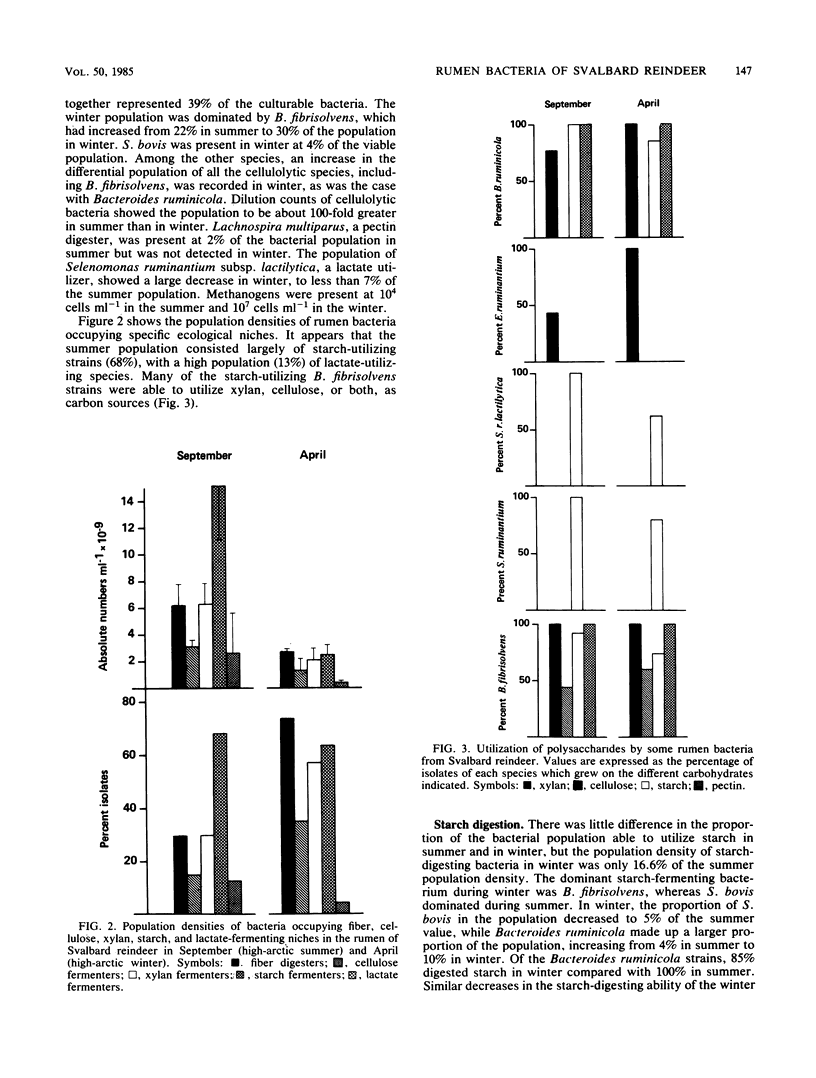
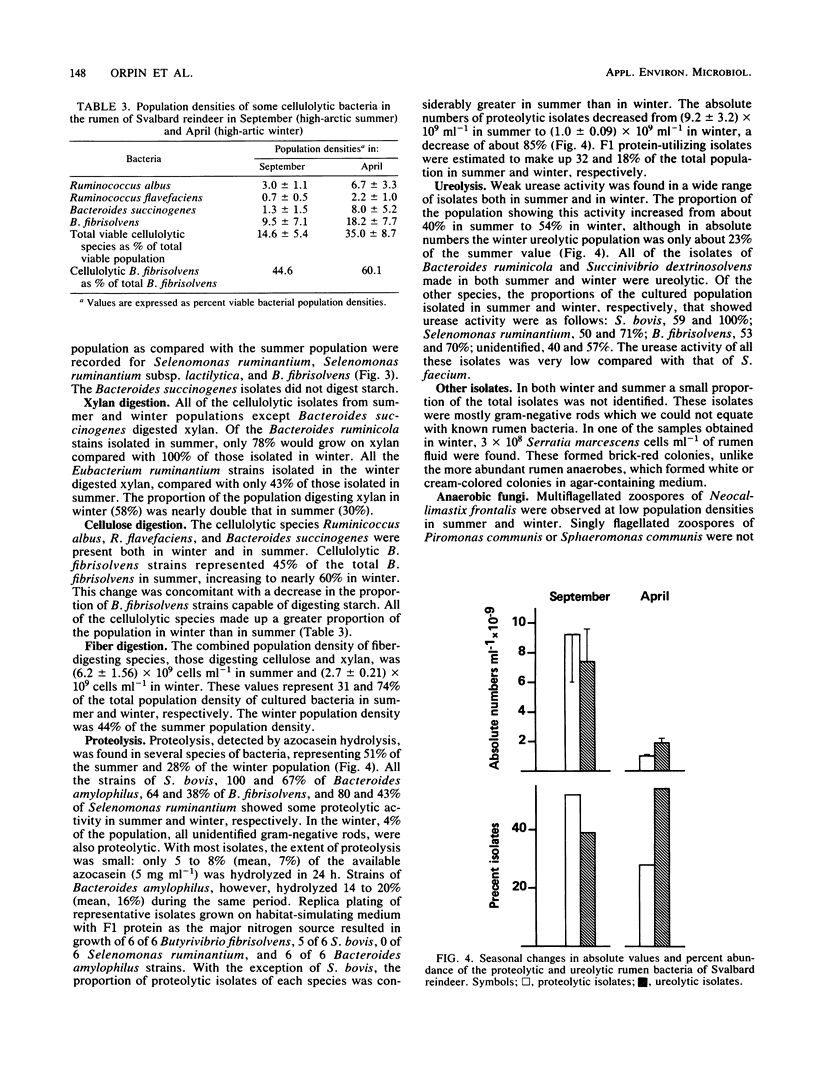
The dominant rumen bacteria in high-arctic Svalbard reindeer were characterized, their population densities were estimated, and ruminal pH was determined in summer, when food quality and availability are good, and in winter, when they are poor. In summer the total cultured viable population density was (2.09 +/- 1.26) X 10(10) cells ml-1, whereas in winter it was (0.36 +/- 0.29) X 10(10) cells ml-1, representing a decrease to 17% of the summer population density. On culture, Butyrivibrio fibrisolvens represented 22% of the bacterial population in summer and 30% in winter. Streptococcus bovis represented 17% of the bacterial population in summer but only 4% in winter. Methanogenic bacteria were present at 10(4) cells ml-1 in summer and 10(7) cells ml-1 in winter. In summer and winter, respectively, the proportions of the viable population showing the following activities were as follows: starch utilization, 68 and 63%; fiber digestion, 31 and 74%; cellulolysis, 15 and 35%; xylanolysis, 30 and 58%; proteolysis, 51 and 28%; ureolysis, 40 and 54%; and lactate utilization, 13 and 4%. The principal cellulolytic bacterium was B. fibrisolvens, which represented 66 and 52% of the cellulolytic population in summer and winter, respectively. The results indicate that the microflora of the rumen of Svalbard reindeer is highly effective in fiber digestion and nitrogen metabolism, allowing the animals to survive under the austere nutritional conditions typical of their high-arctic habitat.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bryant M. P. Nutritional features and ecology of predominant anaerobic bacteria of the intestinal tract. Am J Clin Nutr. 1974 Nov;27(11):1313–1319. doi: 10.1093/ajcn/27.11.1313. [DOI] [PubMed] [Google Scholar]
- Bryant M. P. Nutritional requirements of the predominant rumen cellulolytic bacteria. Fed Proc. 1973 Jul;32(7):1809–1813. [PubMed] [Google Scholar]
- Cook A. R. Urease activity in the rumen of sheep and the isolation of ureolytic bacteria. J Gen Microbiol. 1976 Jan;92(1):32–48. doi: 10.1099/00221287-92-1-32. [DOI] [PubMed] [Google Scholar]
- Edwards T., McBride B. C. New method for the isolation and identification of methanogenic bacteria. Appl Microbiol. 1975 Apr;29(4):540–545. doi: 10.1128/am.29.4.540-545.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FULGHUM R. S., MOORE W. E. ISOLATION, ENUMERATION, AND CHARACTERISTICS OF PROTEOLYTIC RUMINAL BACTERIA. J Bacteriol. 1963 Apr;85:808–815. doi: 10.1128/jb.85.4.808-815.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNGATE R. E. The anaerobic mesophilic cellulolytic bacteria. Bacteriol Rev. 1950 Mar;14(1):1–49. doi: 10.1128/br.14.1.1-49.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlewood G. P., Jones G. A., Mangan J. L. Hydrolysis of leaf Fraction 1 protein by the proteolytic rumen bacterium Bacteroides ruminicola R8/4. J Gen Microbiol. 1981 Apr;123(2):223–232. doi: 10.1099/00221287-123-2-223. [DOI] [PubMed] [Google Scholar]
- Hazlewood G. P., Orpin C. G., Greenwood Y., Black M. E. Isolation of proteolytic rumen bacteria by use of selective medium containing leaf fraction 1 protein (ribulosebisphosphate carboxylase). Appl Environ Microbiol. 1983 Jun;45(6):1780–1784. doi: 10.1128/aem.45.6.1780-1784.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning P. A., van der Walt A. E. Inclusion of xylan in a medium for the enumeration of total culturable rumen bacteria. Appl Environ Microbiol. 1978 Jun;35(6):1008–1011. doi: 10.1128/aem.35.6.1008-1011.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hove K., Jacobsen E. Renal excretion of urea in reindeer. Effect of nutrition. Acta Vet Scand. 1975;16(4):513–519. doi: 10.1186/BF03546644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES G. A., MACLEOD R. A., BLACKWOOD A. C. UREOLYTIC RUMEN BACTERIA. I. CHARACTERISTICS OF THE MICROFLORA FROM A UREA-FED SHEEP. Can J Microbiol. 1964 Jun;10:371–378. doi: 10.1139/m64-050. [DOI] [PubMed] [Google Scholar]
- Jones G. A., Pickard M. D. Effect of titanium (III) citrate as reducing agent on growth of rumen bacteria. Appl Environ Microbiol. 1980 Jun;39(6):1144–1147. doi: 10.1128/aem.39.6.1144-1147.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUBINSKY G. On some parasites of parasitic protozoa. II. Sagittospora cameroni gen. n., sp. n. -a phycomycete parasitizing Ophryoscolecidae. Can J Microbiol. 1955 Oct;1(8):675–684. doi: 10.1139/m55-081. [DOI] [PubMed] [Google Scholar]
- LYTTLETON J. W., TS'O P. O. The localization of fraction I protein of green leaves in the chloroplasts. Arch Biochem Biophys. 1958 Jan;73(1):120–126. doi: 10.1016/0003-9861(58)90246-7. [DOI] [PubMed] [Google Scholar]
- Leedle J. A., Bryant M. P., Hespell R. B. Diurnal variations in bacterial numbers and fluid parameters in ruminal contents of animals fed low- or high-forage diets. Appl Environ Microbiol. 1982 Aug;44(2):402–412. doi: 10.1128/aem.44.2.402-412.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARGHERITA S. S., HUNGATE R. E. SEROLOGICAL ANALYSIS OF BUTYRIVIBRIO FROM THE BOVINE RUMEN. J Bacteriol. 1963 Oct;86:855–860. doi: 10.1128/jb.86.4.855-860.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann S. O. An improved method for determining celluloytic activity in anerobic bacteria. J Appl Bacteriol. 1968 Jun;31(2):241–244. doi: 10.1111/j.1365-2672.1968.tb00363.x. [DOI] [PubMed] [Google Scholar]
- Mead L. J., Jones G. A. Isolation and presumptive identification of adherent epithelial bacteria ("epimural" bacteria) from the ovine rumen wall. Appl Environ Microbiol. 1981 Apr;41(4):1020–1028. doi: 10.1128/aem.41.4.1020-1028.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilssen K. J., Johnsen H. K., Rognmo A., Blix A. S. Heart rate and energy expenditure in resting and running Svalbard and Norwegian reindeer. Am J Physiol. 1984 Jun;246(6 Pt 2):R963–R967. doi: 10.1152/ajpregu.1984.246.6.R963. [DOI] [PubMed] [Google Scholar]
- Nilssen K. J., Sundsfjord J. A., Blix A. S. Regulation of metabolic rate in Svalbard and Norwegian reindeer. Am J Physiol. 1984 Nov;247(5 Pt 2):R837–R841. doi: 10.1152/ajpregu.1984.247.5.R837. [DOI] [PubMed] [Google Scholar]
- Orpin C. G., Munn E. A. The occurrence of bacteriophages in the rumen and their influence on rumen bacterial populations. Experientia. 1974 Sep 15;30(9):1018–1020. doi: 10.1007/BF01938983. [DOI] [PubMed] [Google Scholar]
- Orpin C. G. The characterization of the rumen bacterium Eadie's oval, Magnoovum gen. nov. eadii sp. nov. Arch Microbiol. 1976 Dec 1;111(1-2):155–159. doi: 10.1007/BF00446563. [DOI] [PubMed] [Google Scholar]
- PITTMAN K. A., BRYANT M. P. PEPTIDES AND OTHER NITROGEN SOURCES FOR GROWTH OF BACTEROIDES RUMINICOLA. J Bacteriol. 1964 Aug;88:401–410. doi: 10.1128/jb.88.2.401-410.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pethick D. W., Lindsay D. B., Barker P. J., Northrop A. J. Acetate supply and utilization by the tissues of sheep in vivo. Br J Nutr. 1981 Jul;46(1):97–110. doi: 10.1079/bjn19810013. [DOI] [PubMed] [Google Scholar]
- Roché C., Albertyn H., van Gylswyk N. O., Kistner A. The growth response of cellulolytic acetate-utilizing and acetate-producing butyruvibrios to volatile fatty acids and other nutrients. J Gen Microbiol. 1973 Oct;78(2):253–260. doi: 10.1099/00221287-78-2-253. [DOI] [PubMed] [Google Scholar]
- Russell J. B., Bottje W. G., Cotta M. A. Degradation of protein by mixed cultures of rumen bacteria: identification of Streptococcus bovis as an actively proteolytic rumen bacterium. J Anim Sci. 1981 Jul;53(1):242–252. doi: 10.2527/jas1981.531242x. [DOI] [PubMed] [Google Scholar]
- Russell J. B., Dombrowski D. B. Effect of pH on the efficiency of growth by pure cultures of rumen bacteria in continuous culture. Appl Environ Microbiol. 1980 Mar;39(3):604–610. doi: 10.1128/aem.39.3.604-610.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slyter L. L., Oltjen R. R., Kern D. L., Weaver J. M. Microbial species including ureolytic bacteria from the rumen of cattle fed purified diets. J Nutr. 1968 Feb;94(2):185–192. doi: 10.1093/jn/94.2.185. [DOI] [PubMed] [Google Scholar]
- Stanton T. B., Canale-Parola E. Enumeration and selective isolation of rumen spirochetes. Appl Environ Microbiol. 1979 Nov;38(5):965–973. doi: 10.1128/aem.38.5.965-973.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teather R. M., Wood P. J. Use of Congo red-polysaccharide interactions in enumeration and characterization of cellulolytic bacteria from the bovine rumen. Appl Environ Microbiol. 1982 Apr;43(4):777–780. doi: 10.1128/aem.43.4.777-780.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therion J. J., Kistner A., Kornelius J. H. Effect of pH on growth rates of rumen amylolytic and lactilytic bacteria. Appl Environ Microbiol. 1982 Aug;44(2):428–434. doi: 10.1128/aem.44.2.428-434.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARNER A. C. Enumeration of rumen micro-organisms. J Gen Microbiol. 1962 Apr;28:119–128. doi: 10.1099/00221287-28-1-119. [DOI] [PubMed] [Google Scholar]
- Wood T. M. Cellulose and cellulolysis. World Rev Nutr Diet. 1970;12:227–265. doi: 10.1159/000387589. [DOI] [PubMed] [Google Scholar]
- Wozny M. A., Bryant M. P., Holdeman L. V., Moore W. E. Urease assay and urease-producing species of anaerobes in the bovine rumen and human feces. Appl Environ Microbiol. 1977 May;33(5):1097–1104. doi: 10.1128/aem.33.5.1097-1104.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]




