Abstract
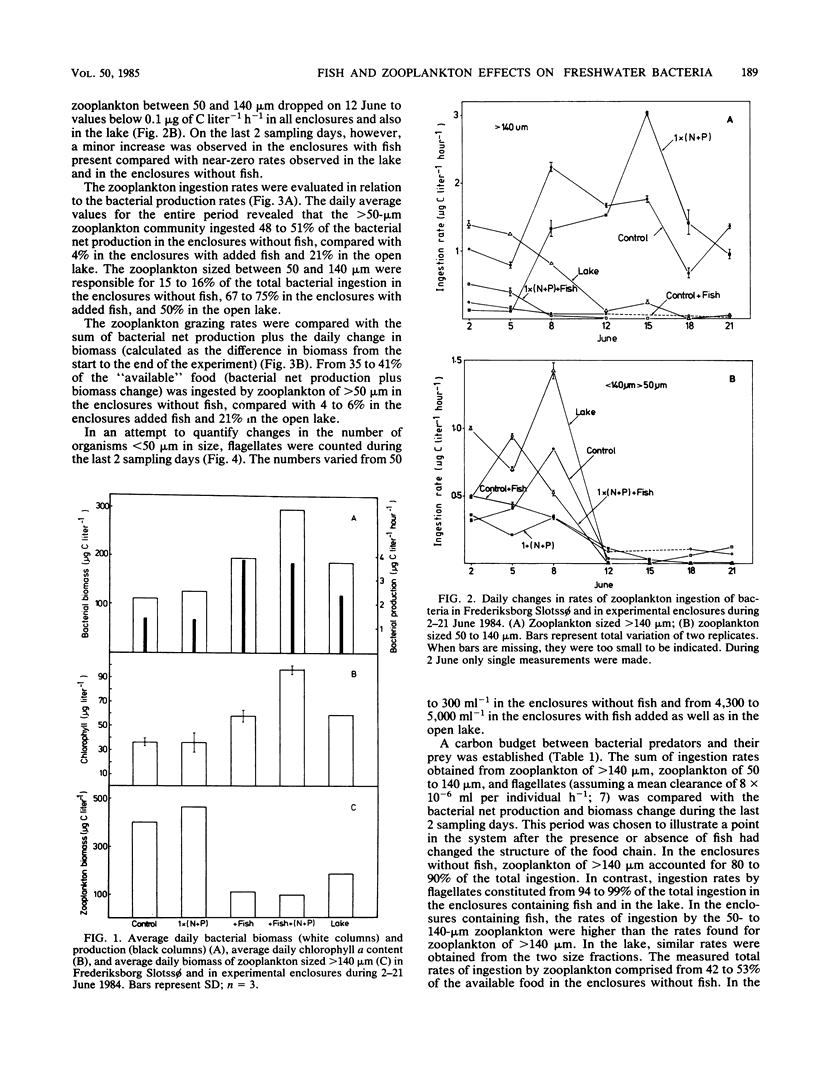
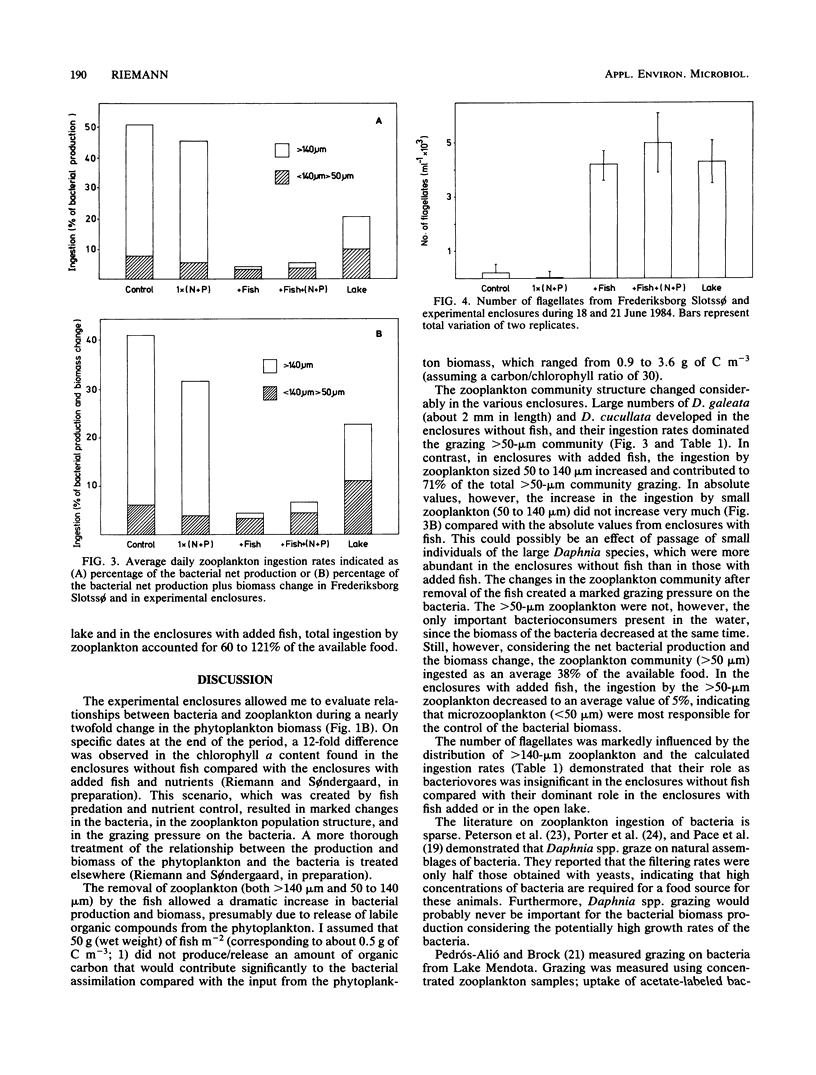
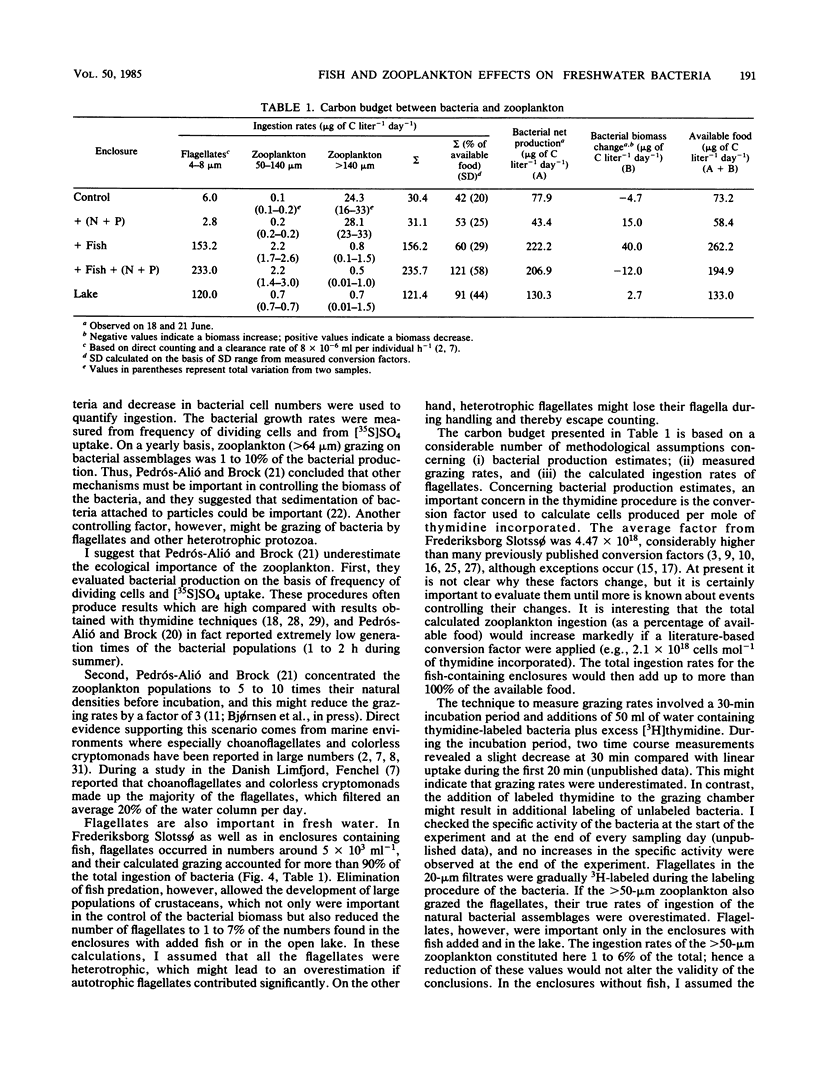
The rates of ingestion of natural bacterial assemblages by natural populations of zooplankton (>50 μm in size) were measured during a 19-day period in eutrophic Frederiksborg Slotssø, Denmark, as well as in experimental enclosures (containing 5.3 m3 of lake water). The fish and nutrients of the enclosures were manipulated. In enclosures without fish, large increases in ingestion by zooplankton >140 μm in size were found (up to 3 μg of C liter−1 h−1), compared with values less than 0.3 μg of C liter−1 h−1 in the enclosures with fish and in the open lake. Daphnia cucullata and D. galeata dominated the community of zooplankton of >140 μm. Ingestion rates for zooplankton between 50 and 140 μm decreased after a period of about 8 days, in all enclosures and in the lake, to values below 0.1 μg of C liter−1 h−1. On the last 2 sampling days, somewhat higher values were observed in the enclosures with fish present. The >50-μm zooplankton ingested 48 to 51% of the bacterial net secondary production in enclosures without fish, compared to 4% in the enclosures with added fish. Considering the sum of bacterial secondary production plus biomass change, 35 to 41% of the available bacteria were ingested by zooplankton of >50 μm in the enclosures without fish, compared with 4 to 6% in the enclosures with added fish and 21% in the open lake. Fish predation reduced the occurrence of zookplankton sized >50 μm and thus left a large proportion of the available bacteria to zooplankton sized <50 μm. In fact, there were 4.6 × 103 to 5.0 × 103 flagellates (4 to 8 μm in size) ml−1 in the enclosures with fish added as well as in the lake, compared with 0.5 × 102 to 2.3 × 102 ml−1 in the enclosures without fish. This link in the food chain was reduced when fish predation on zooplankton was eliminated and a direct route of dissolved organic matter, via the bacteria to the zooplankton, was established.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell R. T., Ahlgren G. M., Ahlgren I. Estimating Bacterioplankton Production by Measuring [H]thymidine Incorporation in a Eutrophic Swedish Lake. Appl Environ Microbiol. 1983 Jun;45(6):1709–1721. doi: 10.1128/aem.45.6.1709-1721.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R. T., Kuparinen J. Assessing phytoplankton and bacterioplankton production during early spring in lake erken, sweden. Appl Environ Microbiol. 1984 Dec;48(6):1221–1230. doi: 10.1128/aem.48.6.1221-1230.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrman J. A., Azam F. Bacterioplankton secondary production estimates for coastal waters of british columbia, antarctica, and california. Appl Environ Microbiol. 1980 Jun;39(6):1085–1095. doi: 10.1128/aem.39.6.1085-1095.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagström A., Larsson U., Hörstedt P., Normark S. Frequency of dividing cells, a new approach to the determination of bacterial growth rates in aquatic environments. Appl Environ Microbiol. 1979 May;37(5):805–812. doi: 10.1128/aem.37.5.805-812.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchman D., Ducklow H., Mitchell R. Estimates of bacterial growth from changes in uptake rates and biomass. Appl Environ Microbiol. 1982 Dec;44(6):1296–1307. doi: 10.1128/aem.44.6.1296-1307.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell C. R., Konopka A. Seasonal bacterial production in a dimictic lake as measured by increases in cell numbers and thymidine incorporation. Appl Environ Microbiol. 1985 Mar;49(3):492–500. doi: 10.1128/aem.49.3.492-500.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray R. E., Hodson R. E. Annual cycle of bacterial secondary production in five aquatic habitats of the okefenokee swamp ecosystem. Appl Environ Microbiol. 1985 Mar;49(3):650–655. doi: 10.1128/aem.49.3.650-655.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrós-Alió C., Brock T. D. Assessing biomass and production of bacteria in eutrophic lake mendota, wisconsin. Appl Environ Microbiol. 1982 Jul;44(1):203–218. doi: 10.1128/aem.44.1.203-218.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemann B., Søndergaard M. Measurements of diel rates of bacterial secondary production in aquatic environments. Appl Environ Microbiol. 1984 Apr;47(4):632–638. doi: 10.1128/aem.47.4.632-638.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintermans J. F., de Mots A. Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim Biophys Acta. 1965 Nov 29;109(2):448–453. doi: 10.1016/0926-6585(65)90170-6. [DOI] [PubMed] [Google Scholar]


