Abstract
TRAIL is a death ligand that induces apoptosis in malignant but not normal cells. Recently the ability of TRAIL to induce proliferation in apoptosis-resistant normal and malignant cells was reported. In this study, we analyzed TRAIL effects in apoptosis sensitive MCF7, OVCAR3 and H460 human tumor cell lines. TRAIL at low concentrations preferentially induced cell proliferation. At 100 ng/ml apoptotic death was readily observed, however surviving cells acquired higher proliferative capacity. TRAIL stimulated production of several cytokines, IL-8, RANTES, MCP-1 and bFGF, and activation of caspases 1 and 8 was essential for this effect. Antibodies to IL-8, RANTES, and bFGF blocked TRAIL-induced cell proliferation and further stimulated apoptosis. For the first time, we report that high TRAIL concentrations induced cell senescence as determined by altered morphology and expression of several senescence markers: SA-β-gal, p21Waf1/Cip1, p16INK4a, and HMGA. Caspase 9 inhibition protected TRAIL-treated cells from senescence, whereas inhibition of caspases 1 and 8 increased yield of SLP cells. In conclusion, in cultured human carcinoma cells, TRAIL therapy results in three functional outcomes, apoptosis, proliferation and senescence. TRAIL induced proapoptotic and prosurvival responses correlate with strength of signaling. TRAIL-induced cytokine production is responsible for its proliferative and prosurvival effects.
Keywords: TRAIL, apoptosis, senescence, cell proliferation, cytokines, caspases
INTRODUCTION
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL/Apo2L) triggers apoptosis via DR4 and DR5 death receptors, forming a death-inducing signaling complex (DISC). This leads to the recruitment and activation of upstream caspase 8 and subsequent activation of effector downstream caspases including caspases 3, 6, and 7 in type I cells [1–3]. In type II cells, apoptosis is mediated by activation of caspase-8 that triggers mitochondria-dependent apoptotic amplification loop by activating Bid, which induces mitochondrial accumulation of Bax, cytochrome c release from mitochondria, and activation of caspases 9, 3, and 7 [1–3].
However, some tumors are resistant to TRAIL-induced apoptosis. TRAIL resistance has been associated with several factors, including downregulation or loss of death receptors or caspase-8 [4], and overexpression of decoy receptors [5] or intracellular protein c-FLIP [6–8].
There is an increasing evidence that TRAIL could also induce various apoptosis–independent biological effects. In normal cells that are generally resistant to the apoptotic effects, TRAIL induces proliferation of vascular smooth muscle cells, synovial fibroblasts and T lymphocytes [9–11]. Similarly, TRAIL treatment stimulated proliferation of apoptosis-resistant malignant cells, such as leukemia, neuroblastoma, renal cell carcinoma, and colon carcinoma cell lines [8, 12, 13]. Moreover, TRAIL promoted metastasis of pancreatic tumors in vivo [14]. In addition, TRAIL can stimulate proinflammatory signaling, leading to increased production of IL-8, IL-1α, IL-1β, and MCP-1 cytokines in normal human endothelial cells and in several apoptosis resistant tumor cell lines [14–17]. However, the significance of TRAIL-induced cytokine production is still not fully understood. It is conceivable that these cytokines may elicit both autocrine and paracrine effects on tumor cells and stromal cells [14, 18, 19]. Several studies indicate that TRAIL-induced cytokine production could counterbalance TRAIL apoptotic effects [16, 20, 21].
TRAIL signaling via cognate receptors activates various signaling pathways, including MAPKs, PKB/Akt- and NF-κB-signaling cascades that could ultimately activate cyclin kinases and stimulate cell cycle [25–27].
Until now, non-apoptotic TRAIL effects were mostly investigated in TRAIL apoptosis-resistant cells. Therefore, it is unclear whether TRAIL can exert its apoptotic and non-apoptotic simultaneously, or the mode of its activity depends on cell characteristics, e.g. histological origin, TRAIL sensitivity/resistance status. We hypothesize that TRAIL activates different signaling pathways and their interplay could simultaneously initiate various biological processes. Interactions and balance of these processes will determine cell fate. In this study, we set to test this hypothesis by simultaneous analysis of multiple biological effects of TRAIL in human carcinoma cells.
We further hypothesize that in addition to already discovered biological effects, TRAIL also could induce cell senescence. Senescence is a cell-cycle arrest that limits cell proliferative capacity and is regulated by p53 (via p21Waf1/Cip1) and/or pRb (p16 INK4a) pathways [28–31]. It is considered that senescence is one of the mechanisms that protect cells from immediate death (reviewed in [32]). Growing experimental evidence indicates that endogenous or exogenous apoptotic stress caused by chemotherapeutic drugs, radiation, environmental insults, or endogenous processes, can induce senescence of tumor cells[29]. TRAIL is well known apoptotic factor; however, effects of TRAIL on cellular senescence have not yet been examined.
In this paper we investigated diverse TRAIL effects in three histologically distinct apoptosis sensitive human tumor cell lines: non-small cells lung cancer (NSCLC) H460, breast carcinoma MCF-7, and ovarian cancer OVCAR-3. To ascertain whether cellular effects of TRAIL depend on the strength of induced signal, we compare cellular responses to low and high concentrations of TRAIL.
We demonstrate that in these cells TRAIL-treatment induce multifactorial signaling network that leads to different functional outcomes: apoptosis, senescence, cells proliferation and elevated production of various cytokines and chemokines. TRAIL-stimulated cytokine production is responsible for the proliferative effects of TRAIL and plays an important role in tumor cell survival. We demonstrate that caspase activation is required for all TRAIL-mediated effects: apoptosis, senescence, production of cytokines and cells proliferation.
MATERIALS AND METHODS
Cell lines
Human ovarian carcinoma OVCAR-3, breast cancer MCF-7 and H460 non-small cell lung cancer (NSCLC) cell lines H460 were obtained from American Type Culture Collection (ATCC, Rockville, MD, USA). Cells were grown in culture media, recommended by ATCC, with 10 % FBS (Millipore Inc., Billerica, MA).
Reagents
TRAIL was purchased from Genentech Inc. (Boston, MA). Neutralizing antibody (Ab) against IL-8 was obtained from Abcam Inc. (Cambridge, MA), anti- IL-10 and RANTES Abs were from BioVision (Montain View, CA); anti-MCP-1, VEGF, b-FGF, and IL-6 Abs were purchased from Calbiochem (EMD Biosciences, Inc, San Diego, CA). The pan-caspase inhibitor, z-VAD-FMK, as well as inhibitors of caspase-1 (z-YVAD-FMK), caspase-3 (z-DEVD-FMK), caspase-6 (z-VEID-FMK) and caspase-8 (z-IETD-FMK), were from Chemicon, Millipore Inc. (Temecula, CA). Fluorescent Poly-Caspases FLICA Apoptosis Detection Kit (FAM-VAD-FMK) was purchased from Immunochemistry Technologies (Bloomington, MN). Anti-p16INK4a Ab was purchased from ZYMED Laboratories (San Francisco, CA), anti-p21WAF1/Cip1 Ab was from Abcam, Inc. (Cambridge, MA), anti-HMGA Ab was from Novus Biologicals (Littleton, Co); anti-β-actin Ab and Hoechst 33342 were from Sigma-Aldrich. Secondary Abs conjugated with Alexa Fluor-488, 546 and 680, were obtained from Molecular Probes (Invitrogen, Carlsbad, CA). FITC-conjugated Annexin V was from Beckman Coulter Inc. (Fullerton, CA).
Cells staining procedure
Cells grown in 96-well plates were fixed in 2% PFA for 20 min, washed in PBS, incubated with 0.1% Triton X-100 for 10 min, washed with PBS containing 1% of BSA (FACS buffer) and incubated with primary Abs against IL-8, RANTES, p16INK4a, β-actin or p21WAF1/Cip1 for 1 h and with secondary Abs conjugated with Alexa 488, or 680 fluorochromes (Molecular Probes/Invitrogen) for 1 h. Next, cells were stained with Hoechst 33342.
To identify individual cells and to optimize focusing cell nuclei were stained with Hoechst 33342, 2 μg/ml, 20 min. All incubation and fixation procedures were performed at room temperature. Cell images were acquired using the Cellomics ArrayScan HCS Reader (20X objective) and analyzed using Target Activation BioApplication Software Module.
Cellomics ArrayScan Automated Imaging
The Cellomics ArrayScan HCS Reader (Cellomics/ThermoFisher, Pittsburgh, PA) was utilized to collect information on distribution of fluorescently labeled components in stained cells. The ArrayScan HCS system scans multiple fields in individual wells to acquire and analyze images of single cells according to defined algorithms. The scanner is equipped with emission and excitation filters (XF93, Omega Optical, Brattleboro, VT, USA) for selectively imaging fluorescent signals. Data were captured, extracted and analyzed with ArrayScan II Data Acquisition and Data Viewer version 3.0 (Cellomics), Quattro Pro version 10.0.0 (Corel, Ottawa, Ontario, Canada), and MS Excel 2002 (Microsoft, Redmond, WA).
Medium conditioned by tumor cells
H460, MCF7, and OVCAR-3 cells grown to 80% confluence in T-75 cell culture flasks were treated with or without 10 ng/ml of TRAIL for 24 h. TRAIL-containing medium was removed, and cells were washed and cultured in TRAIL-free medium for additional 24 h before collecting conditioned medium.
Proliferation assays
Cancer cell lines, OVCAR-3, MCF7 and H460, were plated onto 24-well plates at 2 × 104 cells per well and incubated with 0–1,000 ng/ml of TRAIL for various time intervals (0–72 h). Cell proliferation was assessed by 3H-thymidine incorporation as previously described [33]. In addition, cell proliferation was assessed using CellTiter 96® AQueous One Solution Cell Proliferation Assay (Promega Corp., Madison, WI). In some experiments, cells were counted using the Cellomics ArrayScan HCS Reader after staining with 2 μg/ml of DNA binding dye, Hoechst 33342, for 20 min.
Apoptosis assays
Apoptosis was analyzed by flow cytometry using FITC-conjugated Annexin V and propidium iodine (PI) as described [34]. In some experiments tumor cells were pretreated with different caspase inhibitors for 1–3 hrs prior to TRAIL treatment. To analyze caspase activation, TRAIL treated cells were stained using Fluorescent Poly-Caspases FLICA Apoptosis Detection Kit FAM-VAD-FMK (Immunochemistry Technologies) for 1 h at 37° C in the CO2 incubator. Cells were fixed and stained with 2 μg/ml of Hoechst 33342 for 20 min. Cell images were acquired using the Cellomics ArrayScan HCS Reader (20 X objective) and analyzed using Target Activation BioApplication Software Module.
Senescence assays
Senescence-associated markers SA-β-gal, cyclin-dependent kinase inhibitors, p16INK4a and p21WAF1/Cip1, and HMGA in TRAIL-treated cells were evaluated. SA-β-gal+ cells were determined by bright-field microscopy. Morphological appearance of senescent cells, p16INK4a, p21WAF1/Cip1, and HMGA expression were determined using imaging cytometry as described above.
Multiplex analysis of TRAIL-induced transcription factors and cytokine production by tumor cells
Cytokines and growth factors in medium conditioned by tumor cells were analyzed using multiplexing bead-based sandwich immunoassay technology (xMAP™, Luminex Corp., Austin, TX) using a 38-plex kit from Invitrogen/Biosource (Camarillo, CA) designed for the detection of IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12p40, IL-13, IL-15, IL-17, GM-CSF, IFN-α, IFN-β, TNFα, MCP-1, MCP-2, MCP-3, IP-10, MIP-1α, MIP-1β, RANTES, VEGF, bFGF, EGF, G-CSF, EOTAXIN, HGF, MIG, GROα, TNFRI, TNFRII, DR5, IL-1Rα, sIL-2R, and sIL-6R. Analyses of tumor supernatants were performed in 96-well microplate format according to the protocol by Invitrogen as previously described [35].
A multiplex transcription factor kit was used for measuring activities of NF-kB, YY1, CREB, EGR, SRE, AP-2, NF1, HIF, NFAT, and PPAR according to the protocol by Invitrogen. Nuclear extracts were prepared using the Invitrogen’s Nuclear Extraction Kit according to the manufacturer’s protocol and analyzed using a Bio-Plex plate reader.
Bio-Plex phosphoprotein assay was used for testing phosphoprotein, Akt, ATF-2, ERK1/2, GSK-3a/B, IBM-a, JNK, p38 MAPK, and STAT3, according to the protocol by Bio-Red Laboratories, Inc. (Hercules, CA). Cells were stimulated with TRAIL for 0, 5, 15, 30, 60 min; cell lysates were prepared using Bio-Plex Cell Lyses Kit, and analyzed using 8-Plex Phosphoprotein kit.
Statistical analysis
To normalize results of testing the effects of TRAIL in three different tumor cell lines, all data are presented as a percentage of the corresponding control. All experiments were repeated at least three times and mean ± SE were determined. Comparisons between the values were performed using a two-tailed Student’s t-test. For the comparison of multiple groups, a one- or two-way ANOVA test was applied. For all statistical analyses, the level of significance was set at a probability of P < 0.05.
RESULTS
Effects of TRAIL on apoptosis and proliferation in human carcinoma cell lines
Expression of functional TRAIL death receptors, DR4 and DR5, and decoy receptors DcR1 and DcR2, on the cell surface of three human carcinoma cell lines, H460, OVCAR-3, and MCF7, was evaluated by flow cytometry. All receptors were expressed in all three cell lines in agreement with published observations [33, 36, 37] (data not shown). The apoptotic and proliferative effects of TRAIL were measured in the three human carcinoma cell lines after incubation with 0–100 ng/ml TRAIL for 24 h. TRAIL-induced apoptosis was concentration-dependent (Figure 1A). At a low concentration of 1 ng/ml, TRAIL induced a significantly (p < 0.05) increased cell proliferation as assessed by 3H-thymidine incorporation in H460 and MCF7 cell lines. In contrast, at higher concentrations of TRAIL (10 and 100 ng/ml), 3H-thymidine incorporation was substantially reduced probably due to high proportion of apoptotic cells (Figure 1B). To assess the ability of TRAIL to stimulate cell proliferation and to avoid apoptotic effects of chronic exposure, OVCAR-3, MCF7, and H460 cells were incubated with 1, 10, and 100 ng/mg TRAIL for 4 h, after which medium was replaced with fresh TRAIL-free medium. Short-term TRAIL treatment followed with 120 h incubation in TRAIL-free medium significantly stimulated proliferation in all three cell lines (Figure 1C for OVCAR-3 cells). In cells treated with high concentrations of TRAIL (100 ng/ml) in the first 24 h 3H-thymidine incorporation was reduced probably due to apoptosis (Figure 1C). At later time points, 3H-thymidine incorporation in surviving cells increased, and after 120 h it was significantly above the control group and similar to that found in cells treated with 1–10 ng/ml of TRAIL (Figure 1C). Similar results were observed in MCF7 and H460 cell lines (data not shown).
Figure 1. Effect of TRAIL on apoptosis and proliferation in human tumor cell lines.
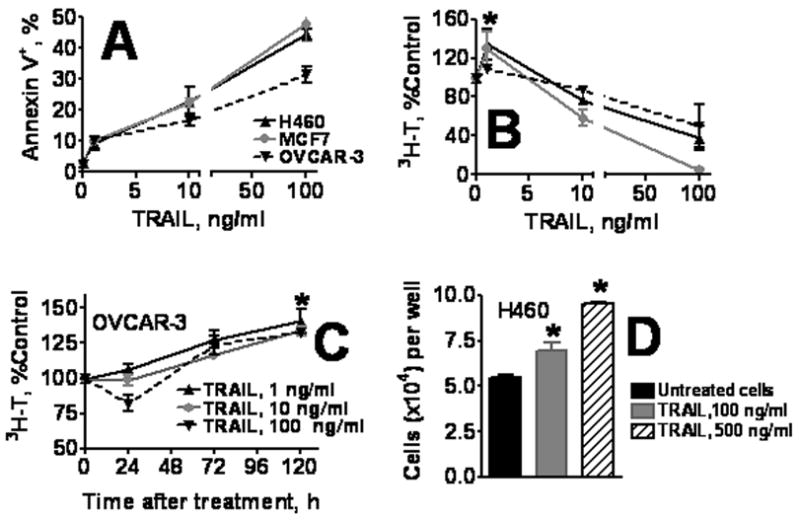
A. TRAIL induces apoptosis in H460, MCF, and OVCAR-3 cells. Cells were incubated with 0–100 ng/ml of TRAIL for 24 h and apoptosis was assessed by Annexin V binding as described in Methods. B–D. Effect of TRAIL on tumor cell proliferation. B, H460, MCF7, and OVCAR-3 cells were treated with TRAIL as in (A) and 72 h later cell proliferation was evaluated by 3H-thymidine incorporation. C. Tumor cell proliferation after short (4 h) TRAIL treatment. OVCAR-3 tumor cells were incubated for 4 h with 0–100 ng/ml TRAIL, washed, and cultured in TRAIL-free medium for 0–120 h. Proliferation was assessed by 3H-thymidine incorporation. D. Proliferation of tumor cells that survived TRAIL treatment. H460 cells were treated for 4 h with 100 or 500 ng/ml TRAIL, washed, and cultured in TRAIL-free medium for 30 h. Viable cells were harvested, counted, and plated onto 96-well plate (2 × 104 viable cells per well). Cells were cultured for 72 h, fixed, stained with Hoechst 33342, and counted using Cellomics ArrayScan HCS Reader. Data are presented as the mean ± SD of one of three independent experiments. In this and following figures, * denotes the statistical significance of results as compared to untreated control; * corresponds to significance on range P < 0.05-0.001
To further assess the ability of TRAIL to stimulate cell proliferation, H460 cells were treated with high concentrations of TRAIL (100 and 500 ng/ml) for 4 h, washed and cultured for 30 h in TRAIL-free medium. TRAIL, (500 ng/ml), killed the majority of cells. Surviving adherent cells were harvested and seeded onto new 96-well plate (at 2 × 104 cells/well). Cells were cultured for 72 h and counted using Cellomics ArrayScan HCS Reader. Cells that survived high concentration of TRAIL proliferated at a higher rate as compared to untreated cells (p < 0.01) (Figure 1D). These results were further confirmed using CellTiter 96® AQueous One Solution Cell Proliferation Assay (data not shown).
Prosurvival effects of medium conditioned by TRAIL-treated tumor cells
The stimulatory effects of TRAIL could be due to increased production of growth factors by treated cells. To test this hypothesis, OVCAR-3, MCF7 or H460 cells were seeded onto semi-permeable membranes in cell culture inserts, and were pre-treated with 10 ng/ml TRAIL for 24 h. Inserts containing TRAIL pre-treated cells were transferred into 24-well plates containing appropriate untreated cells and incubated for 72 h. In the presence of TRAIL pre-treated cells, a substantial (130–140%) stimulation of proliferation of TRAIL untreated OVCAR-3, MCF7 and H460 tumor cells was observed (Figure 2A).
Figure 2. Effects of medium conditioned by TRAIL-treated cells.
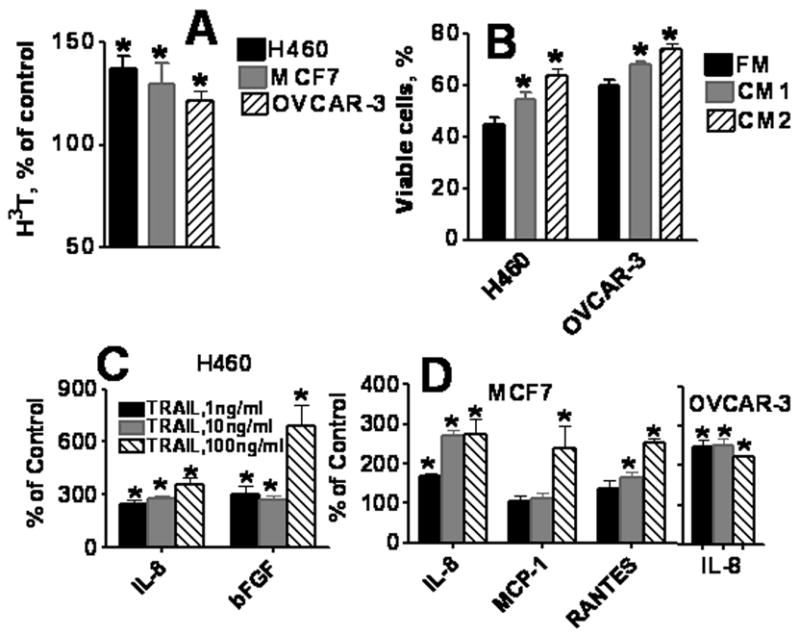
A. Co-culture of TRAIL-treated cells with naive cells. H460, MCF7, and OVCAR-3 cells grown in cell culture inserts were treated with 10 ng/ml TRAIL for 24 h. Washed inserts were placed in 24-well plates seeded with naive tumor cells. Proliferation of bottom cultured cells was evaluated 72 h later by 3H-thymidine incorporation. Data are expressed as percent of control (3H-thymidine incorporation in cells co-cultured with TRAIL untreated cells). B. Antiapoptotic effects of medium conditioned by TRAIL-treated cells. Medium was conditioned by untreated (CM1) and TRAIL-treated (CM2) H460 and OVCAR-3 cells for 24 h. OVCAR-3 and H460 cells were incubated with TRAIL (100 ng/ml) for 24 h in fresh medium (FM), conditioned media (CM1 or CM2). Next, the percentage of apoptotic cells was determined by Annexin V/PI staining and flow cytometry. C–D. Multiplex analysis of cytokines secreted by TRAIL-treated H460 (C), MCF7 and OVCAR-3(D) cells. H460, MCF7 and OVCAR-3 cells were treated with 1–100 ng/ml TRAIL for 24 hours. Conditioned media were collected and concentrations of cytokines and growth factors were measured using multiplex cytokine assay as described in Materials and Methods. Mean of three independent experiments was calculated and data are presented as % increase of cytokines production by TRAIL-treated over untreated control cells. Only factors with significant differences in their concentrations (at least p < 0.05) are included.
We next sought to determine whether conditioned medium collected from TRAIL pre-treated cells offered protection from apoptosis. Media conditioned by H460, MCF7, and OVCAR-3 cells, treated with TRAIL (10 ng/ml) or untreated, were added to naïve cells followed by addition of TRAIL (100 ng/ml). After 24 h, cells were stained with Annexin V/PI and analyzed by flow cytometry. Number of TRAIL-induced apoptotic cells was significantly (P < 0.05) lower when cells were cultured in medium conditioned by OVCAR-3 or H460 cells (Figure 2B).
Taken together, the above studies suggest that tumor cells produce soluble factor(s) that stimulate proliferative and prosurvival responses. TRAIL treatment elevates production of such soluble factors in tumor cells. Therefore, we next analysed concentrations of cytokines, chemokines and growth factors in medium conditioned by TRAIL-treated and untreated cancer cells.
Cytokines secretion by TRAIL-treated human carcinoma cells
Concentrations of 38 cytokines, chemokines and growth factors in medium conditioned by tumor cells were measured using multiplex bead-based sandwich immunoassay. OVCAR-3, H460 and MCF7 cells produced 12 different cytokines and growth factors (IL-6, IL-8, VEGF, IL-10, GROα, FGF-b, RANTES, G-CSF, MCP-1, MIP-1α, IP-10, TNFα) in a cell line-dependent manner (Table 1). Other cytokines and growth factors were produced at very low quantities or were undetectable. IL-6, IL-8 and VEGF were predominant factors produced by H460 and OVCAR-3 cells. Slow growing MCF7 breast tumor cells secreted the lowest levels of cytokines, chemokines and growth factors, except RANTES that was higher in MCF7 than in two other tested cell lines (Table 1).
Table 1.
Levels of Soluble Factors Produced by Human Tumor Cell Lines
| Tumor cells producing factors | H460 | OVCAR-3 | MCF7 | |
|---|---|---|---|---|
| Mean ± SD (pg/1 × 106 cells/ml) | ||||
| 1 | IL-6 | 11897.0 ± 538.6 | 2680.9 ± 236.6 | 10.1 ±1.1 |
| 2 | IL-8 | 9728.6 ± 738.3 | 2516.8± 241.4 | 35.8 ±1.2 |
| 3 | IL-10 | 9.4 ± 2.5 | 12.9 ± 4.1 | 1.9 ± 0.6 |
| 4 | GROα | 54.82.7 | 0.2± 0.1 | 0.2± 0.1 |
| 5 | VEGF | 1038.4 ± 60.9 | 5863.62 ± 160.4 | 678.5 ± 39.9 |
| 6 | bFGF | 86.7 ± 6.0 | 8.75 ± 2.3 | 2.82 ± 0.35 |
| 7 | RANTES | 13.5 ± 0.1 | 2.51 ± 0.8 | 53.5 ± 5.5 |
| 8 | G-CSF | 63.9 ±10.9 | 8.7 ± 1.2 | 1.1 ± 0.2 |
| 9 | MCP-1 | 38.5 ± 4.0 | 18.2 ± 4.0 | 19.4 ± 4.1 |
| 10 | MIP-1a | 13.1 ± 3.1 | 21.4 ± 3.7 | 6.9 ± 0.7 |
| 11 | IP-10 | 0.5 ± 0.1 | 1.31 ± 0.4 | 1.9 ± 0.1 |
| 12 | TNF-a | 0.3 ±0.1 | 1.18 ± 0.21 | 1.2 ± 0.3 |
| 13 | TNF-RI | 77.8± 6.2 | 708.2± 62.9 | 390.4± 20.2 |
| 14 | TNF-RII | 5.9± 0.2 | 275.9± 4.3 | 4.0± 1.3 |
| 15 | sIL-6R | 50. 9± 9.2 | 402.6 ± 16.2 | 431.3± 25.9 |
Next, we determined whether treatment of tumor cells with TRAIL could affect cytokine production. Analysis of cytokines in medium conditioned by tumor cells treated with 1-100 ng/ml TRAIL for 24 h, demonstrated that TRAIL treatment significantly upregulated secretion of IL-8 in all three human carcinoma cell lines (Figure 2C–D). In addition, increased production of RANTES (CCL5) and MCP-1 (CCL2) was found in MCF7 breast cancer cells, whereas secretion of bFGF was elevated in H460 lung cancer cells. TRAIL, at a concentration 100 ng/ml, induced highest stimulation of bFGF, MCP-1 and RANTES (Figure 2C, D).
TRAIL-induced cytokines provide prosurvival and proliferative signals in tumor cells
To determine, which TRAIL-induced factors are responsible for stimulation of tumor cell proliferation and survival, we tested various neutralizing antibodies for their ability to abrogate TRAIL-induced stimulation of tumor cell proliferation. Tumor cells were treated with TRAIL (1 ng/ml) in the presence of neutralizing antibodies against IL-8, MCP-1, RANTES, bFGF, and IL-6 (100 ng/ml). In the presence of control IgG, TRAIL increased 3H-thymidine incorporation (Figure 3). Neutralizing Abs against IL-8 and RANTES significantly (p < 0.05) abrogated TRAIL-induced stimulation of 3H-thymidine incorporation in all three carcinoma cell lines (Figure 3A–C). Anti-bFGF Ab had similar effect in H460 cells (Figure 3A). Of note, only anti-RANTES Ab reduced proliferation of both untreated as well as TRAIL-treated cells, suggesting the importance of RANTES in regulation of tumor cell proliferation (Figure 3). The three tested cell lines are high producers of IL-6, however, TRAIL did not increase production of IL-6, and anti-IL-6 Ab did not alter TRAIL-induced proliferation of OVCAR-3 and MCF7 (Figure 3B, C) and H460 cells (data not shown), indicating that IL-6 is not involved in TRAIL-induced autocrine stimulation of cell proliferation.
Figure 3. Effects of neutralizing anti-cytokine antibodies on TRAIL-induced proliferation (A–C) and apoptosis (D, E).
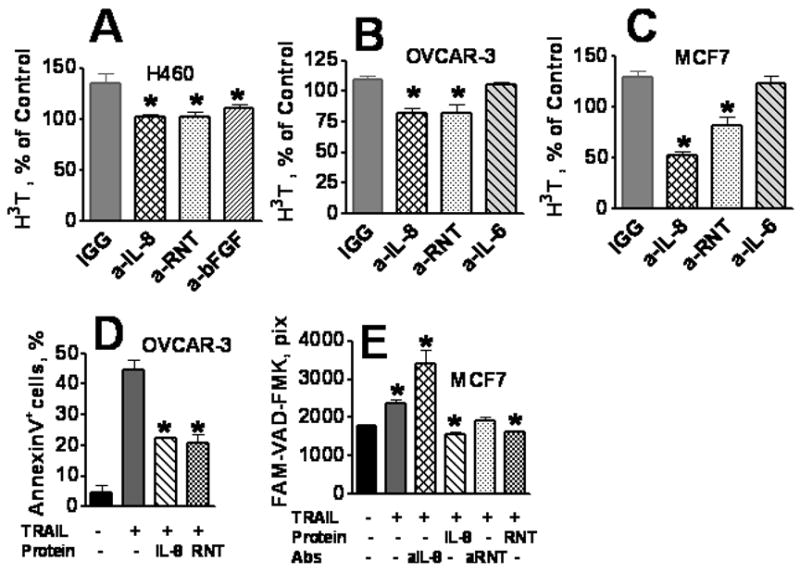
H460 (A), OVCAR-3 (B) and MCF7 (C) cells were pretreated with neutralizing antibodies against IL-8, RANTES (RNT), IL-6, and bFGF or IgG isotype control (at 100 ng/ml) for 1 h before TRAIL (1 ng/ml) were added. After 72 h incubation, 3H-thymidine incorporation assay was performed. Data were normalized against 3H-thymidine incorporation in naive TRAIL untreated cells. D, OVCAR-3 were incubated with recombinant human IL-8 or RANTES(RNT) at 100 ng/ml, for 1 h, followed by incubation with 100ng/ml TRAIL for 30 h. The percentage of apoptotic cells was determined by Annexin V/PI staining and flow cytometry. E, MCF7 cells were incubated with anti-IL-8, anti-RANTES (RNT) neutralizing antibodies, or recombinant human IL-8 and RANTES (RNT) at 100 ng/ml, 1 h and then TRAIL was added (or not added) for 6 h. Caspase activation (apoptosis) was assessed by incubation of the cells with FAM-VAD-FMK. Cell images were acquired using the Cellomics ArrayScan HCS Reader (20X objective) and analyzed using Target Activation BioApplication Software Module. Anti-IL-8, anti-RANTES neutralizing antibodies, or recombinant human IL-8 and RANTES did not affect the spontaneous levels of caspase activation in untreated tumor cells.
We next analyzed the effects of recombinant IL-8 and RANTES on TRAIL-induced apoptosis as assessed by AnnexinV and by FAM-VAD-FMK assays as described in Methods. Recombinant IL-8 significantly protected OVCAR-3 and MCF7 cells from the apoptotic effects of TRAIL, whereas blocking endogenous IL-8 with neutralizing Ab augmented TRAIL-induced apoptosis (Figure 3E). Recombinant RANTES inhibited TRAIL-induced apoptosis (Figure 3D, E), however the effect of anti-RANTES neutralizing Ab was not significant (Figure 3E). These data suggest that IL-8 and RANTES provide proliferative signals and protect tumor cells from TRAIL-induced apoptosis.
TRAIL-induced activation of signaling pathways
Stimulation of cytokines and chemokines production is mediated via activation of several transcription factors (TF) [38]. We analyzed the ability of TRAIL to activate 10 TFs using multiplexing xMAP technology. H460, MCF7, and OVCAR-3 tumor cells were treated with TRAIL (10 ng/ml) and cell lysates or nuclear extracts were collected 5, 15, 30 and 60 min after treatment. Figure 4A demonstrates TRAIL effects on TF activity after 5 and 30 min of treatment. No significant changes in activity of YY1, CREB, SRE, HIF, AP-2 and NFAT transcription factors were found in any cell line (data not shown). TRAIL significantly (p < 0.05) increased the activity of NF-κB, EGR, NF-1, and PPAR in OVCAR-3 cells, and NF-κB and PPAR activity in MCF7 cells, whereas in H460 cells only PPAR was significantly activated (Figure 4A). Phosphorylation of IκB-α leads to activation of NF-κB that could induce various genes encoding cytokines, chemokines and growth factors [39]. TRAIL treatment increased phosphorylation of IκB-α in OVCAR3 and MCF7 cells (Figure 4B). In comparison to other cell lines, H460 cells have low levels of IκB-α phosphorylation that did not significantly increase following TRAIL treatment (Figure 4B). However, H460 cells had significantly higher level of phospho-AKT that was further stimulated by TRAIL. No changes in AKT phosphorylation were induced by TRAIL in MCF7 and OVCAR3 cells (Figure 4B). These data indicate that TRAIL signaling could activate different pathways in different tumor cell lines and stimulate production of different chemokines and growth factors in these cells.
Figure 4. TRAIL-induced activation of signaling pathways.
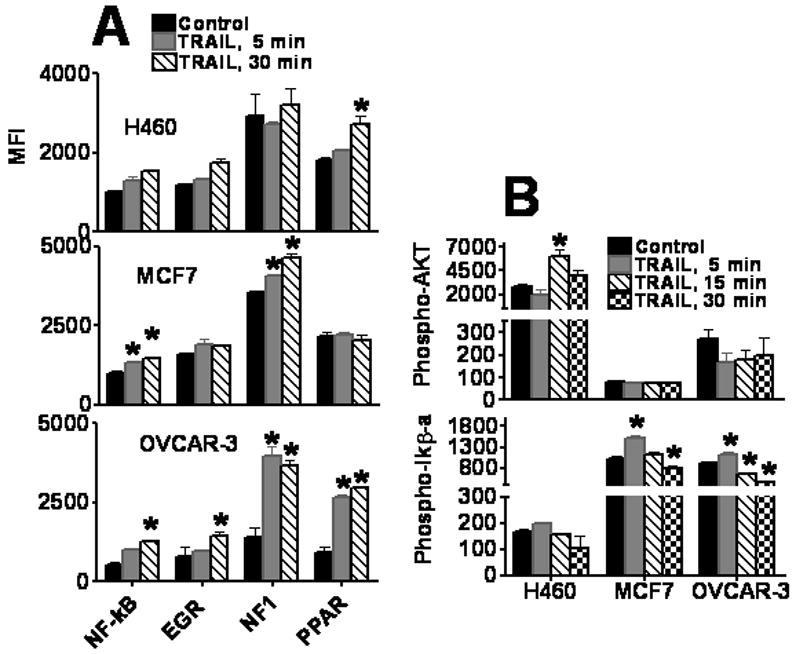
A. Transcription factors expression in TRAIL-treated tumor cells. H460, OVCAR-3, and MCF7 cells were treated with 10 ng/ml of TRAIL for 0, 5, and 30 min. Nuclear extracts were prepared and transcription factor analysis was performed using 10-plex kit. Only transcription factors with significant differences after the TRAIL treatment (at least p < 0.05) are presented. B, C. TRAIL-induced phosphorylation of AKT and IkB-a in H460, MCF7, and OVCAR-3 tumor cells. Tumor cells were treated with 10 ng/ml of TRAIL for 0–30 min. Cell lysates were prepared and analyzed using 8-Plex phosphoprotein kit as described in Methods.
Caspase regulate TRAIL-induced cytokine secretion in tumor cells
TRAIL signaling leads to activation of caspases, as assessed by FAM-VAD-FMK assay, and upregulation of cytokines (Figure 5A for IL-8). Therefore, next we utilized caspase inhibitors to assess whether blocking of upstream or downstream caspases affects TRAIL-induced cytokine production. Cells were pre-treated with inhibitors of caspases 1 (z-YVAD-FMK), 8 (z-IETD-FMK), 3 (z-DEVD-FMK) and 6 (z-VEID-FMK), and then TRAIL (10 ng/ml) was added for 24 h. Inhibitors of caspases 1 and 8 were able to significantly (p < 0.05) block TRAIL-induced up regulation of IL-8 in OVCAR-3 cells, whereas inhibitors of caspases 3 and 6 showed no effect on IL-8 production (Figure 5B). Similar results were obtained in H460 and MCF7 cells (data not shown). TRAIL stimulated production of RANTES in MCF7 cells. Inhibition of caspases 1 and 8, but not 3 and 6, abrogated TRAIL-induced RANTES production (Figure 5C). Inhibition of caspases 1 and 8 slightly reduced IL-8 and RANTES production by TRAIL-untreated tumor cells, whereas inhibition of caspase 3 and 6 had no effect on the cytokines secretion (data not presented).
Figure 5. The role of caspases in TRAIL-induced cytokine production.
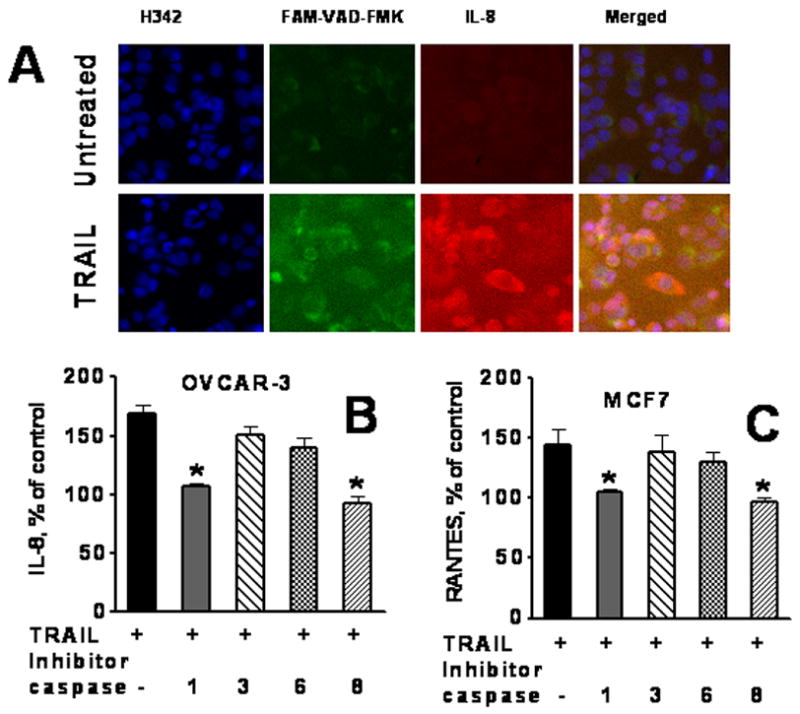
A. Images of immunofluorescently stained OVCAR-3 cells for caspases and expression of IL-8 OVCAR-3 cells, growing in 96 well plates, were treated with TRAIL 10 ng/ml for 4h, and then FAM-VAD-FMK was added for the 1 h. Cells were fixed, permeabilazed and incubated with primary Abs against IL-8, and with secondary Alexa Fluor 688-conjugated Abs. Cell nuclei were stained with Hoechst 33342. Cell images were acquired using the Cellomics ArrayScan HCS Reader (20X objective). B, C. Effect of caspase inhibitors on IL-8 (B) and RANTES (C) production in TRAIL-treated cells. OVCAR-3 and MCF7 cells were pretreated with 20 μM of caspase inhibitors for 2 h, TRAIL was added to a final concentration of 10 ng/ml for 24 h. Inhibitors used: caspase 1 inhibitor, z-YVAD-FMK; caspase 8 inhibitor, z-IETD-FMK; caspase 3 inhibitor, z-DEVD-FMK; and pan-caspase inhibitor, z-VAD-FMK. Cytokines in conditioned medium were analyzed using multiplex cytokine assay.
TRAIL induced senescence-like phenotype (SLP) of tumor cells
Growing evidence indicate that some tumor cells treated with chemotherapeutic drugs could escape apoptosis by developing cell-cycle arrest leading to senescence [40, 41]. We hypothesize that TRAIL is capable to induce senescence that could help tumor cells to escape apoptosis.
Senescent phenotype can be characterized by increased expression of p16 INK4a, p21 Waf1/Cip1, and SA-β-gal [28-30] as well as high mobility group A (HMGA) proteins accumulation. [31]. HMGA proteins are essential structural components of senescence-associated heterochromatic foci (SAHFs). HMGA proteins cooperate with the p16 INK4a promote SAHF formation and proliferative arrest and stabilize senescence by contributing to the repression of proliferation-associated genes[31]. H460, OVCAR-3 and MCF-7 cells were treated with 500 ng/ml TRAIL for 4h. Some surviving cells became enlarged and flattened. Staining with Hoechst 33342 and image analysis revealed that treatment with high concentration of TRAIL significantly induced the number of cells with enlarged nuclei that was assessed by image cytometry (Cellomics) (Figure 6A). No such effects were observed after treatment of tumor cells with lower concentrations of TRAIL (1-10 ng/ml). Large flattened cells were positive for SA-β-gal (Figure 6D, groups 1 and 2). Staining of TRAIL-treated and untreated cells with Hoechst 33342 and anti-β-actin Ab confirmed the appearance of enlarged cells in TRAIL-treated, but not in untreated cells (Figure 6D, groups 3 and 4). Upregulated expression of HMGA, p16, and p21 was observed in TRAIL treated tumor cells as compared with untreated cells (Figure 6B–C also Figure 6D, groups 5–10). These data strongly indicate that high doses of TRAIL can induce senescence in tumor cells that escaped apoptosis.
Figure 6. TRAIL induces senescence like phenotype (SLP) in tumor cells.
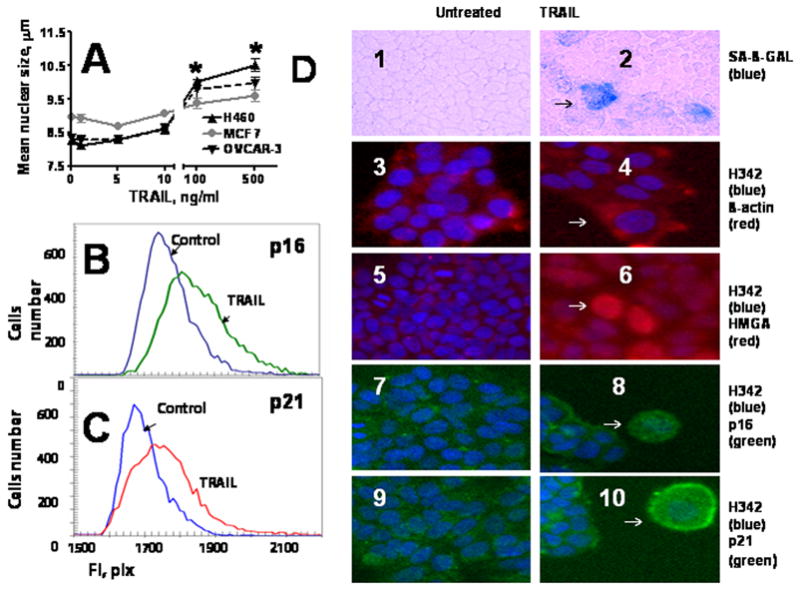
A. Size of nuclei of tumor cells treated with TRAIL. H460, MCF7 and OVCAR-3 cells were treated with 500 ng/ml TRAIL for 4 h, washed, and cultured in TRAIL-free medium for 72 h. Cells were stained with Hoechst 3342, and nuclei were analyzed by Cellomics ArrayScan HCS Reader. B–C. Analysis of SLP markers (p16 and p21) expression in MCF7 and OVCAR-3 cells. Cells were incubated with 500 ng/ml TRAIL for 4 h, washed and cultured for 72 h in fresh medium. Cells were fixed and stained with primary antibodies against p16 or p21 and secondary Alexa Fluor 488 conjugated antibodies. Cell images were acquired using Cellomics ArrayScan HCS Reader (20X objective) and analyzed using the Target Activation BioApplication Software Module. Expression of p16 in MCF7 cells (B) and p21 in OVCAR-3 cells (C) treated with TRAIL was plotted against those in untreated control cells. D. Morphology and markers of senescence phenotype in TRAIL-treated cells. Tumor cells were treated as in 5B,C: 1–2, H460 tumor cells stained with SA-β-gal (light microscope, 10× magnification); 3–10, Immunofluorescent images (20X objective): 3–4, OVCAR-3 cells stained for β-actin (red) and Hoechst 33342 (blue); 5–6, MCF7 cells stained for HMGA (red) and Hoechst 33342 (blue); 7–8, MCF7 cells stained for p16 (green) and Hoechst 33342(blue); 9–10, OVCAR-3 cells stained for p21 (green) and Hoechst 333421 (blue).
Effect of caspase inhibition on SLP in TRAIL-treated cells
To test possible involvement of caspases in TRAIL-induced generation of senescent cells, MCF7 cells were treated with caspase inhibitors, and expression of p21, p16 and HMGA in TRAIL-treated and untreated cells was analyzed using Cellomics ArrayScan HCS Reader. Caspase inhibitors did not effect on SLP markers in untreated cells (data not presented), whereas differentially regulated p21, p16 and HMGA expression in TRAIL-treated cells. Inhibition of apical caspase 8 significantly increased expression of p21, p16 and HMGA in TRAIL-treated cells (Figure 7A). Similar effects were observed when tumor cells were pretreated with an inhibitor of caspase 1 (data not shown). In contrast, pretreatment of tumor cells with pan-caspase inhibitor z-VAD-FMK and inhibitors of caspases 9 completely blocked TRAIL-induced up regulation of p16 and p21, and slightly blocked HMGA accumulation (Figure 7). In fact, the inhibitor of caspase 9 down regulated expression of p16 and p21 below levels found in untreated MCF7 cells (Figure 7). Similar effects were observed when tumor cells were treated with TRAIL in the presence of inhibitors of caspases 3,4,5,6 (data are not shown).
Figure 7. Role of caspases in TRAIL-induced cell senescence.
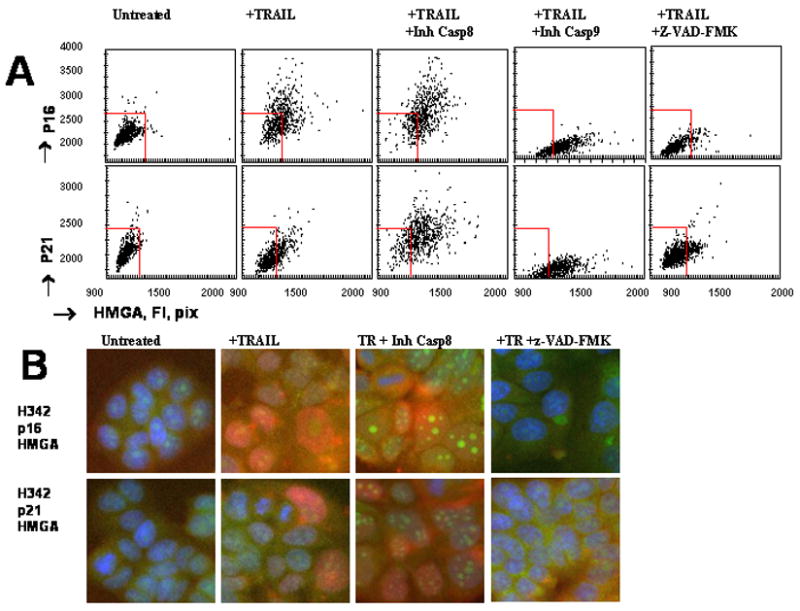
A, Effect of caspase inhibitors on p16, p21 and HMGA expression in TRAIL-treated cells. MCF7 cells were pretreated with 20μM of inhibitors of caspase 8, z-IETD-FMK; caspase 9, LEHD-FMK; or pan-caspase inhibitor z-VAD-FMK for 2 h and incubated with TRAIL (500 ng/ml) for 4 h. Culture medium was replaced with the fresh TRAIL-free medium containing caspase inhibitors. After 72 h in culture, cells were stained for p16, p21 and HMGA as described in Figure 6. Cell images were acquired using the Cellomics ArrayScan HCS Reader (20X objective) and analyzed using the Target Activation BioApplication Software Module. Fluorescence intensity (pix) of p16 and p21 was plotted against fluorescence intensity (pix) of HMGA. Each point represents a single cell. The red squires show the boundaries of the major cellular population of untreated cells. B. Effects of caspase inhibitors on expression of SLP markers in TRAIL-treated cells. Representative images of MCF7 cells stained for HGMA (red), p16 (green) or p21 (green) and Hoechst 33342 stained nuclei (blue) are shown.
DISCUSSION
In this report, we demonstrate that TRAIL-treatment induces multifactorial signaling network leading to various functional outcomes: apoptosis, senescence, cells proliferation, and elevated production of various cytokines and chemokines, in three histologically distinct apoptosis sensitive human tumor cell lines: NSCLC line H460, breast carcinoma MCF-7 and ovarian cancer OVCAR-3 line and that the different responses of tumor cells to TRAIL depend on the concentration of TRAIL.
At low concentration TRAIL induces proliferation of apoptosis sensitive cells. At high concentration, TRAIL is preferentially inducing apoptosis; however surviving cells acquire higher proliferative capacity. Several lines of evidence indicate that TRAIL treatment of normal human endothelial cells and apoptosis resistant tumor cell lines can stimulates production of proinflammatory cytokines and chemokines, e.g. IL-1, IL-8, and MCP-1 [14–17]. We found that TRAIL stimulates production of IL-8 in all three tested human tumor cell lines, RANTES in MCF-7 cells, and bFGF in H460 cells. It remains unclear whether the observed differences could be attributed to the induction of different transcription factors in these cells or other molecules that positively and negatively control expression of cytokine genes. The observed TRAIL induced activation of NF-κB and Akt could be important for tumor cells survival and proliferation [8, 42, 43]. The involvement of caspase 8 in apoptosis signaling is crucial in TRAIL induced apoptosis but its involvement in the proliferation signaling is overlapping with caspase 1 and thus loss of caspase 8 can block apoptosis but not proliferation [1–4]. Our data as well as previously reported [16, 17] indicate that TRAIL-induced activation of caspases 1 and 8 is essential for up regulation of cytokines.. However, the significance of TRAIL-induced cytokine production is not fully understood. We report here that media conditioned by TRAIL-treated tumor cells stimulates tumor cell proliferation and protects them from TRAIL-induced apoptosis. Furthermore, disruption of IL-8, RANTES, and FGF-b cells signaling abrogates TRAIL-induced proliferation and potentiates apoptosis, indicating a pivotal role of chemokines and growth factors in proliferative and prosurvival effects of TRAIL. The ability of recombinant IL-8 to protect tumor cells from TRAIL-induced apoptosis has been previously reported [16]. Initially, IL-8 and RANTES were identified for their ability to stimulate migration of proinflammatory cells [44]. Recent studies demonstrated that these chemokines have broader biological effects and are able to stimulate proliferation of malignant and endothelial cells to stimulate tumor blood vessel network formation [45-48] and increased blood levels of IL-8 and RANTES is often associated with poor prognosis in cancer patients [46–48].
For the first time we demonstrated that TRAIL can induce a senescence-like phenotype (SLP) in three carcinoma cell lines that was induced only with high doses of TRAIL. It is suggested that cell senescence helps tumor to escape drug-induced apoptosis [32]. Some cells that escape apoptosis became senescent and it is unclear whether they could return to initial status and most likely they could eventually died. We found that senescence is differentially regulated by caspases. Pancaspase inhibitor z-VAD-FMK and inhibitors of caspases 3, 4, 5, 6, and 9 abrogated the induction of senescent cells, whereas inhibition of upstream caspases 1 and 8 induced cell senescence. Thus, induction of a senescence phenotype as well as apoptosis and stimulation of proliferation might represent a common response of carcinoma cells to TRAIL.
Our data suggest that different effects of TRAIL might depend on the strength of signaling. At high concentrations TRAIL may induce a strong signaling that preferentially lead to development of apoptosis and senescence. Weaker signaling induced by low doses of TRAIL fails to induce a substantial apoptotic effects and cell senescence but rather activated pathways stimulating production of growth factors and cells proliferation. However, even at high concentrations, TRAIL could also stimulate production of growth factors in surviving cells. All TRAIL-induced biological effects are interrelated and blocking one signaling pathway could increase the outcome of another pathway.
The general model of multiple effects induced by TRAIL in tumor cells is presented in Figure 8. TRAIL signaling via DR4/5 receptors triggers the apoptotic pathway via activation of upstream caspases 1, 8, and 9 that then activate downstream caspases as well as induction of NF-κB/AKT pathway leading to stimulation of cytokine production. Cytokines by binding to their cognate receptors on tumor cells could provide proliferative signals that could counter apoptotic effects of TRAIL. In addition, TRAIL-induced stimulation of tumor cell proliferation could be a result of involvement of other mechanisms, such as activation cyclin kinases and stimulation cell cycle [25–27]. Moreover, activation of downstream caspases could trigger cell-cycle arrest and development of senescence in some of the surviving cells (Figure 8). Thus, senescence could present a mechanism of escape of apoptotic death induced by TRAIL. Indeed, blocking activation of downstream caspases inhibited not only apoptosis, but also senescence. On the other hand, inhibition of caspases 1 and 8 increased TRAIL-induced senescence that coincided with blocking production of chemokines and growth factors. This might indicate the protective role of cytokines against both apoptosis and irreversible growth arrest/SLP formation (Figure 8). TRAIL-induced cytokine production signaling has lower threshold that apoptosis induction and could be triggered by lower concentrations of TRAIL.
Figure 8.
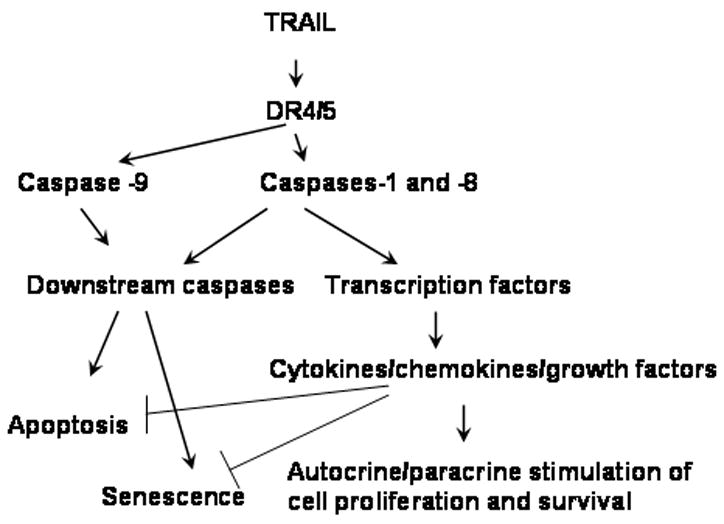
Model of TRAIL triggered pathways and biological effects.
The ability of TRAIL to kill tumor but not normal cells makes it an attractive agent for cancer therapy. However, several clinical trials failed to demonstrate its therapeutic potentials. This failure could be attributed to numerous factors. The long-term result of TRAIL therapy could be the selection of TRAIL resistant cell variants. However, the short-term effects may include stimulation of cell proliferation by low concentrations of TRAIL. Since final intratumoral concentration of TRAIL may vary depending on the tumor size, distribution of blood vessels, blood flow, capillary permeability, interstitial pressure etc. [51], several tumor regions could be exposed to low concentrations of TRAIL. However, even at high concentrations TRAIL may increase production of soluble factors that could have autocrine and paracrine effects and minimize therapeutic effects of TRAIL. Thus, targeting prosurvival cytokines could be a strategy for improving the efficacy of TRAIL-based therapy.
Acknowledgments
We thank Ligita Griniene, Brian Nolan and Liudmilla Velikokhatnaya for the technical support. We are grateful for the helpful advice of members of the University of Pittsburgh Cancer Institute Writing Group, in particular Drs. Merrill Egorin and Pamela Hershberger, for critical evaluation of the manuscript. This work was supported by NIH grants RO1 CA098642, R01 CA108990, and Avon (NIH/NCI) (AEL), as well as by grants from Susan Komen Foundation (AEL), and DOD BC051720 grant (EG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert A, DeForge L, Koumenis IL, Lewis D, Harris L, Bussiere J, Koeppen H, Shahrokh Z, Schwall RH. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999;104:155–162. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kischkel FC, Lawrence DA, Chuntharapai A, Schow P, Kim KJ, Ashkenazi A. Apo2L/TRAIL-dependent recruitment of endogenous FADD and caspase-8 to death receptors 4 and 5. Immunity. 2000;12:611–620. doi: 10.1016/s1074-7613(00)80212-5. [DOI] [PubMed] [Google Scholar]
- 3.Zhang L, Fang B. Mechanisms of resistance to TRAIL-induced apoptosis in cancer. Cancer Gene Ther. 2005;12:228–237. doi: 10.1038/sj.cgt.7700792. [DOI] [PubMed] [Google Scholar]
- 4.Eggert A, Grotzer MA, Zuzak TJ, Wiewrodt BR, Ikegaki N, Brodeur GM. Resistance to TRAIL-induced apoptosis in neuroblastoma cells correlates with a loss of caspase-8 expression. Med Pediatr Oncol. 2000;35:603–607. doi: 10.1002/1096-911x(20001201)35:6<603::aid-mpo24>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 5.Kim K, Fisher MJ, Xu SQ, el-Deiry WS. Molecular determinants of response to TRAIL in killing of normal and cancer cells. Clin Cancer Res. 2000;6:335–346. [PubMed] [Google Scholar]
- 6.Kreuz S, Siegmund D, Scheurich P, Wajant H. NF-kappaB inducers upregulate cFLIP, a cycloheximide-sensitive inhibitor of death receptor signaling. Mol Cell Biol. 2001;21:3964–3973. doi: 10.1128/MCB.21.12.3964-3973.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nesterov A, Lu X, Johnson M, Miller GJ, Ivashchenko Y, Kraft AS. Elevated AKT activity protects the prostate cancer cell line LNCaP from TRAIL-induced apoptosis. J Biol Chem. 2001;276:10767–10774. doi: 10.1074/jbc.M005196200. [DOI] [PubMed] [Google Scholar]
- 8.Baader E, Toloczko A, Fuchs U, Schmid I, Beltinger C, Ehrhardt H, Debatin KM, Jeremias I. Tumor necrosis factor-related apoptosis-inducing ligand-mediated proliferation of tumor cells with receptor-proximal apoptosis defects. Cancer Res. 2005;65:7888–7895. doi: 10.1158/0008-5472.CAN-04-4278. [DOI] [PubMed] [Google Scholar]
- 9.Secchiero P, Zerbinati C, Rimondi E, Corallini F, Milani D, Grill V, Forti G, Capitani S, Zauli G. TRAIL promotes the survival, migration and proliferation of vascular smooth muscle cells. Cell Mol Life Sci. 2004;61:1965–1974. doi: 10.1007/s00018-004-4197-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morel J, Audo R, Hahne M, Combe B. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) induces rheumatoid arthritis synovial fibroblast proliferation through mitogen-activated protein kinases and phosphatidylinositol 3-kinase/Akt. J Biol Chem. 2005;280:15709–15718. doi: 10.1074/jbc.M414469200. [DOI] [PubMed] [Google Scholar]
- 11.Ehrlich S, Infante-Duarte C, Seeger B, Zipp F. Regulation of soluble and surface-bound TRAIL in human T cells, B cells, and monocytes. Cytokine. 2003;24:244–253. doi: 10.1016/s1043-4666(03)00094-2. [DOI] [PubMed] [Google Scholar]
- 12.Ehrhardt H, Fulda S, Schmid I, Hiscott J, Debatin KM, Jeremias I. TRAIL induced survival and proliferation in cancer cells resistant towards TRAIL-induced apoptosis mediated by NF-kappaB. Oncogene. 2003;22:3842–3852. doi: 10.1038/sj.onc.1206520. [DOI] [PubMed] [Google Scholar]
- 13.Milani D, Zauli G, Rimondi E, Celeghini C, Marmiroli S, Narducci P, Capitani S, Secchiero P. Tumour necrosis factor-related apoptosis-inducing ligand sequentially activates pro-survival and pro-apoptotic pathways in SK-N-MC neuronal cells. J Neurochem. 2003;86:126–135. doi: 10.1046/j.1471-4159.2003.01805.x. [DOI] [PubMed] [Google Scholar]
- 14.Trauzold A, Siegmund D, Schniewind B, Sipos B, Egberts J, Zorenkov D, Emme D, Roder C, Kalthoff H, Wajant H. TRAIL promotes metastasis of human pancreatic ductal adenocarcinoma. Oncogene. 2006;25:7434–7439. doi: 10.1038/sj.onc.1209719. [DOI] [PubMed] [Google Scholar]
- 15.Li JH, Kirkiles-Smith NC, McNiff JM, Pober JS. TRAIL induces apoptosis and inflammatory gene expression in human endothelial cells. J Immunol. 2003;171:1526–1533. doi: 10.4049/jimmunol.171.3.1526. [DOI] [PubMed] [Google Scholar]
- 16.Abdollahi T, Robertson NM, Abdollahi A, Litwack G. Identification of interleukin 8 as an inhibitor of tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in the ovarian carcinoma cell line OVCAR3. Cancer Res. 2003;63:4521–4526. [PubMed] [Google Scholar]
- 17.Siegmund D, Klose S, Zhou D, Baumann B, Roder C, Kalthoff H, Wajant H, Trauzold A. Role of caspases in CD95L- and TRAIL-induced non-apoptotic signalling in pancreatic tumour cells. Cell Signal. 2007;19:1172–1184. doi: 10.1016/j.cellsig.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 18.Varfolomeev E, Maecker H, Sharp D, Lawrence D, Renz M, Vucic D, Ashkenazi A. Molecular determinants of kinase pathway activation by Apo2 ligand/tumor necrosis factor-related apoptosis-inducing ligand. J Biol Chem. 2005;280:40599–40608. doi: 10.1074/jbc.M509560200. [DOI] [PubMed] [Google Scholar]
- 19.Ishimura N, Isomoto H, Bronk SF, Gores GJ. Trail induces cell migration and invasion in apoptosis-resistant cholangiocarcinoma cells. Am J Physiol Gastrointest Liver Physiol. 2006;290:G129–136. doi: 10.1152/ajpgi.00242.2005. [DOI] [PubMed] [Google Scholar]
- 20.Secchiero P, Gonelli A, Carnevale E, Corallini F, Rizzardi C, Zacchigna S, Melato M, Zauli G. Evidence for a proangiogenic activity of TNF-related apoptosis-inducing ligand. Neoplasia. 2004;6:364–373. doi: 10.1593/neo.03421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi C, Kutsch O, Park J, Zhou T, Seol DW, Benveniste EN. Tumor necrosis factor-related apoptosis-inducing ligand induces caspase-dependent interleukin-8 expression and apoptosis in human astroglioma cells. Mol Cell Biol. 2002;22:724–736. doi: 10.1128/MCB.22.3.724-736.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Almasan A, Ashkenazi A. Apo2L/TRAIL: apoptosis signaling, biology, and potential for cancer therapy. Cytokine Growth Factor Rev. 2003;14:337–348. doi: 10.1016/s1359-6101(03)00029-7. [DOI] [PubMed] [Google Scholar]
- 23.Falschlehner C, Emmerich CH, Gerlach B, Walczak H. TRAIL signalling: decisions between life and death. Int J Biochem Cell Biol. 2007;39:1462–1475. doi: 10.1016/j.biocel.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 24.Lin A, Karin M. NF-kappaB in cancer: a marked target. Semin Cancer Biol. 2003;13:107–114. doi: 10.1016/s1044-579x(02)00128-1. [DOI] [PubMed] [Google Scholar]
- 25.Joyce D, Albanese C, Steer J, Fu M, Bouzahzah B, Pestell RG. NF-kappaB and cell-cycle regulation: the cyclin connection. Cytokine Growth Factor Rev. 2001;12:73–90. doi: 10.1016/s1359-6101(00)00018-6. [DOI] [PubMed] [Google Scholar]
- 26.Meloche S, Pouyssegur J. The ERK1/2 mitogen-activated protein kinase pathway as a master regulator of the G1- to S-phase transition. Oncogene. 2007;26:3227–3239. doi: 10.1038/sj.onc.1210414. [DOI] [PubMed] [Google Scholar]
- 27.Li Q, Zhu GD. Targeting serine/threonine protein kinase B/Akt and cell-cycle checkpoint kinases for treating cancer. Curr Top Med Chem. 2002;2:939–971. doi: 10.2174/1568026023393318. [DOI] [PubMed] [Google Scholar]
- 28.Roninson IB. Tumor cell senescence in cancer treatment. Cancer Res. 2003;63:2705–2715. [PubMed] [Google Scholar]
- 29.Campisi J. Aging, tumor suppression and cancer: high wire-act! Mech Ageing Dev. 2005;126:51–58. doi: 10.1016/j.mad.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 30.Zheng QH, Ma LW, Zhu WG, Zhang ZY, Tong TJ. p21Waf1/Cip1 plays a critical role in modulating senescence through changes of DNA methylation. J Cell Biochem. 2006;98:1230–1248. doi: 10.1002/jcb.20838. [DOI] [PubMed] [Google Scholar]
- 31.Narita M, Krizhanovsky V, Nunez S, Chicas A, Hearn SA, Myers MP, Lowe SW. A novel role for high-mobility group a proteins in cellular senescence and heterochromatin formation. Cell. 2006;126:503–514. doi: 10.1016/j.cell.2006.05.052. [DOI] [PubMed] [Google Scholar]
- 32.Schmitt CA. Cellular senescence and cancer treatment. Biochim Biophys Acta. 2007;1775:5–20. doi: 10.1016/j.bbcan.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 33.Siervo-Sassi RR, Marrangoni AM, Feng X, Naoumova N, Winans M, Edwards RP, Lokshin A. Physiological and molecular effects of Apo2L/TRAIL and cisplatin in ovarian carcinoma cell lines. Cancer Lett. 2003;190:61–72. doi: 10.1016/s0304-3835(02)00579-7. [DOI] [PubMed] [Google Scholar]
- 34.Gastman B, Wang K, Han J, Zhu ZY, Huang X, Wang GQ, Rabinowich H, Gorelik E. A novel apoptotic pathway as defined by lectin cellular initiation. Biochem Biophys Res Commun. 2004;316:263–271. doi: 10.1016/j.bbrc.2004.02.043. [DOI] [PubMed] [Google Scholar]
- 35.Gorelik E, Landsittel DP, Marrangoni AM, Modugno F, Velikokhatnaya L, Winans MT, Bigbee WL, Herberman RB, Lokshin AE. Multiplexed immunobead-based cytokine profiling for early detection of ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:981–987. doi: 10.1158/1055-9965.EPI-04-0404. [DOI] [PubMed] [Google Scholar]
- 36.Marini P, Schmid A, Jendrossek V, Faltin H, Daniel PT, Budach W, Belka C. Irradiation specifically sensitises solid tumour cell lines to TRAIL mediated apoptosis. BMC Cancer. 2005;5:5. doi: 10.1186/1471-2407-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh TR, Shankar S, Chen X, Asim M, Srivastava RK. Synergistic interactions of chemotherapeutic drugs and tumor necrosis factor-related apoptosis-inducing ligand/Apo-2 ligand on apoptosis and on regression of breast carcinoma in vivo. Cancer Res. 2003;63:5390–5400. [PubMed] [Google Scholar]
- 38.Richmond A. Nf-kappa B, chemokine gene transcription and tumour growth. Nat Rev Immunol. 2002;2:664–674. doi: 10.1038/nri887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andreakos E, Williams RO, Wales J, Foxwell BM, Feldmann M. Activation of NF-kappaB by the intracellular expression of NF-kappaB-inducing kinase acts as a powerful vaccine adjuvant. Proc Natl Acad Sci U S A. 2006;103:14459–14464. doi: 10.1073/pnas.0603493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmitt CA, Fridman JS, Yang M, Lee S, Baranov E, Hoffman RM, Lowe SW. A senescence program controlled by p53 and p16INK4a contributes to the outcome of cancer therapy. Cell. 2002;109:335–346. doi: 10.1016/s0092-8674(02)00734-1. [DOI] [PubMed] [Google Scholar]
- 41.Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 42.Ravi R, Bedi GC, Engstrom LW, Zeng Q, Mookerjee B, Gelinas C, Fuchs EJ, Bedi A. Regulation of death receptor expression and TRAIL/Apo2L-induced apoptosis by NF-kappaB. Nat Cell Biol. 2001;3:409–416. doi: 10.1038/35070096. [DOI] [PubMed] [Google Scholar]
- 43.Whang YE, Yuan XJ, Liu Y, Majumder S, Lewis TD. Regulation of sensitivity to TRAIL by the PTEN tumor suppressor. Vitam Horm. 2004;67:409–426. doi: 10.1016/S0083-6729(04)67021-X. [DOI] [PubMed] [Google Scholar]
- 44.Ben-Baruch A. The multifaceted roles of chemokines in malignancy. Cancer Metastasis Rev. 2006;25:357–371. doi: 10.1007/s10555-006-9003-5. [DOI] [PubMed] [Google Scholar]
- 45.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li A, Varney ML, Singh RK. Expression of interleukin 8 and its receptors in human colon carcinoma cells with different metastatic potentials. Clin Cancer Res. 2001;7:3298–3304. [PubMed] [Google Scholar]
- 47.Azenshtein E, Luboshits G, Shina S, Neumark E, Shahbazian D, Weil M, Wigler N, Keydar I, Ben-Baruch A. The CC chemokine RANTES in breast carcinoma progression: regulation of expression and potential mechanisms of promalignant activity. Cancer Res. 2002;62:1093–1102. [PubMed] [Google Scholar]
- 48.Vaday GG, Peehl DM, Kadam PA, Lawrence DM. Expression of CCL5 (RANTES) and CCR5 in prostate cancer. Prostate. 2006;66:124–134. doi: 10.1002/pros.20306. [DOI] [PubMed] [Google Scholar]
- 49.Bar-Eli M. Role of interleukin-8 in tumor growth and metastasis of human melanoma. Pathobiology. 1999;67:12–18. doi: 10.1159/000028045. [DOI] [PubMed] [Google Scholar]
- 50.Rebbaa A, Zheng X, Chou PM, Mirkin BL. Caspase inhibition switches doxorubicin-induced apoptosis to senescence. Oncogene. 2003;22:2805–2811. doi: 10.1038/sj.onc.1206366. [DOI] [PubMed] [Google Scholar]
- 51.Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer. 2006;6:583–592. doi: 10.1038/nrc1893. [DOI] [PubMed] [Google Scholar]


