Abstract
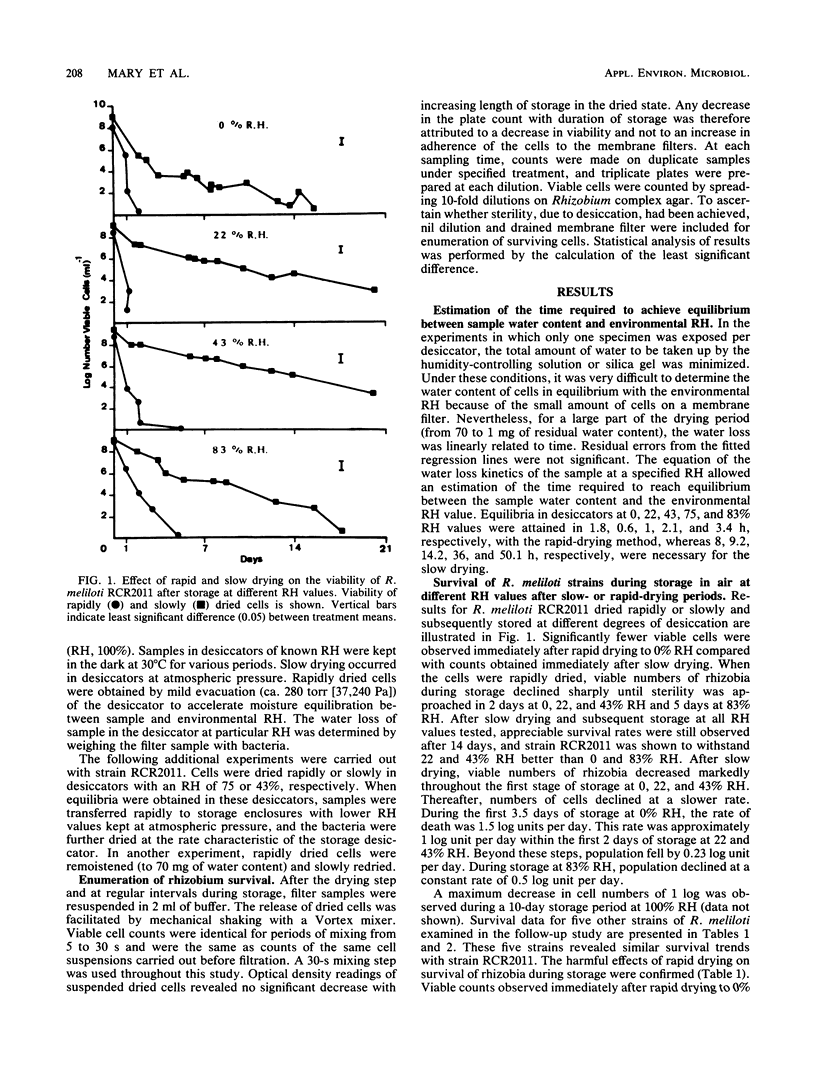
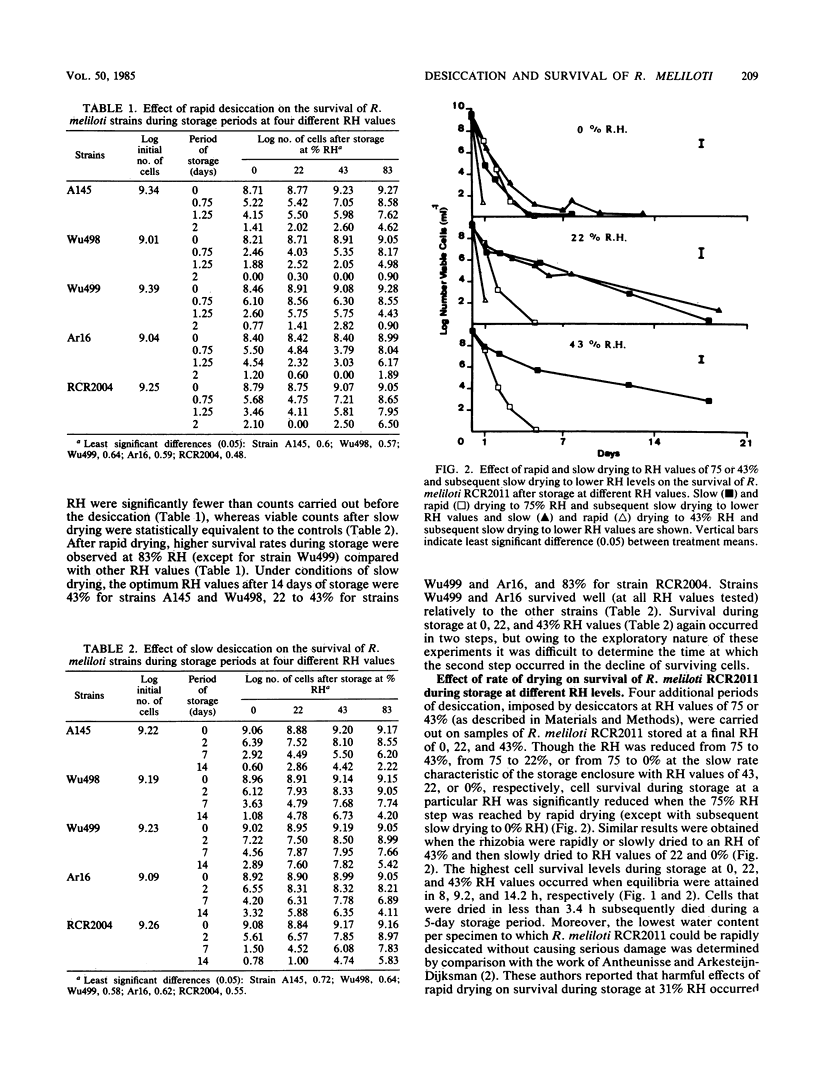
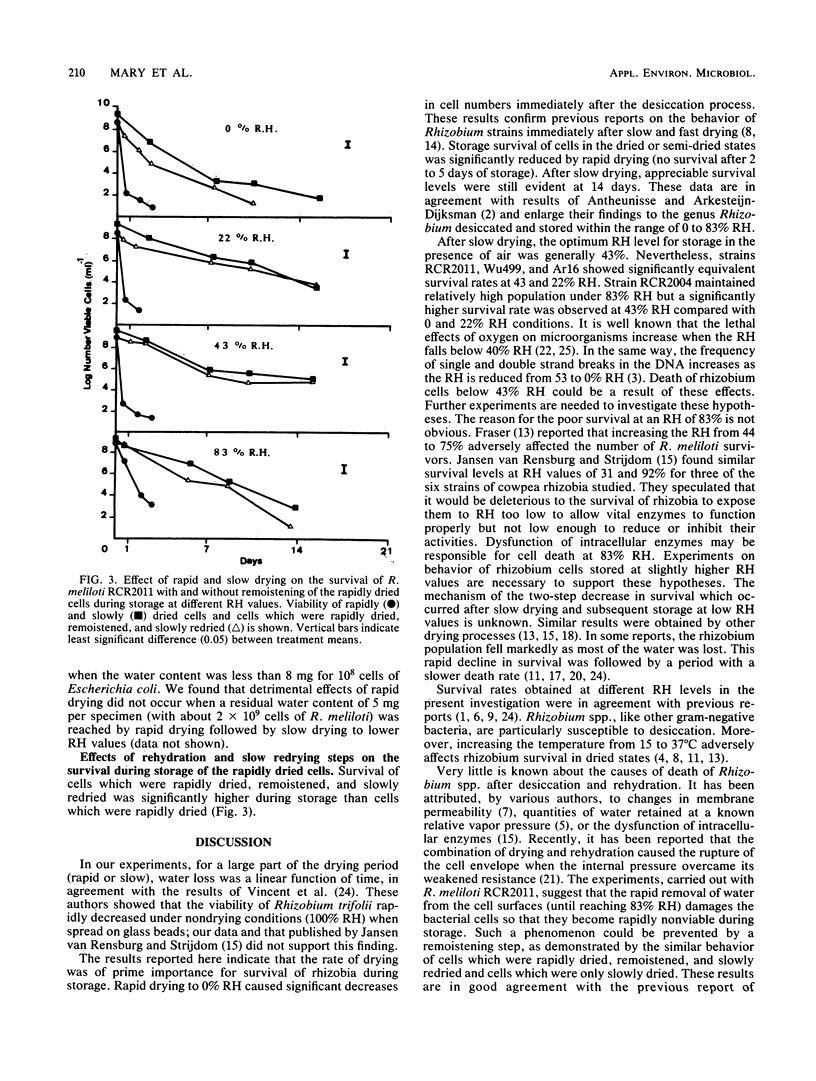
An investigation was made of the survival of six strains of Rhizobium meliloti filtered on membrane filters and held in atmospheres of controlled relative humidities (RH) of from 0 to 100% at 30°C in the presence of air. The rate of water loss in the desiccator was determined by the humidity-controlling solution used. Drying was accelerated by a mild evacuation of the desiccator during the drying step. Survival rates of R. meliloti strains were much higher after slow drying to 0% RH than immediately after rapid drying. Fast drying (drying period less than 3.4 h) was shown to adversely affect the tolerance to storage at all RH values tested (no survival after 2 to 5 days of storage). When survival during storage was measurable (after slow drying), the optimum RH values for storage were 43% for strains A145 and Wu498, 22 to 43% for strains RCR2011, Wu499, and Ar16, and 83% for strain RCR2004. The most favorable drying periods were 8, 9.2, 14.2, and 50.1 h for the subsequent storage of strain RCR2011 at RH values of 0, 22, 43, and 83%, respectively. The damaging effects of rapid drying on the tolerance of strain RCR2011 to storage at different RH values could be prevented either by rehydration and subsequent slow redrying or incomplete rapid drying followed by slow drying. It is suggested that R. meliloti strains are susceptible to desiccation stresses. However, the quantitative differences among strains appear to be large enough to permit selection with regard to tolerance to desiccation and storage in dried states.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antheunisse J., Arkesteijn-Dijksman L. Rate of drying and the survival of microorganisms. Antonie Van Leeuwenhoek. 1979;45(2):177–184. doi: 10.1007/BF00418582. [DOI] [PubMed] [Google Scholar]
- Asada S., Takano M., Shibasaki I. Deoxyribonucleic acid strand breaks during drying of Escherichia coli on a hydorohobic filter membrane. Appl Environ Microbiol. 1979 Feb;37(2):266–273. doi: 10.1128/aem.37.2.266-273.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonkerd N., Weaver R. W. Survival of cowpea rhizobia in soil as affected by soil temperature and moisture. Appl Environ Microbiol. 1982 Mar;43(3):585–589. doi: 10.1128/aem.43.3.585-589.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao W. L., Alexander M. Mineral soils as carriers for Rhizobium inoculants. Appl Environ Microbiol. 1984 Jan;47(1):94–97. doi: 10.1128/aem.47.1.94-97.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson F., Reuszer H. W. Persistence of Rhizobium japonicum on the Soybean Seed Coat Under Controlled Temperature and Humidity. Appl Environ Microbiol. 1978 Jan;35(1):94–96. doi: 10.1128/aem.35.1.94-96.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delattre J. M., Deleplanque A. Etude des caractères physiologiques et biochimiques de 63 souches isolées de nodules de légumineuses. Ann Inst Pasteur Lille. 1971;22:215–230. [PubMed] [Google Scholar]
- Kremer R. J., Peterson H. L. Effects of Carrier and Temperature on Survival of Rhizobium spp. in Legume Inocula: Development of an Improved Type of Inoculant. Appl Environ Microbiol. 1983 Jun;45(6):1790–1794. doi: 10.1128/aem.45.6.1790-1794.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osa-Afiana L. O., Alexander M. Differences among cowpea rhizobia in tolerance to high temperature and desiccation in soil. Appl Environ Microbiol. 1982 Feb;43(2):435–439. doi: 10.1128/aem.43.2.435-439.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCOTT W. J. The effect of residual water on the survival of dried bacteria during storage. J Gen Microbiol. 1958 Dec;19(3):624–633. doi: 10.1099/00221287-19-3-624. [DOI] [PubMed] [Google Scholar]
- Webb S. J. The influence of oxygen and inositol on the survival of semidried microorganisms. Can J Microbiol. 1967 Jul;13(7):733–742. doi: 10.1139/m67-097. [DOI] [PubMed] [Google Scholar]


