SUMMARY
Neurobiological studies of stress often focus on the hippocampus where cortisol binds with different affinities to two types of corticosteroid receptors, i.e., mineralocorticoid receptor (MR) and glucocorticoid receptor (GR). The hippocampus is involved in learning and memory, and regulates the neuroendocrine stress response, but other brain regions also play a role, especially prefrontal cortex. Here we examine MR and GR expression in adult squirrel monkey prefrontal cortex and hippocampus after exposure to social stress in infancy or adulthood. In situ hybridization histochemistry with 35S-labeled squirrel monkey riboprobes and quantitative film autoradiography were used to measure the relative distributions of MR and GR mRNA. Distinct cortical cell layer-specific patterns of MR expression differed from GR expression in three prefrontal regions. The relative distributions of MR and GR also differed in hippocampal Cornu Ammonis (CA) regions. In monkeys exposed to adult social stress compared to the no-stress control, GR expression was diminished in hippocampal CA1 (P=0.021), whereas MR was diminished in cell layer III of ventrolateral prefrontal cortex (P=0.049). In contrast, exposure to early life stress diminished GR but not MR expression in cell layers I and II of dorsolateral prefrontal cortex (P’s<0.048). Similar reductions likewise occurred in ventrolateral prefrontal cortex, but the effects of early life stress on GR expression in this region were marginally not significant (P=0.053). These results provide new information on regional differences and the long-term effects of stress on MR and GR distributions in corticolimbic regions that control cognitive and neuroendocrine functions.
Keywords: Mineralocorticoid receptor, Glucocorticoid receptor, Prefrontal cortex, Hippocampus, Stress, Squirrel monkey
INTRODUCTION
Stressful experiences generally trigger a neuroendocrine cascade that stimulates secretion of cortisol from the adrenal cortex (Smith and Vale, 2006). Cortisol diffuses into the brain and binds with different affinities to two types of corticosteroid receptor, i.e., Type I or mineralocorticoid receptor (MR) and Type II or glucocorticoid receptor (GR). MR has a high binding affinity, is extensively occupied most of the time, and regulates the transcription of genes involved in tonic neurotrophic effects (Woolley et al., 1991; McCullers and Herman, 1998; Gass et al., 2000; Rozeboom et al., 2007). GR has a low binding affinity, is mainly occupied during periods of stress, and regulates the transcription of genes involved in neurodegenerative effects (Kim and Diamond, 2002; Sousa and Almeida, 2002; Morsink et al., 2006; Landfield et al., 2007). The relative balance between MR and GR distributions is therefore important for understanding how stress levels of cortisol affect brain regions that play a role in cognitive and neuroendocrine functions (Lupien and McEwen, 1997; Lopez et al., 1998; Holsboer, 2000; de Kloet et al., 2005).
Although both MR and GR are expressed in human (Watzka et al., 2000; Webster et al., 2002; Xing et al., 2004) and monkey (Patel et al., 2000; Sanchez et al., 2000; Pryce et al., 2005) prefrontal cortex, cell layer-specific differences in the relative balance between MR and GR prefrontal distributions have received little attention. In rats, for example, early life stress down regulates GR but not MR expression in prefrontal cortex analyzed as one contiguous region (Avishai-Eliner et al., 1999; Ladd et al., 2004). GR expression in a similar region of rat prefrontal cortex is likewise down regulated by chronic stress conditions in adulthood (Mizoguchi et al., 2003). Here we examine prefrontal cortical cell layer-specific patterns in MR and GR expression in adult squirrel monkeys exposed to chronic social stress in infancy or adulthood. Stress-induced changes in MR and GR expression are also examined in regions of the squirrel monkey hippocampus.
METHODS
Experimental design
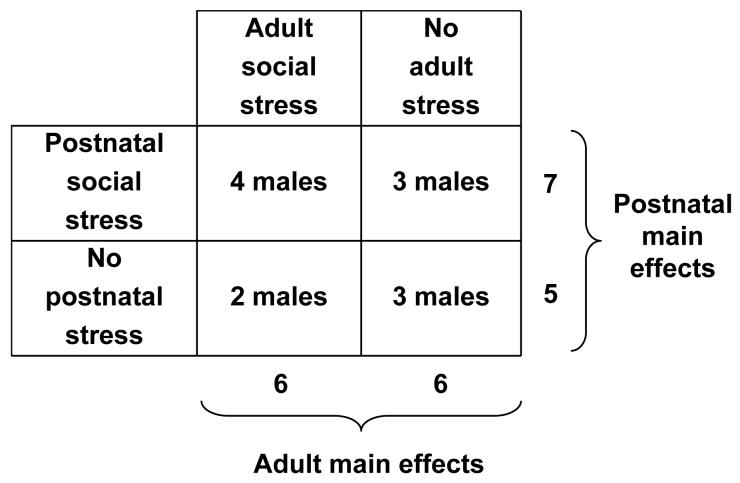
Twelve male squirrel monkeys (Saimiri sciureus) that were born and raised at the Stanford University Animal Research Facility were randomized to the following treatment conditions in adulthood at ∼9 years of age (range 7.2-10.6 years). In one condition, monkeys were exposed to six intermittent social separations that each lasted three weeks in duration. During each separation, monkeys were individually housed and could see, hear, smell, but not touch other unfamiliar monkeys. After each intermittent separation, new male pairs were subsequently formed and maintained for nine weeks. New pair formations (Coe et al., 1982) and social separations (Lyons et al., 1999) are known to increase cortisol levels in adult squirrel monkeys. In the no-stress control condition, adult monkeys were housed with the same male companion in stable same-sex pairs. As part of prior studies, the monkeys from each adult treatment condition were exposed, from 2.5 to 5.5 months of age, to postnatal psychosocial stress or no-stress conditions described elsewhere in detail (Lyons et al., 2002). Randomization of monkeys to the adult conditions was stratified by prior postnatal conditions to provide similar size samples in the 2 × 2 factorial design (Figure 1). Monkeys were maintained throughout the study with lights on:lights off at 0700:1900 hours, with unlimited access to food and water. There were no significant weight differences between groups or following intermittent separations. All procedures were conducted in accordance with the Animal Welfare Act, and were approved by Stanford University’s Administrative Panel on Laboratory Animal Care.
Figure 1.
Schematic representation of the experimental design and subject numbers.
Tissue collection and in situ hybridization
Brain tissues were collected 12-weeks after the end of the last adult social separation while all monkeys were housed in pairs. Monkeys were anesthetized with an intramuscular injection of 10 mg/kg ketamine, followed by euthanasia with an intravenous overdose of 120 mg/kg pentobarbital. Craniotomies were performed, brains were removed, and the left and right cerebral hemispheres were separated by a mid-sagittal incision. Left cerebral hemispheres were placed in a custom-designed acrylic brain matrix, and coronally cut into blocks that were frozen in isopentane on dry ice at -40°C. One block contained all prefrontal tissue from the anterior commissure to the frontal pole, and another block included the entire hippocampus. All brain tissue collections occurred between 08:00-09:30 hours to control for diurnal variation in MR and GR expression (Holmes et al., 1997).
Twenty micrometer thick tissue sections cut on a Leica CM1900 cryostat were thaw-mounted onto Superfrost Plus glass slides and stored at -80°C. Selected slides were fixed for 1 hr in 4% paraformaldehyde before processing for in situ hybridization histochemistry. GR was measured using a previously characterized 390 base antisense probe (cRNA) directed against nucleotides 376-765 (spanning amino acids 4-133) of squirrel monkey GR cDNA (Genbank #AF041834). MR was measured with a previously characterized 392 base cRNA directed against nucleotides 1-392 (spanning amino acids 1-130) of squirrel monkey MR cDNA (Genbank #AF245224). Both cDNAs were cloned in bifunctional vectors that permitted anti-sense and sense strand riboprobe construction from bacterial promoters. Riboprobe synthesis and hybridization were carried out as described elsewhere (Patel et al., 2000). Slides were exposed to Biomax MR film for 1 to 3 days along with [14C]-radiolabeled standards (Amersham) to assure that specific signal did not exceed the linear range of the film. Hybridization controls included incubation of tissue with sense-strand constructs or RNase pretreatment. As previously reported (Patel et al., 2000), no signal was generated in either control condition.
Image analysis
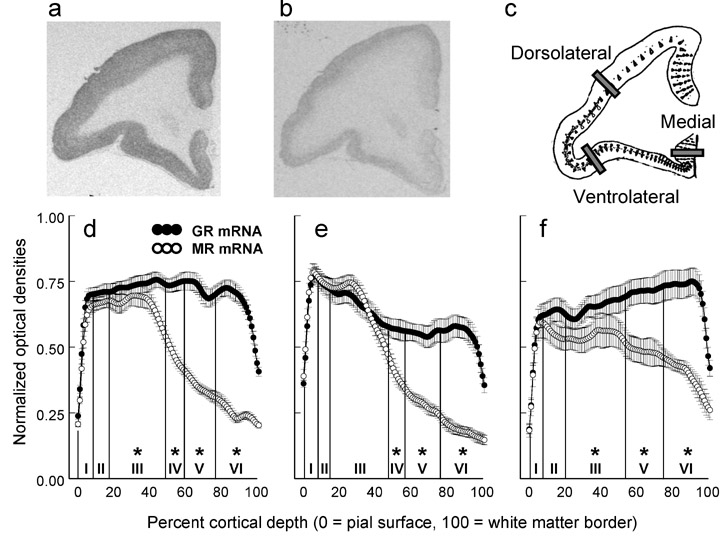
Digital grayscale images of the radiographic films were analyzed using NIH Image v1.62 software without knowledge of the treatment conditions. Prefrontal GR and MR expression were examined in adjacent 1-in-40 serial sections randomly selected from each monkey at the genu of the corpus callosum. On each section three line transects were placed perpendicular to the pial surface and traversed the full width of prefrontal cortical tissue (Figure 2a-c). The medial transect was positioned to sample subcallosal agranular cortex lacking a well-developed cell layer IV (Brodman Areas 25). The dorsolateral transect was positioned over dysgranular cortex between Areas 6 and 8. The ventrolateral transect was positioned to sample granular cortex with a well-developed cell layer IV (Area 11/44). Specific regions were determined from Nissl stained sections and cytoarchitectonic maps of squirrel monkey prefrontal cortex.
Figure 2.
Relative distributions of GR and MR expression in prefrontal cortex. Representative images of (a) GR expression, (b) MR expression, and (c) the locations of line profile transects depicted on Rosabal’s (1967) drawing of squirrel monkey prefrontal cortex. Profile plots of GR and MR densities are presented for the (d) ventrolateral, (e) dorsolateral, and (f) medial transects (N = 12 adult monkeys; mean ± SEM). Asterisks signify significant cell layer-specific differences in the relative distributions of GR and MR expression (P<0.05).
Along the entire length of each line transect from the pial surface to the edge of white matter continuous optical densities were collected using NIH image software. These data were used to construct profile plots linearly interpolated to a common anatomical scale (i.e., percentage of total cortical width) as described for a similar analysis of GR (Webster et al., 2002) and MR (Xing et al., 2004) expression in human prefrontal cortex. Cortical cell layer-specific measures were derived from the percentage of total cortical width representing each layer based on the criteria of Rosabal (1967) for squirrel monkey prefrontal cortex. The percentages of total cortical width for each cell layer in ventrolateral prefrontal cortex were as follows: layer I (1-10%), layer II (11-18%), layer III (19-51%), layer IV (52-61%), layer V (61-76%), and layer VI (77-100%). The percentages of total cortical width for each cell layer in dorsolateral prefrontal cortex were: layer I (1-9%), layer II (10-14%), layer III (14-48%), layer IV (49-57%), layer V (58-77%), and layer VI (78-100%). The percentages of total cortical width for each cell layer in medial prefrontal cortex were: layer I (1-9%), layer II (10-21%), layer III (22-56%), layer IV was not present, layer V (57-75%), and layer VI (76-100%). The validity of these laminar specifications was confirmed by measuring the depth of each boundary on Nissl stained sections adjacent to those that were used for MR and GR hybridizations. Optical density data were background corrected, normalized to the maximum value for each region-specific riboprobe assay to control for inter-assay variability, and averaged across two serial sections per monkey for each regional transect and type of receptor to serve as the units for statistical analysis.
Optical density data for GR and MR were also collected from adjacent 1-in-40 serial sections randomly selected to encompass the entire rostro-caudal extent of the hippocampus, i.e., 8-12 sections per monkey for each type of receptor. On each section, we manually traced the borders of dentate gyrus, CA1, and CA2-3 regions. The anatomic level of analysis was determined from Nissl stained sections and a squirrel monkey brain atlas (Gergen and MacLean, 1962). Optical density data were background corrected, normalized to the maximum value for each region-specific riboprobe assay to control for inter-assay variability, and averaged across 8-12 serial sections per monkey for each hippocampal region and type of receptor to serve as the units for statistical analysis.
Statistical analysis
A single mixed factor four-way analysis of variance (ANOVA) was used to simultaneously examine GR and MR expression along each prefrontal transect. Adult and postnatal stress treatment conditions were considered between-subjects factors, and cortical layer and receptor type were the within-subjects factors. An identical ANOVA was used to examine GR and MR in hippocampus with region included in the analysis instead of cortical cell layer. Statistically significant interactions discerned by the omnibus ANOVAs were subsequently deconstructed and analyzed by two-way ANOVAs to assess adult and postnatal stress effects. Body weights were compared by Student’s t-test. All test statistics are evaluated with two-tailed probabilities (P<0.05).
RESULTS
Prefrontal cortical cell layer-specific differences in GR and MR expression
A significant receptor type-by-cortical layer interaction was discerned for all three prefrontal regions (ventrolateral, F(5,40)=118.95, P<0.001; dorsolateral, F(5,40)=51.76, P<0.001; medial, F(4,32)=13.48, P<0.001). Both GR and MR were clearly expressed in cortical cell layers I-III, but differences in the relative distributions of GR and MR expression emerged in the deeper cell layers (Figure 2d-f). MR expression was consistently diminished in cortical cell layers V and VI, as the relative balance between MR and GR shifted in favor of GR expression in the deeper cortical cell layers.
Stress-dependent differences in prefrontal MR and GR expression
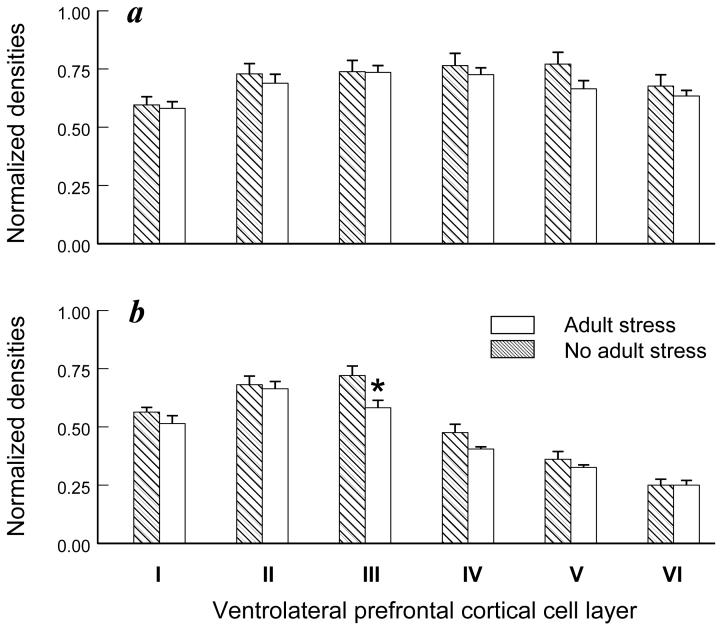
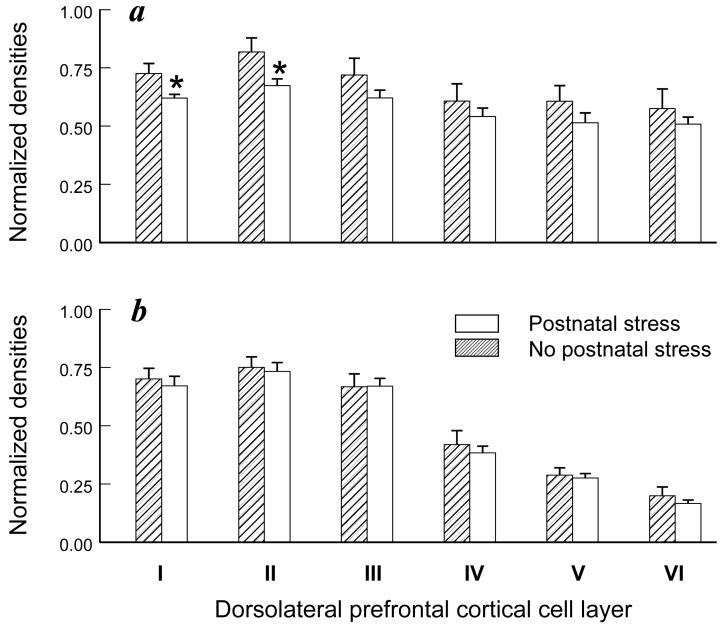
A significant adult social stress-by-receptor-by-cortical layer interaction was discerned for the ventrolateral prefrontal cortex (F(5,40)=78.21, P<0.001). Adult stress effects were not significant for GR expression in the ventrolateral region (Figure 3a), but MR expression in layer III was significantly diminished (-19%) by adult stress exposure compared to the no-stress condition (F(1,8)=5.39, p=0.049; Figure 3b). Conversely, exposure to postnatal stress diminished GR but not MR expression in layer I (-14%; F(1,8)=5.97; P=0.04) and layer II (-18%; F(1,8)=5.44, p=0.048) of dorsolateral prefrontal cortex (Figure 4a-b). A similar reduction in GR expression in layer I was also discerned in ventrolateral prefrontal cortex (data not shown), but the postnatal stress effects in this region were not significant (F(1,8)=5.16; P=0.053). No other stress-dependent main effects or interactions were discerned in prefrontal cortex.
Figure 3.
Adult stress effects on GR and MR expression in ventrolateral prefrontal cortex. Cortical cell layer-specific densities of (a) GR and (b) MR are presented for six adult monkeys from the adult stress condition and six adult monkeys from the adult no-stress condition (mean ± SEM). Asterisk signifies a significant adult stress-dependent main effect (P<0.05).
Figure 4.
Postnatal stress effects on GR and MR expression in adult dorsolateral prefrontal cortex. Cortical cell layer-specific densities of (a) GR and (b) MR are presented for 7 adult monkeys previously exposed to postnatal stress and 5 adult monkeys from the postnatal no-stress condition (mean ± SEM). Asterisks signify significant postnatal stress-dependent main effects (P<0.05).
Hippocampal MR and GR expression
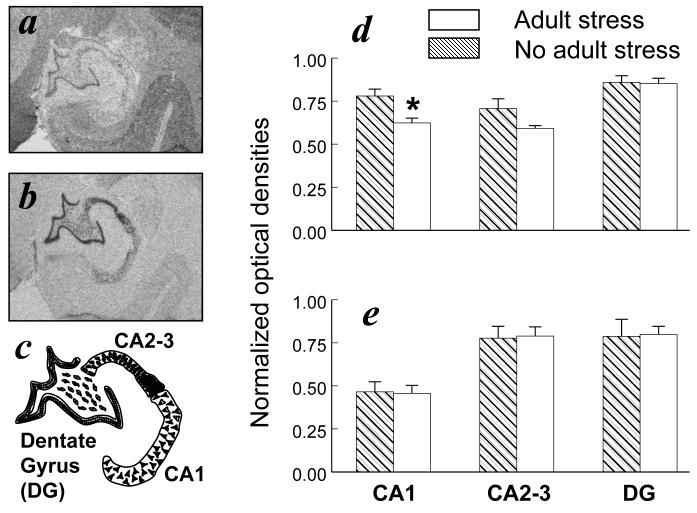
A significant adult social stress-by-receptor-by-region interaction was discerned for the hippocampus (F(2,16)=5.38, P=0.016). GR expression in hippocampal CA1 was significantly diminished (-20%) in monkeys exposed to adult social stress compared to the no-stress condition (F(1,8)=8.20, P=0.021). A similar reduction in GR expression was evident in CA2-3 (Figure 5), but the adult stress effect in this region was not statistically significant (F(1,8)=3.27; P=0.108). No other stress-dependent main effects or interactions were discerned, but regional differences in the relative balance between MR and GR distributions were evident in the hippocampus (F(2,8)=125.68, P<0.001). MR expression was prevalent in dentate gyrus and CA2-3 with lower levels in CA1, whereas GR expression was similar in all hippocampal regions (Figure 5).
Figure 5.
Adult stress effects on GR and MR expression in hippocampus. Representative images of (a) GR expression, (b) MR expression, and (c) the boundaries used to delineate each hippocampal region. Region-specific densities of (d) GR and (e) MR are presented for six adult monkeys from the adult stress condition and six adult monkeys from the adult no-stress condition (mean ± SEM). Asterisk signifies a significant adult stress-dependent main effect (P<0.05).
DISCUSSION
This study indicates that profile plots of MR differ from GR expression in prefrontal cortex. The relative distributions of MR and GR expression also differ in certain regions of the hippocampus. Exposure to adult social stress down regulates GR in hippocampal CA1, and down regulates MR expression in cell layer III of ventrolateral prefrontal cortex. In contrast, exposure to early life stress down regulates GR but not MR expression in cell layers I and II of dorsolateral prefrontal cortex. Similar reductions likewise occur in ventrolateral prefrontal cortex, but the effects of early life stress on GR expression in this region were marginally not significant. These results provide new information on regional differences and stress effects on MR and GR expression in corticolimbic brain circuits.
Previous studies of corticosteroid receptor expression in brain tissues have largely relied on rodents in which the neocortex is not as highly developed as that in humans and other primates. Consequently, prefrontal cortical cell layer-specific differences in the relative distributions of MR and GR expression have not received much attention. In a broad survey of adult brain tissues opportunistically collected from four squirrel monkeys, we first described distinct laminar patterns of MR and GR expression in prefrontal cortex (Patel et al., 2000). Here we provide profile plots of MR and GR expression in prefrontal cortical tissues collected from 12 different adult squirrel monkeys (Figure 2). These plots confirm that GR is expressed throughout all layers of prefrontal cortex, whereas MR expression is prevalent in layers I-III. Pyramidal cells in these superficial layers project to cortical locations (Hutsler et al., 2005). In deeper regions of prefrontal cortex, MR but not GR expression declines to very low levels in cell layers V and VI that project to subcortical targets. Similar profiles have been reported in recent studies of MR (Xing et al., 2004) and GR (Webster et al., 2002) expression in human prefrontal cortex, but MR and GR profiles differ in prefrontal cortex of rats. Rat GR is expressed in all prefrontal cortical layers with a slight preponderance in layers II and V (Bizon et al., 2001). On the other hand, MR is not highly expressed in the prefrontal cortex of rats (Ahima et al., 1991).
Regional distributions of hippocampal MR and GR also differ in squirrel monkeys relative to rats. Both MR and GR are clearly expressed in rat and squirrel monkey dentate gyrus, but robust species differences emerge in certain CA regions. MR in rats is consistently expressed throughout all hippocampal CA regions (Herman et al., 1989; Han et al., 2005), whereas squirrel monkey MR is prevalent in CA2 and CA3 with lower levels in CA1 (Figure 5b, e). Conversely, squirrel monkey GR is expressed at similar levels in all CA regions (Figure 5a, d), whereas GR in rats is expressed in CA1 with very low levels of expression in CA3 (Herman et al., 1989; Han et al., 2005). The relative distributions of hippocampal MR and GR expression in squirrel monkeys differ from rats but are similar to humans, baboons, marmoset monkeys, and two different macaques (Seckl et al., 1991; Brooke et al., 1994b; Sanchez et al., 2000; Neal et al., 2003; Pryce et al., 2005).
Robust differences between primates and rodents in MR and GR expression warrant attention because these differences are larger than those induced in rodents by exposure to stressors that are known to alter cognitive and neuroendocrine functions. When neonatal rats are repeatedly separated from their mother and later studied as adults, they exhibit an increased neuroendocrine stress response, resistance to dexamethasone suppression, and diminished GR expression in frontal cortex compared to controls (Ladd et al., 2004). In similar fashion, neonatal rats raised by mothers that display low levels of licking and grooming develop a subsequent stress reactive phenotype with decreased hippocampal GR expression and methylation of a cytosine residue in the GR promoter (Meaney and Szyf, 2005; Kaffman and Meaney, 2007). Early life stressors that affect maternal availability in squirrel monkeys (Lyons et al., 2002) do not alter GR or MR expression in the hippocampus, but down regulate GR expression in cell layers I and II of dorsolateral prefrontal cortex (Figure 4). Similar reductions in GR occur in cell layer I of ventrolateral prefrontal cortex, but these effects of early life stress are marginally not significant. That early life stress permanently down regulates GR expression in layer I is of interest because this layer has a very high concentration of dendritic terminals and plays an important role in neocortical development (Marin-Padilla, 1998).
In addition to enduring postnatal effects, we found that exposure to adult social stress down regulates MR but not GR expression in cell layer III of ventrolateral prefrontal cortex (Figure 3). This prefrontal region in primates is comprised of granular cortex that is functionally (Dias et al., 1996; Birrell and Brown, 2000) and cytoarchitecturally (Uylings et al., 2003) similar to dorsal medial prefrontal cortex in rats. During cortical development, cell layers II and III mature later and establish a broad network of corticocortical projections (Hutsler et al., 2005). Because of these intra- and inter-hemispheric connections with various cortical locations, cell layer III is thought to be involved in complex neurocognitive functions. In ventromedial prefrontal cortex of monkeys, for example, cell layer III projects to temporal lobe regions involved in aspects of learning and memory (Petrides and Pandya, 2002).
Consistent with numerous studies of rats (Kitraki et al., 1999; Paskitti et al., 2000; Karandrea et al., 2002), adult social stress also down regulates squirrel monkey GR expression in hippocampal CA1 (Figure 5). Chronic exposure to adult social stress decreases hippocampal GR but not MR ligand binding, and increases dexamethasone resistance in cynomolgus macaques (Brooke et al., 1994a). Resistance to dexamethasone suppression is thought to reflect stress sensitization as down regulation of hippocampal GR impairs glucocorticoid feedback inhibition and facilitates increased activation of the hypothalamic-pituitary-adrenal axis (HPA) by subsequent stress exposure (Sapolsky and Plotsky, 1990; Marti et al., 1994; Bhatnagar and Dallman, 1998). As described elsewhere in greater detail (Karssen et al., 2007), exposure to adult social stress in our study appeared to sensitize the HPA axis as squirrel monkey cortisol levels were greater after the fifth compared to the first adult social separation. After the initial increases in cortisol during each social separation, cortisol levels returned to baseline and then increased again for a short time thereafter during new pair formations. In a follow-up study of the same monkeys we did not, however, find evidence for impaired glucocorticoid feedback inhibition in response to exogenous cortisol (Lyons et al., 2007). Social housing after each separation may have prevented the expected deficit in glucocorticoid feedback, but social housing was not sufficient to restore normal levels of hippocampal GR expression.
Although early experiences can permanently alter GR expression in the forebrain of rats (Ladd et al., 2004; Meaney and Szyf, 2005; Kaffman and Meaney, 2007), little is known about the duration of adult experience-dependent changes in MR or GR expression. Down regulation of GR expression in hippocampus (Kitraki et al., 1999; Paskitti et al., 2000; Isgor et al., 2004) and prefrontal cortex (Mizoguchi et al., 2003) persists for a long time after termination of adult stress in rats. In squirrel monkeys, MR and GR expression in certain regions of hippocampus and prefrontal cortex are diminished 12 weeks after the final separation when all adult monkeys are socially housed in pairs. Further research is needed to determine whether adult social stress-induced changes in corticosteroid receptor expression derive from an epigenetic mechanism like that found in neonatal rats (Meaney and Szyf, 2005; Kaffman and Meaney, 2007).
These results should be interpreted in the context of potential limitations. Our findings for male monkeys may or may not hold true for females. Small samples diminished the power to detect potentially interesting interactions between postnatal and adult stress effects. Small sample sizes may also have contributed to our observation that in much of squirrel monkey prefrontal cortex, MR and GR expression are relatively resistant to change induced by social stress. Our study was not powered to detect small differences, but it is noteworthy that rodent studies support the observation that MR and GR expression regulation in hippocampus are regionally specific (Herman et al., 1989) and rigorously defended against change (Herman et al., 1999). We did not examine MR or GR protein in this squirrel monkey study, but MR and GR mRNA levels correspond with protein levels in rats, marmosets, and rhesus monkeys (Sanchez et al., 2000; Pryce et al., 2005). Co-localization of MR and GR mRNA within the same cells has not been established for prefrontal cortex, but our data suggest that co-localization more likely occurs in cell layers I-III, which primarily project to cortical locations, compared to layers V and VI which project to subcortical targets.
In summary, this study provides new information on regional differences and stress effects on MR and GR distributions in primate prefrontal cortex. The relative balance between MR and GR distributions is important for understanding how stress levels of cortisol affect corticolimbic regions involved in cognitive and neuroendocrine functions. The presence of two corticosteroid receptors with different affinities for cortisol affords tremendous flexibility, but also creates opportunities for MR/GR imbalance and dysregulation (de Kloet et al., 2005). Cortical layer-specific differences in MR and GR distributions should be considered in studies of stress and prefrontal cortex-dependent functions.
Acknowledgements
We gratefully acknowledge P. Buckmaster for help with tissue collection, M. Dar for help with image analysis, and C. Buckmaster and C. Pontrello for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ahima R, Krozowski Z, Harlan R. Type I corticosteroid receptor-like immunoreactivity in the rat CNS: distribution and regulation by corticosteroids. J. Comp. Neurol. 1991;313:522–538. doi: 10.1002/cne.903130312. [DOI] [PubMed] [Google Scholar]
- Avishai-Eliner S, Hatalski CG, Tabachnik E, Eghbal-Ahmadi M, Baram TZ. Differential regulation of glucocorticoid receptor messenger RNA (GR-mRNA) by maternal deprivation in immature rat hypothalamus and limbic regions. Brain Res. Dev. Brain Res. 1999;114:265–268. doi: 10.1016/s0165-3806(99)00031-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar S, Dallman M. Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neurosci. 1998;84:1025–1039. doi: 10.1016/s0306-4522(97)00577-0. [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J. Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizon JL, Helm KA, Han JS, Chun HJ, Pucilowska J, Lund PK, Gallagher M. Hypothalamic-pituitary-adrenal axis function and corticosterone receptor expression in behaviourally characterized young and aged Long-Evans rats. Eur. J. Neurosci. 2001;14:1739–1751. doi: 10.1046/j.0953-816x.2001.01781.x. [DOI] [PubMed] [Google Scholar]
- Brooke SM, de Haas-Johnson AM, Kaplan JR, Manuck SB, Sapolsky RM. Dexamethasone resistance among nonhuman primates associated with a selective decrease of glucocorticoid receptors in the hippocampus and a history of social instability. Neuroendocrinol. 1994a;60:134–140. doi: 10.1159/000126743. [DOI] [PubMed] [Google Scholar]
- Brooke SM, de Haas-Johnson AM, Kaplan JR, Sapolsky RM. Characterization of mineralocorticoid and glucocorticoid receptors in primate brain. Brain Res. 1994b;637:303–307. doi: 10.1016/0006-8993(94)91249-1. [DOI] [PubMed] [Google Scholar]
- Coe CL, Franklin D, Smith ER, Levine S. Hormonal responses accompanying fear and agitation in the squirrel monkey. Physiol. Behav. 1982;29:1051–1057. doi: 10.1016/0031-9384(82)90297-9. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat. Rev. Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996;380:69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- Gass P, Kretz O, Wolfer DP, Berger S, Tronche F, Reichardt HM, Kellendonk C, Lipp HP, Schmid W, Schutz G. Genetic disruption of mineralocorticoid receptor leads to impaired neurogenesis and granule cell degeneration in the hippocampus of adult mice. EMBO Rep. 2000;1:447–451. doi: 10.1093/embo-reports/kvd088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gergen JA, MacLean PD. A Stereotaxic Atlas of the Squirrel Monkey’s Brain (Saimiri sciureus) Public Health Service; Bethesda: 1962. [Google Scholar]
- Han F, Ozawa H, Matsuda K, Nishi M, Kawata M. Colocalization of mineralocorticoid receptor and glucocorticoid receptor in the hippocampus and hypothalamus. Neurosci. Res. 2005;51:371–381. doi: 10.1016/j.neures.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Herman JP, Patel PD, Akil H, Watson SJ. Localization and regulation of glucocorticoid and mineralocorticoid receptor messenger RNAs in the hippocampal formation of the rat. Mol. Endocrinol. 1989;3:1886–1894. doi: 10.1210/mend-3-11-1886. [DOI] [PubMed] [Google Scholar]
- Herman JP, Watson SJ, Spencer RL. Defense of adrenocorticosteroid receptor expression in rat hippocampus: effects of stress and strain. Endocrinology. 1999;140:3981–3991. doi: 10.1210/endo.140.9.6962. [DOI] [PubMed] [Google Scholar]
- Holmes MC, French KL, Seckl JR. Dysregulation of diurnal rhythms of serotonin 5-HT2C and corticosteroid receptor gene expression in the hippocampus with food restriction and glucocorticoids. J. Neurosci. 1997;17:4056–4065. doi: 10.1523/JNEUROSCI.17-11-04056.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharm. 2000;23:477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- Hutsler JJ, Lee DG, Porter KK. Comparative analysis of cortical layering and supragranular layer enlargement in rodent carnivore and primate species. Brain Res. 2005;1052:71–81. doi: 10.1016/j.brainres.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Isgor C, Kabbaj M, Akil H, Watson SJ. Delayed effects of chronic variable stress during peripubertal-juvenile period on hippocampal morphology and on cognitive and stress axis functions in rats. Hippocampus. 2004;14:636–648. doi: 10.1002/hipo.10207. [DOI] [PubMed] [Google Scholar]
- Kaffman A, Meaney MJ. Neurodevelopmental sequelae of postnatal maternal care in rodents: clinical and research implications of molecular insights. J. Child Psychol. Psychiatry. 2007;48:224–244. doi: 10.1111/j.1469-7610.2007.01730.x. [DOI] [PubMed] [Google Scholar]
- Karandrea D, Kittas C, Kitraki E. Forced swimming differentially affects male and female brain corticosteroid receptors. Neuroendocrinol. 2002;75:217–226. doi: 10.1159/000054713. [DOI] [PubMed] [Google Scholar]
- Karssen AM, Her S, Li JZ, Patel PD, Meng F, Bunney WE, Jr., Jones EG, Watson SJ, Akil H, Myers RM, Schatzberg AF, Lyons DM. Stress-induced changes in primate prefrontal profiles of gene expression. Mol. Psychiatry. 2007 doi: 10.1038/sj.mp.4002095. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat. Rev. Neurosci. 2002;3:453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- Kitraki E, Karandrea D, Kittas C. Long-lasting effects of stress on glucocorticoid receptor gene expression in the rat brain. Neuroendocrinol. 1999;69:331–338. doi: 10.1159/000054435. [DOI] [PubMed] [Google Scholar]
- Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Plotsky PM. Long-term adaptations in glucocorticoid receptor and mineralocorticoid receptor mRNA and negative feedback on the hypothalamo-pituitary-adrenal axis following neonatal maternal separation. Biol. Psychiatry. 2004;55:367–375. doi: 10.1016/j.biopsych.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Landfield PW, Blalock EM, Chen KC, Porter NM. A new glucocorticoid hypothesis of brain aging: implications for Alzheimer’s disease. Curr. Alzheimer Res. 2007;4:205–212. doi: 10.2174/156720507780362083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez JF, Chalmers DT, Little KY, Watson SJ. A.E. Bennett Research Award. Regulation of serotonin1A, glucocorticoid, and mineralocorticoid receptor in rat and human hippocampus: implications for the neurobiology of depression. Biol. Psychiatry. 1998;43:547–573. doi: 10.1016/s0006-3223(97)00484-8. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS. The acute effects of corticosteroids on cognition: integration of animal and human model studies. Brain Res. Brain Res. Rev. 1997;24:1–27. doi: 10.1016/s0165-0173(97)00004-0. [DOI] [PubMed] [Google Scholar]
- Lyons DM, Afarian H, Schatzberg AF, Sawyer-Glover A, Moseley ME. Experience-dependent asymmetric variation in primate prefrontal morphology. Behav. Brain Res. 2002;136:51–59. doi: 10.1016/s0166-4328(02)00100-6. [DOI] [PubMed] [Google Scholar]
- Lyons DM, Parker KJ, Zeitzer JM, Buckmaster CL, Schatzberg AF. Preliminary Evidence that Hippocampal Volumes in Monkeys Predict Stress Levels of Adrenocorticotropic Hormone. Biol. Psychiatry. 2007 doi: 10.1016/j.biopsych.2007.03.012. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons DM, Wang OJ, Lindley SE, Levine S, Kalin NH, Schatzberg AF. Separation induced changes in squirrel monkey hypothalamic-pituitary-adrenal physiology resemble aspects of hypercortisolism in humans. Psychoneuroendocrinol. 1999;24:131–142. doi: 10.1016/s0306-4530(98)00065-1. [DOI] [PubMed] [Google Scholar]
- Marin-Padilla M. Cajal-Retzius cells and the development of the neocortex. Trends. Neurosci. 1998;21:64–71. doi: 10.1016/s0166-2236(97)01164-8. [DOI] [PubMed] [Google Scholar]
- Marti O, Gavalda A, Gomez F, Armario A. Direct evidence for chronic stress-induced facilitation of the adrenocorticotropin response to a novel acute stressor. Neuroendocrinol. 1994;60:1–7. doi: 10.1159/000126713. [DOI] [PubMed] [Google Scholar]
- McCullers DL, Herman JP. Mineralocorticoid receptors regulate bcl-2 and p53 mRNA expression in hippocampus. Neuroreport. 1998;9:3085–3089. doi: 10.1097/00001756-199809140-00031. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Szyf M. Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome. Dialogues Clin. Neurosci. 2005;7:103–123. doi: 10.31887/DCNS.2005.7.2/mmeaney. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi K, Ishige A, Aburada M, Tabira T. Chronic stress attenuates glucocorticoid negative feedback: involvement of the prefrontal cortex and hippocampus. Neurosci. 2003;119:887–897. doi: 10.1016/s0306-4522(03)00105-2. [DOI] [PubMed] [Google Scholar]
- Morsink MC, Steenbergen PJ, Vos JB, Karst H, Joels M, De Kloet ER, Datson NA. Acute activation of hippocampal glucocorticoid receptors results in different waves of gene expression throughout time. J. Neuroendocrinol. 2006;18:239–252. doi: 10.1111/j.1365-2826.2006.01413.x. [DOI] [PubMed] [Google Scholar]
- Neal CR, Patel PD, Akil H, Watson SJ. Mineralocorticoid and glucocorticoid receptor mRNA in the hippocampal formation of the developing baboon (Papio) Soc. for Neurosci. 2003 Abstr. 506.1. [Google Scholar]
- Paskitti ME, McCreary BJ, Herman JP. Stress regulation of adrenocorticosteroid receptor gene transcription and mRNA expression in rat hippocampus: time-course analysis. Brain Res. Mol. Brain Res. 2000;80:142–152. doi: 10.1016/s0169-328x(00)00121-2. [DOI] [PubMed] [Google Scholar]
- Patel PD, Lopez JF, Lyons DM, Burke S, Wallace M, Schatzberg AF. Glucocorticoid and mineralocorticoid receptor mRNA expression in squirrel monkey brain. J. Psychiatr. Res. 2000;34:383–392. doi: 10.1016/s0022-3956(00)00035-2. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Comparative cytoarchitectonic analysis of the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey. Eur. J. Neurosci. 2002;16:291–310. doi: 10.1046/j.1460-9568.2001.02090.x. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Feldon J, Fuchs E, Knuesel I, Oertle T, Sengstag C, Spengler M, Weber E, Weston A, Jongen-Relo A. Postnatal ontogeny of hippocampal expression of the mineralocorticoid and glucocorticoid receptors in the common marmoset monkey. Eur. J. Neurosci. 2005;21:1521–1535. doi: 10.1111/j.1460-9568.2005.04003.x. [DOI] [PubMed] [Google Scholar]
- Rozeboom AM, Akil H, Seasholtz AF. Mineralocorticoid receptor overexpression in forebrain decreases anxiety-like behavior and alters the stress response in mice. Proc. Natl. Acad. Sci. U. S. A. 2007;104:4688–4693. doi: 10.1073/pnas.0606067104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez MM, Young LJ, Plotsky PM, Insel TR. Distribution of corticosteroid receptors in the rhesus brain: relative absence of glucocorticoid receptors in the hippocampal formation. J. Neurosci. 2000;20:4657–4668. doi: 10.1523/JNEUROSCI.20-12-04657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Plotsky PM. Hypercortisolism and its possible neural bases. Biol. Psychiatry. 1990;27:937–952. doi: 10.1016/0006-3223(90)90032-w. [DOI] [PubMed] [Google Scholar]
- Seckl JR, Dickson KL, Yates C, Fink G. Distribution of glucocorticoid and mineralocorticoid receptor messenger RNA expression in human postmortem hippocampus. Brain Res. 1991;561:332–337. doi: 10.1016/0006-8993(91)91612-5. [DOI] [PubMed] [Google Scholar]
- Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin. Neurosci. 2006;8:383–395. doi: 10.31887/DCNS.2006.8.4/ssmith. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa N, Almeida OF. Corticosteroids: sculptors of the hippocampal formation. Rev. Neurosci. 2002;13:59–84. doi: 10.1515/revneuro.2002.13.1.59. [DOI] [PubMed] [Google Scholar]
- Uylings HB, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behav. Brain Res. 2003;146:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Watzka M, Bidlingmaier F, Beyenburg S, Henke RT, Clusmann H, Elger CE, Schramm J, Klingmuller D, Stoffel-Wagner B. Corticosteroid receptor mRNA expression in the brains of patients with epilepsy. Steroids. 2000;65:895–901. doi: 10.1016/s0039-128x(00)00205-1. [DOI] [PubMed] [Google Scholar]
- Webster MJ, Knable MB, O’Grady J, Orthmann J, Weickert CS. Regional specificity of brain glucocorticoid receptor mRNA alterations in subjects with schizophrenia and mood disorders. Mol. Psychiatry. 2002;7:985–994. doi: 10.1038/sj.mp.4001139. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Gould E, Sakai RR, Spencer RL, McEwen BS. Effects of aldosterone or RU28362 treatment on adrenalectomy-induced cell death in the dentate gyrus of the adult rat. Brain Res. 1991;554:312–315. doi: 10.1016/0006-8993(91)90207-c. [DOI] [PubMed] [Google Scholar]
- Xing GQ, Russell S, Webster MJ, Post RM. Decreased expression of mineralocorticoid receptor mRNA in the prefrontal cortex in schizophrenia and bipolar disorder. Int. J. Neuropsychopharmacol. 2004;7:143–153. doi: 10.1017/S1461145703004000. [DOI] [PubMed] [Google Scholar]