Abstract
The α9β1 integrin accelerates cell migration through binding of spermidine/spermine acetyltransferase (SSAT) to the α9 cytoplasmic domain. We now show that SSAT enhances α9-mediated migration specifically through catabolism of spermidine and/or spermine. Because spermine and spermidine are effective blockers of K+ ion efflux through inward-rectifier K+ (Kir) channels, we examined the involvement of Kir channels in this pathway. The Kir channel inhibitor, barium, or knockdown of a single subunit, Kir4.2, specifically inhibited α9-dependent cell migration. α9β1 and Kir4.2 colocalized in focal adhesions at the leading edge of migrating cells and inhibition or knockdown of Kir4.2 caused reduced persistence and an increased number of lamellipodial extensions in cells migrating on an α9β1 ligand. These results identify a pathway through which the α9 integrin subunit stimulates cell migration by localized polyamine catabolism and modulation of Kir channel function.
The α9β1 integrin is widely expressed and binds to a number of ligands, including the extracellular matrix protein tenascin-C, the vascular cell adhesion molecule, VCAM-1, and several members of the ADAM family (1–3). α9β1 mediates enhanced cell migration, an effect that specifically depends on the α9 cytoplasmic domain (4, 5). The closely related integrin α4 subunit also accelerates cell migration, through reversible interaction with the scaffolding protein, paxillin (6, 7). The mechanism by which the α9 subunit cytoplasmic domain accelerates cell migration is completely different (4, 5), and requires binding of the enzyme, spermidine/spermine-N1-acetyltransferase (SSAT) (5). The mechanisms by which SSAT binding modulates migration were until now unknown. SSAT specifically catalyzes catabolism of the higher order polyamines, spermidine and spermine, to the lower order polyamine, putrescine (8), thereby increasing intracellular levels of putrescine, and decreasing those of spermidine and spermine (9, 10).
Spermine and spermidine are potent blockers of outward potassium (K+) currents from inward rectifier K+ (Kir) channels (11–13). These channels conduct larger inward currents at membrane voltages below the resting potential than outward currents at more positive voltages. The long, positively charged polyamines, spermine (+4) or spermidine (+3), mediate rectification by binding to negatively charged residues in the channel pore (12, 14). The catabolized polyamine putrescine (+2) is less effective at blocking outward flow of K+ ions (11, 12). Although Kir channels have been implicated in the regulation of many different physiological processes, including heart rate and membrane excitability (15), there are currently no reports suggesting their involvement in the regulation of cell migration. However, numerous studies have suggested that K+ efflux is a critical factor in modulating cell migration (16–19). In this study, we show that both the catalytic activity of SSAT and the downstream catabolism of polyamines are specifically required for α9β1-mediated migration, and identify a specific role for the Kir channel subunit, Kir4.2, in mediating this effect.
Results
SSAT Catalytic Activity Is Crucial for α9-Dependent Migration.
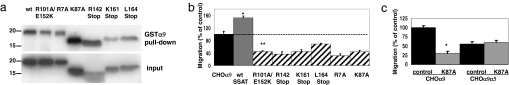
Binding of SSAT to the α9 integrin cytoplasmic domain is required for α9-dependent enhancement of cell migration (5). To systematically evaluate the role of SSAT catalytic activity, we studied six catalytically inactive mutants of SSAT (20, 21) with mutations scattered throughout the protein. Each of these bound to the α9 cytoplasmic domain (Fig. 1a), but expression of any of three point mutants or three C-terminal truncation mutants caused comparable and significant dominant-negative inhibition of migration on the α9-specific ligand, TNfn3RAA (Fig. 1b) (5). A catalytically inactive mutant (K87A) did not affect migration of cells expressing a chimeric α subunit containing the α9 extracellular and transmembrane domains and the α5 integrin cytoplasmic domain (α9α5) (Fig. 1c).
Fig. 1.
Expression of catalytic mutants of SSAT inhibits α9-dependent migration. (a) 35S-labeled wild-type SSAT (wt), R101A/E152K, R7A, K87A point mutants, and truncation mutants containing C-terminal stop codons at R142 (R142Stop), K161 (K161Stop), or L164 (L164Stop) were produced by in vitro transcription, mixed with α9 cytoplasmic domain fused to GST and glutathione Sepharose 4B, and bound proteins were analyzed by SDS/PAGE and autoradiography. (b) CHO cells expressing α9 integrin subunit alone or with SSAT catalytic mutants migrated for 3 h across filters coated with 5 μg/ml TNfn3RAA. *, P = 2 × 10−9; **, P = 1.5 × 10−15. (c) Migration of CHO cells expressing α9 or α9α5 integrin subunits alone, or with the SSAT catalytic point mutant K87A, analyzed after 3 h as in b. Data in b and c are expressed as mean ± SD. *, P = 0.001.
α9-Dependent Migration Requires the Presence and Catabolism of Polyamines.
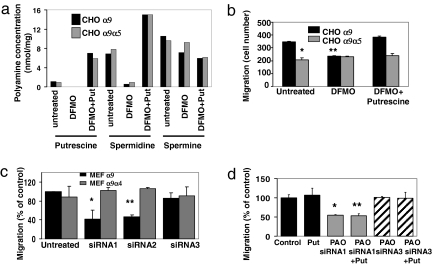
SSAT acetylates its polyamine substrates, spermine and spermidine, the rate-limiting step in polyamine catabolism (12, 13). To examine the specific role of polyamines in α9-mediated migration, we treated cells with α-difluoromethylornithine (DFMO), an inhibitor of the enzyme ornithine decarboxylase (ODC) [supporting information (SI) Fig. S1a]. ODC catalyzes the conversion of the polyamine precursor ornithine to the low order polyamine putrescine. Chinese hamster ovary (CHO) cells were treated for 2 days with 5 mM DFMO, and polyamine levels were analyzed by high-performance liquid chromatography (22). As previously reported in ref. 23, DFMO substantially reduced both spermidine and putrescine levels, whereas spermine levels were unaffected. Addition of putrescine (100 μM) to DFMO-treated cells restored the levels of spermidine and putrescine (Fig. 2a). Migration of cells expressing the wild-type α9 integrin subunit was significantly inhibited by DFMO, whereas migration of cells expressing the α9α5 chimera was not affected (Fig. 2b). Putrescine rescued the DFMO-induced decrease in migration (Fig. 2b), demonstrating the dependence of α9 integrin-mediated migration on polyamines.
Fig. 2.
Modulation of polyamine levels affects α9-dependent enhanced migration. (a) Levels of polyamines from cells treated for 2 d with 5 mM DFMO with or without 100 μM putrescine. (b) α9- or α9α5-expressing CHO cells were treated as in a, and migration on TNfn3RAA was measured after 3 h. *, P = 2.2 × 10−10; **, P = 9.8 × 10−8. (c) α9- or α9α4-expressing MEFs were treated or not with three different siRNAs to PAO and induced to migrate on TNfn3RAA. *, P = 2.0 × 10−4; **, P = 0.014. (d) Migration of α9-transfected MEF cells treated with siRNA to PAO as in c, with or without additional treatment with 100 μM putrescine. *, P = 8 × 10−16; **, P = 1.1 × 10−16.
Enhancement of Migration Is Not due to Increased Concentrations of Acetylated Polyamine Intermediates.
The acetylation of spermine and spermidine by SSAT causes temporary increases in acetylated polyamine intermediates. To determine whether these intermediates or the subsequent catabolism of higher order polyamines is responsible for α9-mediated migration, we evaluated the effects of knockdown of polyamine oxidase (PAO), the enzyme responsible for catabolism of acetylated polyamines. Two of three PAO siRNAs significantly decreased mRNA levels in mouse embryonic fibroblasts (MEFs) and each of these significantly decreased migration of cells expressing wild-type α9 (Fig. 2c). siRNA3, which was ineffective in reducing PAO mRNA levels, had only a minor effect on migration (Fig. 2c), and no siRNA affected the migration of cells expressing an α9α4 chimera (Fig. 2c). Addition of putrescine to siRNA-treated cells did not rescue the effects of PAO knockdown (Fig. 2d).
Inward Rectifier Potassium Channel Function Is Important for α9 Integrin-Mediated Migration.
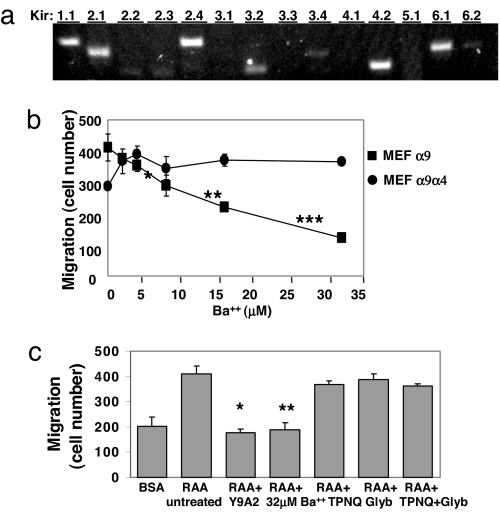
One well characterized function of spermine and spermidine is inward rectification of Kir channels (12). We therefore evaluated the expression levels of Kir channel subunits in α9-expressing MEFs. MEFs expressed at least eight Kir channel subunits, including Kir 1.1, 2.1, 2.4, 3.2, 3.4, 4.2, 6.1, and 6.2 (Fig. 3a).
Fig. 3.
Kir channel involvement in α9-dependent migration. (a) mRNA from MEFα9 cells was analyzed by RT-PCR by using primers to the indicated Kir subunits. (b) α9- and α9α4-expressing MEFs were induced to migrate on TNfn3RAA in the presence or absence of a range of Ba2+ concentrations, or the anti-α9β1 mAb, Y9A2. *, P = 1.06 × 10−5; **, P = 7.6 × 10−12; ***, P = 1.25 × 10−14. (c) MEFα9 cells were pretreated or not with 50 μg/ml Y9A2, 32 μM Ba2+, 100 nM TPNQ, 1 mM Glyb, or both TPNQ and Glyb and then induced to migrate on either 1% BSA (untreated cells) or TNfn3RAA. *, P = 1.4 × 10−15; **, P = 1.8 × 10−15.
To investigate the role of Kir channels, we treated cells with barium (Ba2+), at concentrations that specifically inhibit Kir channel function (24) (Fig. S2b; also see SI Materials and Methods). In cells expressing wild-type α9, Ba2+ caused a concentration-dependent inhibition of migration on TNfn3RAA, with effects of 32 μM similar to those of α9β1-blocking antibody (Fig. 3b). Ba2+ had no effect on migration of α9α4- or α9α5-expressing cells (data not shown) or migration on the nonspecific ligand plasma fibro nectin (Fig. S3a), and had no effect on adhesion to either substrate (Fig. S3b).
We also examined the effects of tertiapin-Q (TPNQ), an inhibitor of Kir1.1 and Kir3.x channels (25), or glybenclamide (Glyb), an inhibitor of ATP-activated K+ channels (26, 27), which exist as heteromultimers of sulfonylurea receptor subunits and Kir6.1 or Kir6.2 subunits (28). For each inhibitor, concentrations were chosen that were substantially (>10 times) higher than the IC50 described for the relevant targets (25, 29). Neither of these inhibitors alone or in combination had any effect on migration on TNfn3RAA, suggesting that contributions of Kir1.1, 3.1, 3.2, 3.4, 6.1, and 6.2 do not explain α9β1-dependent enhancement of migration (Fig. 3c).
Kir4.2 Is Crucial for Enhancement of Migration.
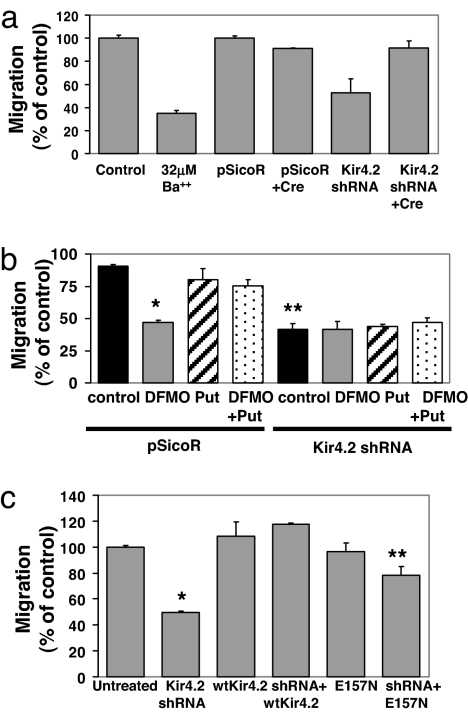
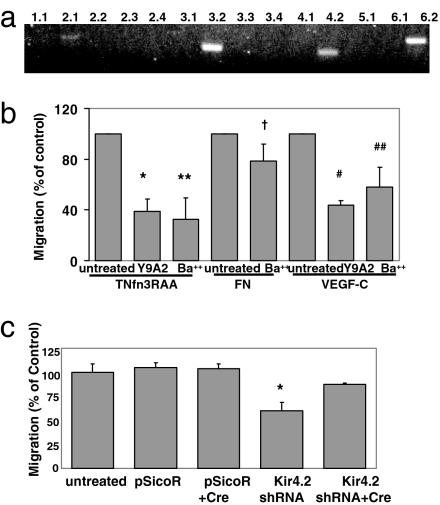
The sole member of the Kir4.x subfamily expressed in MEFs is Kir4.2. To determine involvement of this subunit in α9-dependent migration, we knocked down Kir4.2 expression by using small hairpin RNA (shRNA) (30). Kir4.2 shRNA caused >90% reduction in Kir4.2 mRNA and infection with adenovirus cre restored Kir4.2 mRNA levels (Fig. S4a). Knockdown of Kir4.2 significantly inhibited migration of α9-expressing cells (Fig. 4a), but not α9α4-expressing cells (Fig. S4b), whereas the control vector had no effect on either cell type. Additional treatment of shRNA-expressing cells with a combination of tertiapin Q (100 nM) and glybenclamide (1 μM) did not further reduce α9-dependent migration, providing additional evidence that members of the 1.x, 3.x, and 6.x families of Kir channels do not significantly contribute to this response (Fig. S4d). shRNA excision completely reversed the inhibition due to knockdown of Kir4.2 (Fig. 4a).
Fig. 4.
Effect of knockdown of Kir4.2 on α9-dependent enhanced migration. (a) MEFα9 cells expressing pSicoR or shRNA to Kir4.2 were treated overnight or not with cre recombinase for shRNA excision before addition to TNfn3RAA-coated Transwell filters. Some control cells were treated with 32 μM Ba2+. Migration after 3 h is expressed as a percentage of migration of untreated cells. *, P = 0.0007; **, P = 0.02. (b) Control MEFα9 cells or cells expressing either pSicoR or Kir4.2 shRNA were treated for 2 d with 5 mM DFMO, 100 μM putrescine, or both and induced to migrate across Transwell filters coated with Tnfn3RAA. *, P = 0.04; **, P = 0.07. (c) Migration on Tnfn3RAA of wild-type or E157N mutant Kir4.2-expressing MEFα9 cells treated or not with Kir4.2 shRNA. *, P = 2.0 × 10−17; **, P = 9.1 × 10−6.
Treatment with DFMO did not cause any additional inhibition of migration in α9-expressing cells with knockdown of Kir4.2 (Fig. 4b). In addition, treatment with putrescine either alone or for reconstitution of polyamine levels in DFMO-treated cells did not modulate migration of cells expressing shRNA to Kir4.2. Expression of wild-type Kir4.2 completely rescued migration, whereas equivalent expression of a mutant with impaired rectification (E157N) (Figs. S2a and S4e) only partially restored migration (Fig. 4c). Expression of either wild-type or mutant proteins did not affect migration of α9α4-expressing cells (Fig. S4f). These data support a role for Kir4.2 channel rectification downstream of SSAT-mediated catabolism of polyamines.
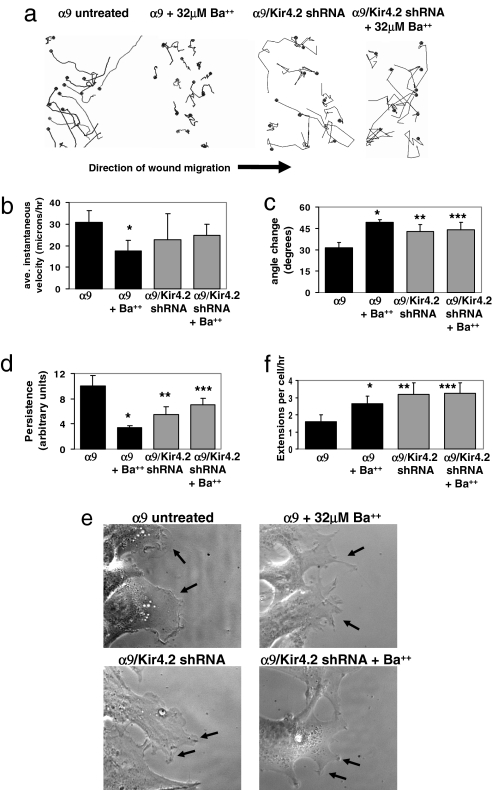
To determine how Kir4.2 modulates cell migration, we performed scratch wound assays. α9-expressing cells demonstrated increases in migration persistence, with a corresponding decrease in angle changes during migration (Fig. 5 a–d). In contrast, cells treated with 32 μM Ba2+ and MEFα9 cells expressing shRNA to Kir4.2 showed significantly less persistent migration. Higher magnification imaging (×630) revealed that untreated MEFα9 cells generally developed single broad lamellae at the leading edge, whereas cells treated with Ba2+ or expressing shRNA to Kir4.2 extended multiple processes, a likely explanation for their decrease in migratory persistence and their increase in angle changes during migration (Fig. 5 e and f).
Fig. 5.
Morphological analysis of α9-dependent scratch wound migration. (a) Confluent MEFα9 cells or cells expressing shRNA to Kir4.2 were wounded with a pipette tip in the presence or absence of 32 μM Ba2+, and then imaged every 10 min for 15 h. Representative tracks of cell centroids (black dots) migrating into the wound space are shown. (b) Mean instantaneous velocity of the cells treated in a. *, P = 2.0 × 10−5. (c) Mean change in the angle of migration relative to the initial direction, as analyzed in b. *, P = 0.0003; **, P = 0.004; ***, P = 0.004. (d) Mean persistence in cell migration of the same cells analyzed in b and c. *, P = 0.0001; **, P = 0.007; ***, P = 0.009. (e) Images of individual cells and lamellipodia at the wound edge (arrows). (f) Quantification of the mean number of lamellipodial extensions >2 μm in width in cells treated as in a–d and imaged every 2 min for 2 h. *, P = 0.0008; **, P = 0.0008; ***, P = 0.0004.
Kir4.2 Channel Function Is Required for α9-Dependent Migration of Microvascular Endothelial Cells.
Microvascular endothelial cells express the α9β1 integrin (31), and adhere to and migrate on α9β1 ligands (31). Human microvascular endothelial cells (HMVECs) expressed four Kir subunits that are also expressed in MEFs, including Kir4.2 (Fig. 6a). Ba2+ significantly decreased HMVEC migration on the α9β1 integrin ligands TNfn3RAA or VEGF-C, but had little effect on HMVEC migration on fibronectin (Fig. 6b). Knockdown of human Kir4.2 significantly inhibited migration on TNfn3RAA (Fig. 6c and Fig. S5), and this effect was reversed by shRNA excision (Fig. 6c and Fig. S5).
Fig. 6.
Involvement of Kir4.2 subunits in α9-dependent cell migration of HMVECs. (a) RT-PCR of Kir channel subunits expressed in HMVECs. (b) HMVECs were treated with the α9β1 integrin-blocking antibody Y9A2 or with 32 μM Ba2+, and induced to migrate across filters coated with 5 μg/ml TNfn3RAA or FN, or 3 μg/ml VEGF-C. *, P = 0.01; **, P = 0.03; †, P = 0.14; #, P = 8.2 × 10−8; ##, P = 0.0005. (c) Uninfected HMVECs or cells expressing either pSicoR or Kir4.2 shRNA alone or also expressing Cre were induced to migrate across filters coated with 5 μg/ml TNfn3RAA. *, P = 0.30; **, P = 2.6 × 10−11.
The α9 Integrin and Kir4.2 Subunits Colocalize in Migrating Cells.
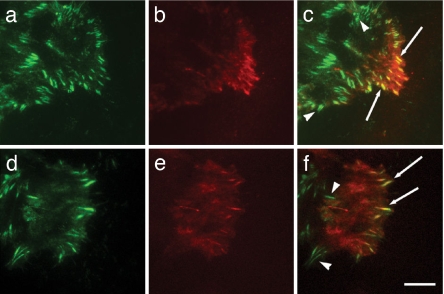
For SSAT associated with the α9 cytoplasmic domain to functionally modulate Kir4.2 by altering the local concentrations of polyamines, α9 and Kir4.2 would need to be in close physical apposition. We therefore evaluated whether the α9β1 integrin and Kir4.2 subunit are colocalized in migrating cells by using total internal reflection fluorescence (TIRF) microscopy on cells expressing GFP-α9 integrin and mCherry-Kir4.2 fusion proteins. α9-GFP was present in focal adhesion-like structures throughout the migrating cells (Fig. 7 a and d). Kir4.2-mCherry was strikingly restricted to the advancing portion of migrating cells (Fig. 7 b and e) and was clearly colocalized with GFP-α9 in numerous focal adhesions at the leading edge (Fig. 7 c and f, arrows).
Fig. 7.
The α9 integrin and Kir4.2 codistribute in focal adhesions at the leading edge of migrating cells. (a and d) α9 integrin-GFP localization in MEF cells at the edge of a scratch wound. (b and e) Kir4.2-mCherry distribution in the same cells as in a and d. (c) Overlay of α9-GFP and Kir4.2-mCherry images in a and b. (f) Overlay of α9-GFP and Kir4.2-mCherry images in d and e. Arrows, Colocalization of α9-GFP and Kir4.2-mCherry. Arrowheads, α9 integrin localization alone. (Scale bar, 10 μm.)
Discussion
We have previously reported that direct interaction between the integrin α9 cytoplasmic domain and SSAT is required for α9β1-dependent enhancement of cell migration. We now show that this effect depends on the acetyltransferase activity of SSAT. Knockdown of PAO, the enzyme responsible for cleaving acetylated polyamines, also specifically inhibits α9β1-dependent enhanced migration, and this effect is not rescued by exogenous putrescine, demonstrating that this response depends on the loss of higher order polyamines rather than on the production of acetylated intermediates or increases in the concentration of putrescine. However, our finding that pharmacologic inhibition of ODC, the enzyme responsible for synthesis of the polyamine putrescine, also specifically inhibits α9β1-dependent enhancement of migration, does not support a simple model by which global reduction in higher order polyamines enhances cell migration. We favor the hypothesis that, by localizing SSAT activity, α9β1 mediates spatially restricted dynamic regulation of polyamine levels and that this process is required for enhanced cell migration. Testing this hypothesis will require analysis of localized polyamine flux.
Because rectification by Kir channels is one of the few cytosolic functions known to be differentially regulated by higher and lower order polyamines, we sought evidence that these channels were involved in α9β1-dependent enhancement of migration. Strikingly, the effects of shRNA knockdown and rescue by expression of a single Kir channel subunit, Kir4.2, and Ba2+, at concentrations that inhibit Kir channel function, suggest that the effects of polyamine catabolism on currents through channels containing this subunit likely explain the role of SSAT in α9β1-dependent enhancement of cell migration.
Polyamines mediate inward rectification of Kir channels by binding to critical negatively charged amino acid residues in the channel pore (14). The spacing of these charged residues favors interactions with higher order polyamine molecules (spermine or spermidine), whereas putrescine, which contains only two amino groups, is too short to effectively interact with multiple residues (12, 14). This size difference is thought to explain the differential ability of lower and higher order polyamines to mediate rectification of Kir channels (12, 13). Because spermine and spermidine are the most effective blockers of K+ efflux, their local catabolism to putrescine would be predicted to permit more K+ efflux in channels in close proximity to α9-bound SSAT. This would suggest a mechanism by which spatially restricted impairment of rectification and therefore increased K+ efflux enhances the rate of cell migration. However, our observation that DFMO treatment, which resulted in substantial reductions in cellular spermidine levels, inhibited α9β1-mediated cell migration suggests that global impairment in Kir channel rectification might not produce the same effect. Similarly, if global impairment of inward rectification could enhance cell migration, we would have expected enhanced migration in cells expressing a weakly rectifying Kir4.2 mutant (E157N). However, this mutant was less effective than wild-type Kir4.2 in enhancing migration. We hypothesize that this is due to the loss of spatial restriction of localized K+ efflux changes at sites of α9β1 and SSAT colocalization (i.e., the leading edge of migrating cells).
Potassium channels have been implicated in the migration of many cell types, including epithelial cells, neutrophils, and fibroblasts (17, 18, 32, 33). In previous reports, the K+ channels that contributed to cell migration were channels that allow K+ efflux. For example, specific inhibition of calcium-activated K+ channels inhibits the migration of MDCK cells, suggesting that the well described role of increased intracellular calcium concentrations in mediating cell migration could result, at least in part, from stimulation of K+ efflux. Interestingly, in migrating MDCK cells, calcium-activated K+ channels are concentrated at the leading edge, just as we found for Kir4.2 in the current study (34). The current study is the first, to our knowledge, to implicate Kir channels in enhancement of cell migration. In contrast to other types of K+ channels, in normal physiological conditions Kir channels permit little K+ efflux. However, SSAT bound to the integrin α9 cytoplasmic domain would be predicted to impair the inward rectification of nearby channels, leading to an increase in K+ efflux through channels in close proximity to α9β1. Accordingly, voltage patch-clamping experiments were attempted to detect changes in potassium efflux through Kir channels in cells migrating on the α9β1 integrin ligands, but these efforts were unsuccessful. We speculate that this is because the effects of catabolized polyamines on rectification are limited to small and specific regions of the leading edge of cells, in which the small numbers of channels involved are below the detection limit for patch clamping.
The mechanisms by which enhanced K+ efflux modulate cell migration remain controversial. One hypothesis is that K+ efflux leads to water efflux and thus causes localized decreases in cell volume that allow retraction of the tails of migrating cells (35). However, this hypothesis would not fit well with the preferential distribution of these channels at the leading edge. Alternatively, it has been suggested that increased K+ efflux may change the membrane potential and create a driving force for calcium (Ca2+) influx. Such an effect has been demonstrated in both cytolytic T lymphocytes and IEC-6 intestinal epithelial cells, in which K+ efflux and the resulting plasma membrane hyperpolarization caused increases in intracellular calcium levels (17, 36). The increase in intracellular Ca2+ levels may be sufficient to activate well described Ca2+-regulated stimulatory pathways for migration (37–41).
There have been previous reports of functional interactions between integrins and K+ channels (42–45). For instance, integrin-mediated adhesion activates the human ether-a-go-go-related K+ channel (46, 47). In endothelial cells, Ca2+-dependent K+ currents have been shown to be modulated by integrin αvβ3-mediated adhesion to fibronectin (45). Our studies identify the involvement of one Kir channel subunit, Kir4.2, downstream of the α9 integrin in a pathway that enhances cell migration. Kir4.2 is present in a wide variety of tissues, including lung and liver, that are also sites of expression of the α9 integrin subunit (48, 49). Thus, it remains plausible that these proteins functionally cooperate in vivo.
By TIRF microscopy, we found the integrin α9β1 diffusely expressed in focal adhesions in migrating cells, but Kir4.2 was concentrated at the leading edge where it colocalized with the integrin in multiple focal adhesions. Although it is difficult to definitively prove the significance of colocalization by TIRF, the spatial pattern seen suggests relevance to events at the leading edge of migrating cells. Our studies of wounded cell monolayers suggest the enhancement of migration mediated by α9β1 and Kir4.2 is due to an increase in migratory persistence, an effect that was correlated with a decrease in the number of leading edge cellular protrusions. Our data thus suggest that the localized increase in K+ efflux mediated by the pathway we describe is either enhancing the formation of a single lamellipod or inhibiting the inappropriate formation of additional cellular extensions. There is precedent for this observation, because decreased persistence in fibroblasts is directly correlated with increased numbers of extensions peripheral to the primary cell axis (50).
Numerous previous reports have indicated roles for polyamines in cell migration (51). For example, polyamine depletion by DFMO decreased wound healing and migration of intestinal epithelial cells, both in vitro and in vivo (52), and these effects were reversed by putrescine or spermidine reintroduction (52). However, expression of integrin α9β1 on the cells used in those experiments was not described, so it is unknown whether the results were dependent on the pathway we describe here.
In summary, the data in this study identify a mechanism of integrin-dependent cell migration, involving direct interaction of the integrin α9 subunit cytoplasmic domain with the acetyltransferase SSAT, localized catabolism of polyamines, and modulation of Kir channel function. These observations provide insights into the general mechanisms regulating cell motility and into the specific mechanisms underlying the unique in vivo functions of the α9β1 integrin.
Materials and Methods
Cell Culture.
CHO and MEF cells expressing α9, α9α4, or α9α5 integrin subunits were generated as described in refs. 4 and 5. Human Phoenix-E (φE) cells (from Gary Nolan, Stanford University, Stanford, CA) were cultured in DMEM containing 10% FBS (HyClone) and 1% penicillin/streptomycin (Invitrogen). HMVECs (Lonza) were cultured in endothelial growth medium (EBM-2) containing EGM-2 MV supplements (Lonza).
Reagents.
BE-3-3-3 (N1,N11-diethylnorspermine tetrahydrochloride) was from Tocris. Putrescine (1,4-diaminobutane) was from Acros Organics. Barium chloride (BaCl2), DFMO (dl-α-difluoromethylornithine hydrochloride), tertiapin-Q, glybenclamide, and fibronectin were from Sigma-Aldrich. Vascular endothelial growth factor-C was from R&D Systems. Mouse monoclonal α9 integrin antibody Y9A2 and the mutant form of the third fibronectin type III repeat in tenascin-C (TNfn3RAA) have been described in refs. 53 and 54.
SSAT Mutant Cell Lines.
Myc-tagged SSAT cDNA was amplified by PCR and cloned into the vector pcDNA3.1+ (Invitrogen). Point mutants R7A, K22A, and K87A were made by using QuikChange Site-Directed Mutagenesis (Stratagene). Truncation mutants were generated by PCR. Mutants were transfected into CHO cells by using Lipofectamine 2000 (Invitrogen), and Western blot analysis with the anti-c-Myc monoclonal antibody 9E10 (Abcam) identified stable clones.
GST-α9 Pull-down Assays.
35S-labeled mutant SSAT proteins were produced by the addition of SSAT mutant cDNA and l-[35S]methionine to rabbit reticulocyte lysates (Promega) and assayed for binding to GST-α9 cytoplasmic domain as described in ref. 5.
Polyamine Analysis.
Polyamine levels were determined by reverse-phase HPLC by using postcolumn derivatization with o-phthaldialdehyde and fluorescence detection, as described in ref. 22.
siRNA Construction and Transfection.
Small interfering RNAs were designed by using Ambion web-based software. siRNAs (siRNA1, AATGGCCTTTTGGAAGAGACG; siRNA2, AAGAGGCAGTACACCAGTTTC; siRNA3, AAGGAAAGGCTCCTAGAAGGT) were synthesized and transfected into MEFs at 80% confluence by using siPORT Amine Transfection Agent (Ambion).
shRNAs were designed against mouse (target sequence, GAAAGTATGTGGCTGATTT) and human Kir4.2 (target sequence, GAAAGTGAACTCTCATAAT) by using pSicoOligomaker software from Tyler Jacks (Massachusetts Institute of Technology, Cambridge, MA), ligated into the HpaI/XhoI-digested pSicoR plasmid and used to generate lentivirus as described in ref. 30.
shRNA-treated MEFs and HMVECs were infected with adenoviruses to express Cre recombinase and excise shRNA-encoding sequences. Cre adenovirus-containing conditioned medium, a gift from Lily Wu (University of California, Los Angeles, CA) was added at a multiplicity of infection of 10 to cells at 70% confluence.
Real-Time PCR.
Real-time PCR was performed by ABI Prism 7700 (Applied Biosystems). Amplified cDNA was detected with SYBR Green (Invitrogen) and standardized to ROX dye levels. cDNA concentrations were expressed as the number of cycles to threshold (Ct). Ct numbers were normalized to GAPDH in the same samples.
Flow Cytometry.
For GFP expression, cells were trypsinized, washed, resuspended in 100 μl of PBS, and analyzed by using a FACScan flow cytometer (Becton Dickinson). For α9β1 expression, cells were blocked by normal goat serum, then sequentially incubated with Y9A2 and phycoerythrin-labeled goat anti-mouse antibody before analysis.
Adhesion Assays.
Wells were coated with TNfn3RAA or plasma fibronectin at 10 μg/ml for 1 h at 37°C, and adhesion assays were performed as described in ref. 5.
Migration Assays.
Transwell filters (8.0-μm pore; Corning) were coated with TNfn3RAA (10 μg/ml), plasma fibronectin (10 μg/ml), or VEGF-C (3 μg/ml) in DMEM and blocked with 1% BSA. A total of 5 × 104 cells was added to upper chambers, and DMEM containing 1% FBS was added to the bottom chamber for 3 h at 37°C. Filters were fixed with DiffQuik, and stained cells were counted in 10 randomly chosen high-power fields per filter.
Wound assays were performed by scratching confluent monolayers on six-well plates with a pipette tip. Cells were imaged by phase contrast every 10 min for 15 h by using a Leica DM6000B microscope and Leica DFC350FX camera (Leica Microsystems). Instantaneous velocity and angle change from the migration vector at every time point were measured by tracking at least 15 cell centroids by using Image-Pro Plus (Media Cybernetics) software. Persistence was calculated from angle change and distance as described in ref. 55. Extension number of migrating cells was quantified by using the same software from images taken every 2 min for 2 h at ×630. Extensions >2 μm in width were counted in at least 15 cells for each condition.
P values were generated by two-tailed, equal variance Student's t tests for single comparisons or ANOVA analysis (Tukey's post hoc method for normal distribution) for multiple comparisons.
Fluorescent Protein Expression and Microscopy.
Fluorescent proteins were created by ligating EGFP or mCherry sequences to the C terminus of PCR-generated cDNA of the human α9 integrin or Kir4.2 subunit, respectively, in the retroviral vectors pBabe and pWZL. The Kir4.2 E157N mutant was generated by QuikChange Site-Directed Mutagenesis (Stratagene). Retrovirus was used to infect MEFs, which were sorted for expression of both GFP and mCherry. Cell monolayers were scratched with a pipette tip to simulate wounding and imaged 2 h later at ×900 on a total internal reflection fluorescence microscope (Nikon TE2000E) by using a Photometrics Cascade II EM CCD camera and Nikon NIS Elements software, all stationed at the Nikon Imaging Center at University of California (San Francisco, CA).
Supplementary Material
Acknowledgments.
We thank Lily Wu (University of California, Los Angeles) for the cre adenovirus, Clare Waterman-Storer (The Scripps Research Institute, La Jolla, CA) for mCherry constructs, Michael McManus (University of California, San Francisco) for shRNA construction and lentiviral infection assistance, and Lily Jan and Shi-Bing Yang (University of California, San Francisco) for valuable advice about Kir channels. This work was supported by National Institutes of Health Grants HL64353 (to D.S.) and F32 HL079763 (to G.W.d.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 7109.
This article contains supporting information online at www.pnas.org/cgi/content/full/0708044105/DCSupplemental.
References
- 1.Taooka Y, et al. The integrin alpha9beta1 mediates adhesion to activated endothelial cells and transendothelial neutrophil migration through interaction with vascular cell adhesion molecule-1. J Cell Biol. 1999;145:413–420. doi: 10.1083/jcb.145.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yokosaki Y, et al. The integrin alpha(9)beta(1) binds to a novel recognition sequence (SVVYGLR) in the thrombin-cleaved amino-terminal fragment of osteopontin. J Biol Chem. 1999;274:36328–36334. doi: 10.1074/jbc.274.51.36328. [DOI] [PubMed] [Google Scholar]
- 3.Eto K, et al. Functional classification of ADAMs based on a conserved motif for binding to integrin alpha 9beta 1: implications for sperm-egg binding and other cell interactions. J Biol Chem. 2002;277:17804–17810. doi: 10.1074/jbc.M200086200. [DOI] [PubMed] [Google Scholar]
- 4.Young BA, et al. The cytoplasmic domain of the integrin alpha9 subunit requires the adaptor protein paxillin to inhibit cell spreading but promotes cell migration in a paxillin-independent manner. Mol Biol Cell. 2001;12:3214–3225. doi: 10.1091/mbc.12.10.3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen C, et al. Spermidine/spermine N1-acetyltransferase specifically binds to the integrin alpha9 subunit cytoplasmic domain and enhances cell migration. J Cell Biol. 2004;167:161–170. doi: 10.1083/jcb.200312166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han J, et al. Phosphorylation of the integrin alpha 4 cytoplasmic domain regulates paxillin binding. J Biol Chem. 2001;276:40903–40909. doi: 10.1074/jbc.M102665200. [DOI] [PubMed] [Google Scholar]
- 7.Goldfinger LE, et al. Spatial restriction of alpha4 integrin phosphorylation regulates lamellipodial stability and alpha4beta1-dependent cell migration. J Cell Biol. 2003;162:731–741. doi: 10.1083/jcb.200304031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casero RA, Jr, Pegg AE. Spermidine/spermine N1-acetyltransferase—the turning point in polyamine metabolism. FASEB J. 1993;7:653–661. [PubMed] [Google Scholar]
- 9.McCloskey DE, Coleman CS, Pegg AE. Properties and regulation of human spermidine/spermine N1-acetyltransferase stably expressed in Chinese hamster ovary cells. J Biol Chem. 1999;274:6175–6182. doi: 10.1074/jbc.274.10.6175. [DOI] [PubMed] [Google Scholar]
- 10.Vujcic S, et al. Effects of conditional overexpression of spermidine/spermine N1-acetyltransferase on polyamine pool dynamics, cell growth, and sensitivity to polyamine analogs. J Biol Chem. 2000;275:38319–38328. doi: 10.1074/jbc.M003270200. [DOI] [PubMed] [Google Scholar]
- 11.Lopatin AN, Makhina EN, Nichols CG. Potassium channel block by cytoplasmic polyamines as the mechanism of intrinsic rectification. Nature. 1994;372:366–369. doi: 10.1038/372366a0. [DOI] [PubMed] [Google Scholar]
- 12.Oliver D, Baukrowitz T, Fakler B. Polyamines as gating molecules of inward-rectifier K+ channels. Eur J Biochem. 2000;267:5824–5829. doi: 10.1046/j.1432-1327.2000.01669.x. [DOI] [PubMed] [Google Scholar]
- 13.Ficker E, et al. Spermine and spermidine as gating molecules for inward rectifier K+ channels. Science. 1994;266:1068–1072. doi: 10.1126/science.7973666. [DOI] [PubMed] [Google Scholar]
- 14.Bichet D, Haass FA, Jan LY. Merging functional studies with structures of inward-rectifier K+ channels. Nat Rev Neurosci. 2003;4:957–967. doi: 10.1038/nrn1244. [DOI] [PubMed] [Google Scholar]
- 15.Nichols CG, Lopatin AN. Inward rectifier potassium channels. Annu Rev Physiol. 1997;59:171–191. doi: 10.1146/annurev.physiol.59.1.171. [DOI] [PubMed] [Google Scholar]
- 16.Cruse G, et al. Functional KCa3.1 K+ channels are required for human lung mast cell migration. Thorax. 2006;61:880–885. doi: 10.1136/thx.2006.060319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rao JN, et al. Activation of K+ channels and increased migration of differentiated intestinal epithelial cells after wounding. Am J Physiol. 2002;282:C885–C898. doi: 10.1152/ajpcell.00361.2001. [DOI] [PubMed] [Google Scholar]
- 18.Da Silva-Santos JE, et al. The role of ATP-sensitive potassium channels in neutrophil migration and plasma exudation. J Pharmacol Exp Ther. 2002;300:946–951. doi: 10.1124/jpet.300.3.946. [DOI] [PubMed] [Google Scholar]
- 19.Schwab A, et al. K+ channel-dependent migration of fibroblasts and human melanoma cells. Cell Physiol Biochem. 1999;9:126–132. doi: 10.1159/000016309. [DOI] [PubMed] [Google Scholar]
- 20.Coleman CS, Huang H, Pegg AE. Structure and critical residues at the active site of spermidine/spermine-N1-acetyltransferase. Biochem J. 1996;316:697–701. doi: 10.1042/bj3160697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coleman CS, Huang H, Pegg AE. Role of the carboxyl terminal MATEE sequence of spermidine/spermine N1-acetyltransferase in the activity and stabilization by the polyamine analog N1,N12-bis(ethyl)spermine. Biochemistry. 1995;34:13423–13430. doi: 10.1021/bi00041a020. [DOI] [PubMed] [Google Scholar]
- 22.Seiler N, Knoedgen B. Determination of polyamines and related compounds by reversed-phase high-performance liquid chromatography: improved separation systems. J Chromatography. 1985;339:45–57. [Google Scholar]
- 23.Marton LJ, Pegg AE. Polyamines as targets for therapeutic intervention. Annu Rev Pharmacol Toxicol. 1995;35:55–91. doi: 10.1146/annurev.pa.35.040195.000415. [DOI] [PubMed] [Google Scholar]
- 24.Quayle JM, Standen NB, Stanfield PR. The voltage-dependent block of ATP-sensitive potassium channels of frog skeletal muscle by caesium and barium ions. J Physiol (London) 1988;405:677–697. doi: 10.1113/jphysiol.1988.sp017355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin W, Lu Z. Synthesis of a stable form of tertiapin: a high-affinity inhibitor for inward-rectifier K+ channels. Biochemistry. 1999;38:14286–14293. doi: 10.1021/bi991205r. [DOI] [PubMed] [Google Scholar]
- 26.Delaey C, Van de Voorde J. Heterogeneity of the inhibitory influence of sulfonylureas on prostanoid-induced smooth muscle contraction. Eur J Pharmacol. 1997;325:41–46. doi: 10.1016/s0014-2999(97)00097-6. [DOI] [PubMed] [Google Scholar]
- 27.Akar F, et al. Protective effect of cromakalim and diazoxide, and proulcerogenic effect of glibenclamide on indomethacin-induced gastric injury. Eur J Pharmacol. 1999;374:461–470. doi: 10.1016/s0014-2999(99)00277-0. [DOI] [PubMed] [Google Scholar]
- 28.Mikhailov MV, et al. 3-D structural and functional characterization of the purified KATP channel complex Kir6.2-SUR1. EMBO J. 2005;24:4166–4175. doi: 10.1038/sj.emboj.7600877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hernandez-Benito MJ, et al. Suppression of transient outward potassium currents in mouse ventricular myocytes by imidazole antimycotics and by glybenclamide. J Pharmacol Exp Ther. 2001;298:598–606. [PubMed] [Google Scholar]
- 30.Ventura A, et al. Cre-lox-regulated conditional RNA interference from transgenes. Proc Natl Acad Sci USA. 2004;101:10380–10385. doi: 10.1073/pnas.0403954101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vlahakis NE, et al. The lymphangiogenic vascular endothelial growth factors VEGF-C and -D are ligands for the integrin alpha9beta1. J Biol Chem. 2005;280:4544–4552. doi: 10.1074/jbc.M412816200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arcangeli A, et al. HERG- and IRK-like inward rectifier currents are sequentially expressed during neuronal development of neural crest cells and their derivatives. Eur J Neurosci. 1997;9:2596–2604. doi: 10.1111/j.1460-9568.1997.tb01689.x. [DOI] [PubMed] [Google Scholar]
- 33.Schwab A, et al. Cells move when ions and water flow. Pflügers Arch. 2007;453:421–432. doi: 10.1007/s00424-006-0138-6. [DOI] [PubMed] [Google Scholar]
- 34.Schwab A, et al. Subcellular distribution of calcium-sensitive potassium channels (IK1) in migrating cells. J Cell Physiol. 2006;206:86–94. doi: 10.1002/jcp.20434. [DOI] [PubMed] [Google Scholar]
- 35.Schwab A, et al. Polarized ion transport during migration of transformed Madin–Darby canine kidney cells. Pflügers Arch. 1995;430:802–807. doi: 10.1007/BF00386179. [DOI] [PubMed] [Google Scholar]
- 36.Gray LS, et al. The role of K+ in the regulation of the increase in intracellular Ca2+ mediated by the T lymphocyte antigen receptor. Cell. 1987;50:119–127. doi: 10.1016/0092-8674(87)90668-4. [DOI] [PubMed] [Google Scholar]
- 37.Lee J, et al. Regulation of cell movement is mediated by stretch-activated calcium channels. Nature. 1999;400:382–386. doi: 10.1038/22578. [DOI] [PubMed] [Google Scholar]
- 38.Yang S, Huang XY. Ca2+ influx through L-type Ca2+ channels controls the trailing tail contraction in growth factor-induced fibroblast cell migration. J Biol Chem. 2005;280:27130–27137. doi: 10.1074/jbc.M501625200. [DOI] [PubMed] [Google Scholar]
- 39.Lawson MA, Maxfield FR. Ca2+- and calcineurin-dependent recycling of an integrin to the front of migrating neutrophils. Nature. 1995;377:75–79. doi: 10.1038/377075a0. [DOI] [PubMed] [Google Scholar]
- 40.Bilato C, et al. The inhibition of vascular smooth muscle cell migration by peptide and antibody antagonists of the alphavbeta3 integrin complex is reversed by activated calcium/calmodulin- dependent protein kinase II. J Clin Invest. 1997;100:693–704. doi: 10.1172/JCI119582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kimura C, et al. Alterations of Ca2+ mobilizing properties in migrating endothelial cells. Am J Physiol. 2001;281:H745–H754. doi: 10.1152/ajpheart.2001.281.2.H745. [DOI] [PubMed] [Google Scholar]
- 42.McPhee JC, et al. Evidence for a functional interaction between integrins and G protein-activated inward rectifier K+ channels. J Biol Chem. 1998;273:34696–34702. doi: 10.1074/jbc.273.52.34696. [DOI] [PubMed] [Google Scholar]
- 43.Davis MJ, et al. Regulation of ion channels by integrins. Cell Biochem Biophys. 2002;36:41–66. doi: 10.1385/CBB:36:1:41. [DOI] [PubMed] [Google Scholar]
- 44.Arcangeli A, et al. Physical and functional interaction between integrins and hERG potassium channels. Biochem Soc Trans. 2004;32:826–827. doi: 10.1042/BST0320826. [DOI] [PubMed] [Google Scholar]
- 45.Kawasaki J, Davis GE, Davis MJ. Regulation of Ca2+-dependent K+ current by alphavbeta3 integrin engagement in vascular endothelium. J Biol Chem. 2004;279:12959–12966. doi: 10.1074/jbc.M313791200. [DOI] [PubMed] [Google Scholar]
- 46.Arcangeli A. Expression and role of hERG channels in cancer cells. Novartis Found Symp. 2005;266:225–234. [PubMed] [Google Scholar]
- 47.Hofmann G, et al. HERG K+ channels activation during beta(1) integrin-mediated adhesion to fibronectin induces an up-regulation of alpha(v) beta(3) integrin in the preosteoclastic leukemia cell line FLG 29.1. J Biol Chem. 2001;276:4923–4931. doi: 10.1074/jbc.M005682200. [DOI] [PubMed] [Google Scholar]
- 48.Pearson WL, et al. Expression of a functional Kir4 family inward rectifier K+ channel from a gene cloned from mouse liver. J Physiol (London) 1999;514:639–653. doi: 10.1111/j.1469-7793.1999.639ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Palmer EL, et al. Sequence and tissue distribution of the integrin alpha 9 subunit, a novel partner of beta 1 that is widely distributed in epithelia and muscle. J Cell Biol. 1993;123:1289–1297. doi: 10.1083/jcb.123.5.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pankov R, et al. A Rac switch regulates random versus directionally persistent cell migration. J Cell Biol. 2005;170:793–802. doi: 10.1083/jcb.200503152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McCormack SA, Johnson LR. Polyamines and cell migration. J Physiol Pharmacol. 2001;52:327–349. [PubMed] [Google Scholar]
- 52.Rao JN, et al. Ca2+-RhoA signaling pathway required for polyamine-dependent intestinal epithelial cell migration. Am J Physiol. 2001;280:C993–C1007. doi: 10.1152/ajpcell.2001.280.4.C993. [DOI] [PubMed] [Google Scholar]
- 53.Yokosaki Y, et al. The integrin alpha 9 beta 1 mediates cell attachment to a non-RGD site in the third fibronectin type III repeat of tenascin. J Biol Chem. 1994;269:26691–26696. [PubMed] [Google Scholar]
- 54.Yokosaki Y, et al. Identification of the ligand binding site for the integrin alpha9 beta1 in the third fibronectin type III repeat of tenascin-C. J Biol Chem. 1998;273:11423–11428. doi: 10.1074/jbc.273.19.11423. [DOI] [PubMed] [Google Scholar]
- 55.Mandeville JT, Ghosh RN, Maxfield FR. Intracellular calcium levels correlate with speed and persistent forward motion in migrating neutrophils. Biophys J. 1995;68:1207–1217. doi: 10.1016/S0006-3495(95)80336-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.