Abstract
Background
Telavancin is a lipoglycopeptide with multiple mechanisms of action that include membrane-destabilizing effects towards bacterial cells. It shows bactericidal activity against forms of Staphylococcus aureus (phagolysosomal infection) with different resistance phenotypes [methicillin-resistant S. aureus, vancomycin-intermediate S. aureus or vancomycin-resistant S. aureus]. We examine here the uptake, efflux and intracellular distribution of telavancin in eukaryotic cells as well as its potential to induce lysosomal changes (in comparison with vancomycin and oritavancin).
Methods
J774 macrophages and rat embryo fibroblasts were exposed for up to 24 and 72 h to telavancin (5–90 mg/L). The following studies were performed: measurement of 14C-labelled telavancin cellular uptake and subcellular distribution (cell fractionation), determination of pericellular membrane integrity (lactate dehydrogenase release), electron microscopy with morphometric analysis of changes in lysosome size and determination of total phospholipid and cholesterol content.
Results
The uptake of telavancin proceeded linearly as a function of time and concentration in both cell types (clearance rate of ∼10 mL/g of protein/h). Efflux (macrophages) was ∼5.7-fold slower. Telavancin subcellular distribution was superimposable on that of a lysosomal marker (N-acetyl-β-hexosaminidase). It did not cause an increase in the release of lactate dehydrogenase and did not induce significant increases in total phospholipid or cholesterol content. It caused only mild morphological lysosomal alterations (similar to vancomycin and much less than oritavancin by morphometric analysis).
Conclusions
Telavancin is taken up by eukaryotic cells and localizes in lysosomes, causing mild morphological alterations without evidence of lipid metabolism alterations. These data support our observations that telavancin is active against intracellular S. aureus.
Keywords: glycopeptides, cellular pharmacokinetics, lipids, membrane, oritavancin, vancomycin
Introduction
Telavancin is a novel lipoglycopeptide derivative of vancomycin1 with marked bactericidal activity against vancomycin-susceptible and vancomycin-resistant organisms2 due to a multifunctional mechanism of action that combines inhibition of cell wall synthesis and disruption of bacterial cell membrane permability.3 It shows a high penetration in tissues,4 including human alveolar macrophages.5 In a recent study, we showed that telavancin exerts time- and concentration-dependent bactericidal activity against intraphagocytic Staphylococcus aureus, disregarding their resistance phenotypes (methicillin-resistant S. aureus, vancomycin-intermediate S. aureus or vancomycin-resistant S. aureus).6
The present investigation was initiated to further document and rationalize this observation by examining the uptake and subcellular disposition of telavancin in eukaryotic cells. Because previous studies had disclosed marked lysosomal alterations in eukaryotic cells exposed to another lipoglycopeptide, oritavancin,7 we also undertook to assess the impact of telavancin in this context, using vancomycin as a comparator. The study was performed with both phagocytic (J774 macrophages) and non-phagocytic (fibroblasts) cells because this allowed us, in the past, to obtain a comprehensive picture of the behaviour of other antibiotics accumulating in cells and causing specific lysosomal alterations.8–11
Materials and methods
Cells, cell cultures and assessment of membrane integrity
J774 mouse macrophages and rat embryo fibroblasts were cultivated, as previously described,7 in RPMI 1640 or in Dulbecco's modified Eagle's medium, respectively, both supplemented with 10% fetal calf serum (unless stated otherwise). The integrity of the pericellular membrane upon exposure to the antibiotics was assessed by measuring the release of lactate dehydrogenase in the culture medium, as described previously.6
Determination of cellular antibiotic concentration
Cells incubated with 14C-labelled telavancin were washed three times in ice-cold 0.9% NaCl, collected by scraping in distilled water and used for radioactivity determination (liquid scintillation counting) and protein assay (a detailed description of the methodology has been published previously12). In pilot experiments, cell-associated antibiotic was also measured by a microbiological technique (with Micrococcus luteus ATCC 9341 as test organism and antibiotic medium 11).12 A correlation coefficient (R2) of 0.95 was found between the two methods for cells incubated 24 h at concentrations spanning from 10 to 100 mg/L (n = 12).
Cell fractionation studies
Cells were collected by gentle scraping in 0.25 M sucrose/1 mM EGTA/3 mM imidazole pH 7.4, homogenized and fractionated, as described previously.12 In brief, homogenates were separated into an ‘unbroken cells/nuclei’ fraction by a low-speed centrifugation. The resulting cytoplasmic extract was separated into a ‘granules/membranes’ fraction and a final supernatant by high-speed centrifugation. The granule/membrane fraction was then further analysed by isopycnic centrifugation in a sucrose gradient. Protein and [14C]telavancin contents were determined in the fractions in parallel with the activity of marker enzymes of the main organelles, namely, inosine 5′-diphosphatase (E.C. 3.6.1.6.; plasma and endoplasmic reticulum membranes), cytochrome c oxidase (E.C. 1.9.3.1.; mitochondria) and N-acetyl-β-glucosaminidase (E.C. 3.2.1.30.; lysosomes). Results are expressed as the percentage of enzyme activity, protein or drug recovered in each fraction. For isopycnic centrifugation, distributions were standardized for equal density increments ranging from 1.08 to 1.21, as described previously.13
Biochemical studies
Cell sheets were washed three times in ice-cold 0.9% NaCl, collected by scraping in distilled water and lysed by sonication. Total phospholipids and cholesterol were extracted and assayed, as described previously.7 Proteins were assayed by the Folin–Ciocalteau/biuret method.14
Electron microscopy
Sample preparation was performed as described previously.15,16 Observations were made in a Zeiss electron microscope operated at 80 kV. Morphometric analysis was performed on pictures taken at random, using the Image J software available from the NIH web site (http://rsb.info.nih.gov/ij/) and examining a total cell surface of cell profiles of 1000–2000 µm2. For each cell profile, we manually selected the zones occupied by electron-dense material (for which a limiting membrane could not always be clearly recognized) or corresponding to large vesicles filled with heterogeneous material (see Figure 3 for examples). Results were expressed as percentage of the whole cell surface profile, which is numerically identical to the percentage of cell volume.17
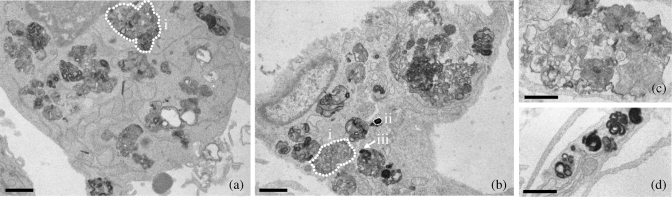
Figure 3.
Selected pictures illustrating typical changes observed in macrophages (a) and fibroblasts (b) after incubation with 90 mg/L telavancin for 24 or 72 h, respectively. Both cells show markedly enlarged and ill-shaped lysosome-like bodies with a pleiotropic material that is highly osmiophilic (often organized in a distorted concentric fashion). (c) Example of loose material and (d) example of highly osmiophilic material organized in multiple separate bodies. Bars are 1 µm. The dotted lines show examples of profiles selected for morphometric analysis: i, a large profile showing mainly a moderately osmiophilic and pleiomorphic material; ii, a small profile showing mainly osmiophilic material [possibly a polar section of a lysosome with mixed content (as in iii), but cut as shown by the arrow].
Materials
Telavancin hydrochloride (powder for microbiological evaluation and >90% purity) and [14C]telavancin trifluoroacetate (33.8 mCi/mmol and radiochemical purity >91%) were supplied by Theravance, Inc. (South San Francisco, CA, USA). The labelled drug was mixed with unlabelled telavancin to obtain a specific activity of 5 mCi/mmol. Stock solutions were prepared at a final concentration of 1–2 mg/L by vigorous vortexing in distilled water (the use of acidified DMSO recommended by the manufacturer was avoided because preliminary experiments disclosed a decrease in cell viability). Oritavancin (supplied as diphosphate salt fully hydrated) was obtained from Intermune (Brisbane, CA, USA). Vancomycin was procured as VANCOCIN® from GlaxoSmithKline, Belgium. Cell culture or microbiology media were from Invitrogen (Paisley, Scotland, UK) and Difco (Sparks, MD, USA). Other reagents were of analytic grade and purchased from E. Merck AG (Darmstadt, Germany) or Sigma-Aldrich-Fluka (St Louis, MO, USA).
Results
Influence of telavancin on pericellular membrane integrity
In preliminary experiments, we measured the release of lactate dehydrogenase, a cytosolic enzyme, from cells exposed to telavancin (90 mg/L, corresponding to the human Cmax18), in comparison with vancomycin (50 mg/L, corresponding to the human Cmax19) and oritavancin (25 mg/L, corresponding to the human Cmax20). No difference in controls (5.0 ± 0.2%) was seen with telavancin (4.9 ± 1.0%) or vancomycin (5.0 ± 1.5%) in J774 macrophages after 24 h of incubation, whereas oritavancin induced a small but significant increase in enzyme release (15.5 ± 1.5%; P < 0.001; n = 3 for all conditions). No significant effect was seen for fibroblasts after 72 h of incubation between controls (13.4 ± 3.7%) and cells incubated with telavancin (16.9 ± 2.5%), vancomycin (17.1 ± 1.3%) or oritavancin (18.2 ± 3.0%; n = 3 for all testing conditions).
Kinetics of uptake and release of telavancin
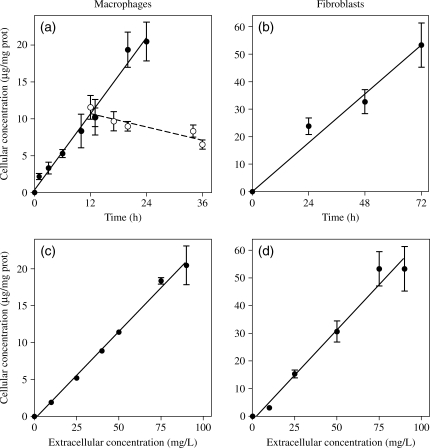
Figure 1(a and b) shows the kinetics of uptake of telavancin in J774 macrophages and fibroblasts incubated with an extracellular concentration of 90 mg/L. The uptake proceeded linearly over time at a rate that was similar in both cell types (see figure captions for details). Figure 1(c and d) examines the uptake of telavancin at increasing extracellular concentrations and at fixed time points (24 h for macrophages and 72 h for fibroblasts). The uptake was linearly related to the extracellular concentration in both cell types, allowing us to calculate a clearance rate for all conditions shown in Figure 1 of ~10 µL/mg of cell protein per h. For macrophages, we also examined the drug efflux (after an initial uptake of 12 h in the presence of 90 mg/L telavancin), which occurred at an apparent rate ∼5.7-fold lower than that of influx (Figure 1a).
Figure 1.
(a and b) Kinetics of uptake of telavancin (filled symbols and continuous line) in J774 macrophages (a) or embryo fibroblasts (b) incubated for the indicated times with an extracellular concentration of 90 mg/L at 37°C in medium supplemented with 10% fetal calf serum. (a) Kinetics of efflux of the drug from J774 macrophages exposed to telavancin (90 mg/L) for 12 h and re-incubated in a drug-free medium for an additional 24 h (open symbols and broken line) are also shown. (c and d) Cellular concentration of telavancin in J774 macrophages (c) or embryo fibroblasts (d) incubated at 37°C for 24 h or 72 h, respectively, in the presence of telavancin at the extracellular concentrations indicated on the abscissa. Results are given as arithmetic means ± SD (n = 3) and analysed by linear regression to calculate the corresponding clearances (µL/mg of protein/h): J774 macrophages, 9.6 ± 0.6 (R2 = 0.98; a) and 10.0 ± 0.4 (R2 = 0.99; c); fibroblasts, 8.2 ± 0.4 (R2 = 0.97; b) and 9.0 ± 0.7 (R2 = 0.98; d).
The influence of serum on telavancin uptake was examined in J774 macrophages by performing experiments in culture medium not supplemented with calf serum. The incubation was limited to 5 h to ensure maintenance of cell viability. Telavancin uptake remained linear over the 10–90 mg/L range of extracellular concentrations, but proceeded at a rate ~1.7-fold faster than in the presence of serum (data not shown).
Subcellular distribution of telavancin
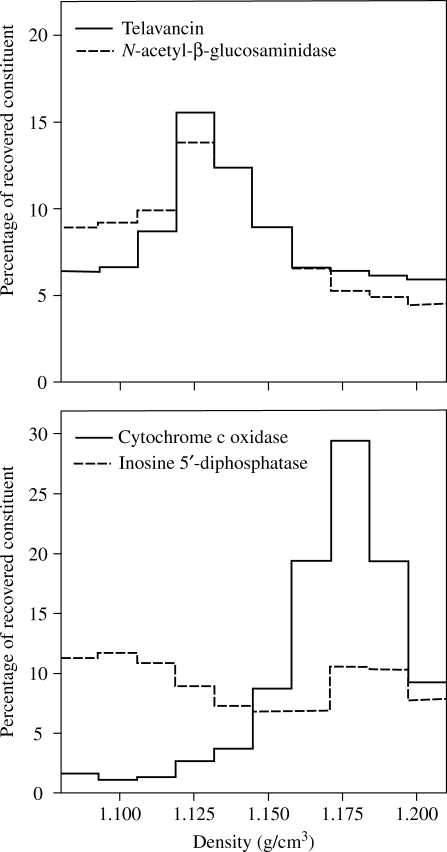
The subcellular distribution of cell-associated telavancin was examined in homogenates obtained from J774 macrophages incubated for 24 h with the drug at an extracellular concentration of 90 mg/L. The distribution of cell-associated telavancin and N-acetyl-β-glucosaminidase (lysosome marker) among the unbroken cells/nuclei, granules/membranes and supernatant fractions was similar (35% and 27%, 55% and 67% and 10% and 6%, respectively). The granule/membrane fraction was therefore subfractionated by isopycnic centrifugation. The density distribution of telavancin, in comparison with that of N-acetyl-β-glucosaminidase (lysosomes), cytochrome c oxidase (mitochondria) and inosine 5′-diphosphatase (plasma/endoplasmic reticulum membranes), is shown in Figure 2. The distribution pattern of telavancin was largely superimposable on that of the N-acetyl-β-glucosaminidase, clearly dissociated from that of cytochrome c oxidase and also distinct from that of inosine 5′-diphosphatase.
Figure 2.
Density distribution of marker enzymes [N-acetyl-β-glucosaminidase (lysosomes), cytochrome c oxidase (mitochondria) and inosine 5′-diphosphatase (plasma and endoplasmic reticulum membranes)] and of telavancin after isopycnic centrifugation in a linear sucrose gradient of a granule fraction prepared from homogenates of J774 cells that were incubated for 24 h with 90 mg/L telavancin at 37°C in medium supplemented with 10% fetal calf serum. The ordinate shows the percentage of each constituent recovered in each fraction.
Ultrastructural alterations
Electron microscopy was used to examine whether telavancin induces morphological alterations in the subcellular organelles of cells incubated in its presence. Figure 3 shows selected pictures of alterations that could be observed in J774 macrophages and fibroblasts (90 mg/L; 24 and 72 h, respectively). There was an enlargement of lysosomes that were filled with a pleiotropic material, made of partly highly osmiophilic, concentric structures and partly of a more loose appearance material.
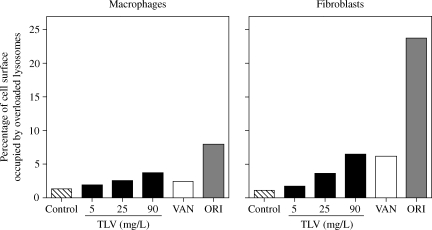
To gain quantitative information on these changes, a morphometric analysis was performed on pictures taken at random. Figure 4 shows the relative abundance of these abnormal lysosomal profiles in cells incubated with three different concentrations of telavancin, in comparison with control cells or cells exposed to 50 mg/L vancomycin or 20 mg/L oritavancin. In macrophages (24 h incubation) as well as in fibroblasts (72 h incubation), telavancin induced a concentration-dependent increase in the percentage of cell volume occupied by overloaded lysosomes. The morphometric analysis showed that: (i) changes induced in fibroblasts were more marked than those in macrophages (which may have been a consequence of the longer incubation time in fibroblasts); (ii) incubation of macrophages with 25 mg/L telavancin or of fibroblasts with 90 mg/L caused changes similar to those seen with 50 mg/L vancomycin in either cell types and (iii) alterations induced by telavancin at the highest concentration tested (90 mg/L) were considerably milder than in cells incubated with 20 mg/L oritavancin in both cell types.
Figure 4.
Morphometric analysis of the material accumulated in macrophages (left-hand panel) or fibroblasts (right-hand panel) after 24 and 72 h of incubation, respectively, in control conditions or in the presence of 5, 25 or 90 mg/L telavancin (TLV), 50 mg/L vancomycin (VAN) or 20 mg/L oritavancin (ORI). Results are expressed as percentage of the cell surface occupied by the electron-dense material and/or large vesicles filled with a material of undetermined nature. Surface analysed was ~2000 µm2 for macrophages and 1000 µm2 for fibroblasts.
Influence of telavancin on cell phospholipid and cholesterol contents
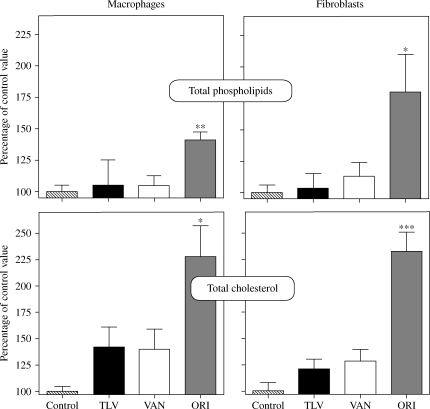
In view of the ultrastructural changes observed, we looked for changes in phospholipids and cholesterol content of cells exposed to 90 mg/L telavancin in comparison with vancomycin (50 mg/L) and oritavancin (25 mg/L). Figure 5 illustrates the data obtained after 24 h in macrophages and 72 h in fibroblasts. Neither telavancin nor vancomycin caused detectable increase in the phospholipid content. In contrast, oritavancin induced significant increases in the phospholipid content in both cell types. Telavancin and vancomycin caused a slight but not significant increase in the cholesterol content, whereas oritavancin again induced a marked, statistically significant increase.
Figure 5.
Total phospholipid content (upper panels) or total cholesterol content (lower panels) of J774 mouse macrophages (left-hand panels) or rat embryo fibroblasts (right-hand panels) exposed for 24 and 72 h, respectively, to glycopeptides at their human Cmax (VAN, vancomycin 50 mg/L; TLV, telavancin 90 mg/L and ORI, oritavancin 25 mg/L). Results are expressed as percentages of control values for total phospholipids and cholesterol. Values are arithmetic means ± SD (n = 6–8). Values for control macrophages and fibroblasts were, respectively, 204 ± 10 and 295 ± 8 nmol/mg of protein for total phospholipids and 72 ± 3 and 130 ± 16 nmol/mg of protein for total cholesterol. Statistical analysis (ANOVA): ***P < 0.001, **P < 0.01, *P < 0.05 when compared with the matching control. Other differences were not significant.
Discussion
The present study provides evidence that telavancin is taken up by cultured macrophages where it becomes associated with lysosomes, as assessed by cell fractionation studies. These results rationalize our previous data, showing that telavancin exerts a marked antibiotic activity against intraphagocytic S. aureus, which is known to develop in the phagolysosomes of infected macrophages.21,22
The accumulation level reached by telavancin in macrophages at 24 h (∼45-fold) is intermediate between that recorded previously in the same conditions for vancomycin (∼8-fold) and oritavancin (∼370-fold).12 Its uptake is not specific to phagocytic cells, as it is also observed with fibroblasts, both cell types taking up the drug at similar rates. The entry of drugs into lysosomes may occur through either diffusion/segregation or pinocytosis,23 as illustrated by the behaviour of macrolides8 and aminoglycosides,24 respectively. Diffusion–segregation seems unlikely in view of the slow efflux of telavancin, as this process is usually considered to be reversible (although binding of the drug to intracellular constituents could explain this slow efflux, as is observed for azithromycin in fibroblasts16). Pinocytosis, therefore, appears more plausible, especially if considering the size of the molecule that would prevent its fast diffusion through membranes. However, the clearance rates recorded here (∼10 µL/mg of protein/h) are ~15- to 30-fold higher than those reported for fluid-phase pinocytosis markers (∼0.7 µL/mg of protein/h in macrophages15 and ∼0.3 µL/mg of protein/h in fibroblasts24). Telavancin uptake, therefore, should involve a process of adsorptive pinocytosis (through binding to cell surface; see discussion and modelling in Silverstein et al.25) However, the lack of saturation of telavancin uptake upon increase of its extracellular concentration is intriguing as this (i) is an hallmark of adsorptive pinocytosis25 and (ii) was observed for oritavancin.12 The initial uptake clearance rate of oritavancin, however, is considerably larger (∼150 µL/mg of protein/h),12 which indicates that binding of telavancin should be much weaker and may not actually show saturation in the concentration range investigated here. A weaker membrane binding of telavancin is actually consistent with its lower lipophilicity when compared with oritavancin (reported logP values of 0.6 for telavancin versus 4.1 for oritavancin) [Advanced Chemistry Development Software Solaris V4.67, Sci Finder Scholar 2006, American Chemical Society, Washington, DC, USA].
Beyond its therapeutic interest, the lysosomal accumulation of telavancin may also be responsible for the morphological alterations observed in cells exposed to the drug. Yet, these changes appear quantitatively minor, as is also the case for vancomycin. Moreover, and in contrast to what is observed with oritavancin,7 telavancin did not significantly affect phospholipid or cholesterol cellular levels. This difference may be related to the lower uptake of telavancin, although we cannot exclude, at this stage, a true difference in the intrinsic capacity of the two molecules to interfere with lipid metabolism. Further studies are required to determine the exact nature of the accumulated material and to decipher the underlying molecular mechanisms. These may be more complex than those evidenced for aminoglycoside antibiotics, which mainly induce an accumulation of phospholipids.26 Interestingly, telavancin was without detectable effect on lactate dehydrogenase release. This suggests that the membrane-destabilizing properties exerted by telavancin towards bacterial membranes,3 which probably contribute to its marked bactericidal effect, involve constituents that are specific to or more abundant in prokaryotic cells.
Independent studies have shown that telavancin accumulates in human alveolar macrophages, reaching a cellular concentration of ~50 mg/L within 8–24 h after systemic administration of therapeutic doses.5,27 In our experiments, macrophages exposed for 24 h at 90 mg/L have an apparent cellular concentration of 4 mg/mL [based on an estimated mean cellular volume of 5 µL/mg of protein (see Van Bambeke et al.12 and references cited therein)]. The lower cellular uptake of telavancin reported in vivo may result from (i) its high protein binding (∼90% to 93%) and (ii) the fluctuation of its serum levels related to its once-daily administration and its half-life of 7.5 h.18 As extensively discussed in previous publications,6,7,12,22 our cellular model suffers from the limitation that it does not take into account these two important pharmacokinetic determinants. This may lead to an overestimation of the accumulation of telavancin versus that of vancomycin, for which the free fraction in vivo oscillates between 10% and 55%.19 Thus, assuming that only the free fraction of telavancin is available for the uptake by macrophages in vivo, its effective extracellular concentration will oscillate between ∼10 and 0.5 mg/L, which, in our model, would create a cellular concentration of ∼0.24 µg/mg of protein (i.e. ∼48 mg/L). At this concentration, which is of the same order of magnitude as that observed in vivo, telavancin does not cause any morphological change in our model.
Taken together, our data further document the tissue-directed pharmacokinetics of lipoglycopeptides.28 As telavancin is clearly superior to vancomycin with respect to bactericidal activity towards both extracellular and intraphagolysosomal S. aureus6 while showing a similar cellular safety profile (as far as lipid metabolism and subcellular morphology are concerned), the data support its potential interest for the treatment of infections where intracellular foci are present.29 Further studies using in vivo models are, however, required to confirm the improved intracellular efficacy and cellular inocuity of telavancin.
Funding
F. V. B. is Maître de Recherches of the Belgian Fonds National de la Recherche Scientifique. Funding: Belgian Fonds de la Recherche Scientifique (FRS–FNRS) and Belgian Fonds de la Recherche Scientifique Médicale (FRSM; grant nos 1.5.223.05 F, 3.4.549.00 F and 3.4.639.04 F); Belgian Federal Science Policy Office (Research project P5/33; research action P5); Fonds Spéciaux de Recherches and Actions de Recherches Concertées of the Université catholique de Louvain; Grant-in-Aid from Theravance, Inc. (for the pharmacokinetic studies with telavancin).
Transparency declarations
F. V. B. and P. M. T. are members of the European Advisory Board of Targanta Inc. (current owner of oritavancin). The remaining authors have none to declare.
Acknowledgements
We thank F. Andries-Renoird, M. C. Cambier, A. Lefevre and M. Vergauwen for their dedicated technical assistance and Dr Y. Guiot for access to electron microscopy facilities.
References
- 1.Leadbetter MR, Adams SM, Bazzini B, et al. Hydrophobic vancomycin derivatives with improved ADME properties: discovery of telavancin (TD-6424) J Antibiot (Tokyo) 2004;57:326–36. doi: 10.7164/antibiotics.57.326. [DOI] [PubMed] [Google Scholar]
- 2.Pace JL, Krause K, Johnston D, et al. In vitro activity of TD-6424 against Staphylococcus aureus. Antimicrob Agents Chemother. 2003;47:3602–4. doi: 10.1128/AAC.47.11.3602-3604.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Higgins DL, Chang R, Debabov DV, et al. Telavancin, a multifunctional lipoglycopeptide, disrupts both cell wall synthesis and cell membrane integrity in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2005;49:1127–34. doi: 10.1128/AAC.49.3.1127-1134.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun HK, Duchin K, Nightingale CH, et al. Tissue penetration of telavancin after intravenous administration in healthy subjects. Antimicrob Agents Chemother. 2006;50:788–90. doi: 10.1128/AAC.50.2.788-790.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gotfried MH, Shaw JP, Benton BM, et al. Intrapulmonary distribution of intravenous telavancin in healthy subjects and effect of pulmonary surfactant on in vitro activities of telavancin and other antibiotics. Antimicrob Agents Chemother. 2008;52:92–7. doi: 10.1128/AAC.00875-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barcia-Macay M, Lemaire S, Mingeot-Leclercq MP, et al. Evaluation of the extracellular and intracellular activities (human THP-1 macrophages) of telavancin versus vancomycin against methicillin-susceptible, methicillin-resistant, vancomycin-intermediate and vancomycin-resistant Staphylococcus aureus. J Antimicrob Chemother. 2006;58:1177–84. doi: 10.1093/jac/dkl424. [DOI] [PubMed] [Google Scholar]
- 7.Van Bambeke F, Saffran J, Mingeot-Leclercq MP, et al. Mixed-lipid storage disorder induced in macrophages and fibroblasts by oritavancin ( LY333328), a new glycopeptide antibiotic with exceptional cellular accumulation. Antimicrob Agents Chemother. 2005;49:1695–700. doi: 10.1128/AAC.49.5.1695-1700.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carlier MB, Garcia-Luque I, Montenez JP, et al. Accumulation, release and subcellular localization of azithromycin in phagocytic and non-phagocytic cells in culture. Int J Tissue React. 1994;16:211–20. [PubMed] [Google Scholar]
- 9.Montenez JP, Van Bambeke F, Piret J, et al. Interactions of macrolide antibiotics (erythromycin A, roxithromycin, erythromycylamine [dirithromycin], and azithromycin) with phospholipids: computer-aided conformational analysis and studies on acellular and cell culture models. Toxicol Appl Pharmacol. 1999;156:129–40. doi: 10.1006/taap.1999.8632. [DOI] [PubMed] [Google Scholar]
- 10.Aubert-Tulkens G, Van Hoof F, Tulkens P. Gentamicin-induced lysosomal phospholipidosis in cultured rat fibroblasts. Quantitative ultrastructural and biochemical study. Lab Invest. 1979;40:481–91. [PubMed] [Google Scholar]
- 11.Tulkens P, Van Hoof F. Comparative toxicity of aminoglycoside antibiotics towards the lysosomes in a cell culture model. Toxicology. 1980;17:195–9. doi: 10.1016/0300-483x(80)90094-3. [DOI] [PubMed] [Google Scholar]
- 12.Van Bambeke F, Carryn S, Seral C, et al. Cellular pharmacokinetics and pharmacodynamics of the glycopeptide antibiotic oritavancin ( LY333328) in a model of J774 mouse macrophages. Antimicrob Agents Chemother. 2004;48:2853–60. doi: 10.1128/AAC.48.8.2853-2860.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Renard C, Vanderhaeghe HJ, Claes PJ, et al. Influence of conversion of penicillin G into a basic derivative on its accumulation and subcellular localization in cultured macrophages. Antimicrob Agents Chemother. 1987;31:410–6. doi: 10.1128/aac.31.3.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 15.Tyteca D, Van Der Smissen P, Mettlen M, et al. Azithromycin, a lysosomotropic antibiotic, has distinct effects on fluid-phase and receptor-mediated endocytosis, but does not impair phagocytosis in J774 macrophages. Exp Cell Res. 2002;281:86–100. doi: 10.1006/excr.2002.5613. [DOI] [PubMed] [Google Scholar]
- 16.Tyteca D, Van Der Smissen P, Van Bambeke F, et al. Azithromycin, a lysosomotropic antibiotic, impairs fluid-phase pinocytosis in cultured fibroblasts. Eur J Cell Biol. 2001;80:466–78. doi: 10.1078/0171-9335-00180. [DOI] [PubMed] [Google Scholar]
- 17.Loud AV. A method for the quantification of cytoplasmic structures. J Cell Biol. 1962;15:481–7. doi: 10.1083/jcb.15.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaw JP, Seroogy J, Kaniga K, et al. Pharmacokinetics, serum inhibitory and bactericidal activity, and safety of telavancin in healthy subjects. Antimicrob Agents Chemother. 2005;49:195–201. doi: 10.1128/AAC.49.1.195-201.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feketi R. Vancomycin, teicoplanin, and the streptogramins: quinupristin and dalfopristin. In: Mandell GE, Bennett JE, Dolin R, editors. Principles and Practice of Infectious Diseases. Philadelphia, PA: Churchill Livingstone; 2000. pp. 382–92. [Google Scholar]
- 20.Braun DK, Chien JK, Farlow DS, et al. Oritavancin ( LY333328): a dose-escalation safety and pharmacokinetics study in patients. Clin Microbiol Infect. 2001;7:P434. [Google Scholar]
- 21.Seral C, Van Bambeke F, Tulkens PM. Quantitative analysis of gentamicin, azithromycin, telithromycin, ciprofloxacin, moxifloxacin, and oritavancin ( LY333328) activities against intracellular Staphylococcus aureus in mouse J774 macrophages. Antimicrob Agents Chemother. 2003;47:2283–92. doi: 10.1128/AAC.47.7.2283-2292.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barcia-Macay M, Seral C, Mingeot-Leclercq MP, et al. Pharmacodynamic evaluation of the intracellular activities of antibiotics against Staphylococcus aureus in a model of THP-1 macrophages. Antimicrob Agents Chemother. 2006;50:841–51. doi: 10.1128/AAC.50.3.841-851.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Duve C, de Barsy T, Poole B, et al. Commentary. Lysosomotropic agents. Biochem Pharmacol. 1974;23:2495–531. doi: 10.1016/0006-2952(74)90174-9. [DOI] [PubMed] [Google Scholar]
- 24.Tulkens P, Trouet A. The uptake and intracellular accumulation of aminoglycoside antibiotics in lysosomes of cultured rat fibroblasts. Biochem Pharmacol. 1978;27:415–24. doi: 10.1016/0006-2952(78)90370-2. [DOI] [PubMed] [Google Scholar]
- 25.Silverstein SC, Steinman RM, Cohn ZA. Endocytosis. Annu Rev Biochem. 1977;46:669–722. doi: 10.1146/annurev.bi.46.070177.003321. [DOI] [PubMed] [Google Scholar]
- 26.Laurent G, Carlier MB, Rollman B, et al. Mechanism of aminoglycoside-induced lysosomal phospholipidosis: in vitro and in vivo studies with gentamicin and amikacin. Biochem Pharmacol. 1982;31:3861–70. doi: 10.1016/0006-2952(82)90303-3. [DOI] [PubMed] [Google Scholar]
- 27.Wong S, Shaw JP, Gotfried M, et al. Abstracts of the Seventeenth European Congress of Clinical Microbiology and Infectious Diseases and the Twenty-fifth International Congress of Chemotherapy, Munich, Germany, 2007. Penetration of telavancin into pulmonary epithelial lining fluid and alveolar macrophages. Abstract O420. [Google Scholar]
- 28.Van Bambeke F. Glycopeptides and glycodepsipeptides in clinical development: a comparative review of their antibacterial spectrum, pharmacokinetics and clinical efficacy. Curr Opin Investig Drugs. 2006;7:740–9. [PubMed] [Google Scholar]
- 29.Lowy FD. Is Staphylococcus aureus an intracellular pathogen? Trends Microbiol. 2000;8:341–3. doi: 10.1016/s0966-842x(00)01803-5. [DOI] [PubMed] [Google Scholar]