Abstract
Schwann cells are remarkably plastic cells that can both form and stably maintain myelin sheaths around axons and also rapidly dedifferentiate upon injury. New findings (Parkinson, D.B., A. Bhaskaran, P. Arthur-Farraj, L.A. Noon, A. Woodhoo, A.C. Lloyd, M.L. Feltri, L. Wrabetz, A. Behrens, R. Mirsky, and K.R. Jessen. 2008. J. Cell Biol. 181:625–637) indicate that the transition between these distinct states of differentiation is directed by the transcription factor Krox-20, which promotes and maintains myelination, and c-Jun, which antagonizes it. Cross-inhibition of these transcription factors serves to switch Schwann cells between the myelinated and dedifferentiated phenotypes, respectively.
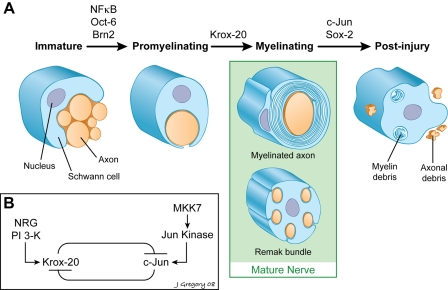
Schwann cells develop in contact with and dependent on axons for signals that promote their genesis and subsequent differentiation (Jessen and Mirsky, 2005). In mature nerves, Schwann cells adopt one of two distinct fates: a nonmyelinating phenotype, in which multiple small-diameter axons are separately enclosed within pockets of the Schwann cell (forming a Remak bundle), or a myelinating phenotype, in which they acquire a one/one association with and subsequently myelinate larger axons that express the promyelinating signal neuregulin 1 (Fig. 1; Nave and Salzer, 2006). Myelinating Schwann cells remain remarkably plastic throughout life. Thus, during Wallerian degeneration, axon transection initiates rapid Schwann cell dedifferentiation (i.e., down-regulation of myelin protein expression and up-regulation of nonmyelin markers) and proliferation (Scherer and Salzer, 2001). These reprogrammed cordons of Schwann cells, termed “bands of Büngner,” provide a cellular substrate for nerve regeneration and subsequent remyelination. The events that underlie these changes in Schwann cell differentiation have been largely obscure. In the current issue, Parkinson et al. (see p. 625) provide important insights into the mechanisms by which injury promotes Schwann cell dedifferentiation. They show that the transcription factor c-Jun is up-regulated upon injury and antagonizes Krox-20, an important promyelinating transcription factor, to promote a switch in the Schwann cell phenotype.
Figure 1.
Regulation of Schwann cell myelination and dedifferentiation. (A) Schwann cell differentiation is regulated by expression of specific transcription factors. Immature Schwann cells receive axonal signals, including neuregulin 1 (NRG), which up-regulate expression of transcription factors including NFκB, Oct-6, and Brn2. These transcription factors promote the promyelinating stage, in which Schwann cells acquire a one/one association with the axon and express early myelin markers. Up-regulation of Krox-20 is required for Schwann cells to form myelin sheaths and express myelin-specific proteins. (In the mature nerve, other Schwann cells that do not express these promyelinating transcription factors remain unmyelinated and segregate off multiple small axons as Remak bundles). Upon injury (i.e., axotomy), there is a rapid up-regulation of c-Jun and Sox-2. The former contributes to the down-regulation of Krox-20 and the dedifferentiation of Schwann cells. (B) Cross-inhibition of Krox-20 and c-Jun promotes a switch in transcriptional complexes. Promyelinating signals from the axon, such as NRG, drive expression of Krox-20, potentially via the phosphatidylinositol 3-kinase pathway. Activation of the JNK pathway during injury is likely to promote expression of c-Jun. The signals that activate this pathway in Schwann cells after injury are not known. These two transcription factors cross-inhibit each other's expression as shown.
Previous work on the regulation of the Schwann cell lineage focused on elucidating the transcriptional cascade that promotes myelination (Jessen and Mirsky, 2005). Among these are factors that promote glial/Schwann cell specification and initial differentiation (e.g., Sox10 and NFκB) and those that promote Schwann cell differentiation to the promyelinating stage (i.e., Oct-6/SCIP and Brn2; Fig. 1). The latter transcription factors, together with Sox10 (Ghislain and Charnay, 2006), are required for the expression of Krox-20 (Egr2), which is essential for Schwann cell myelination. Recently, conditional inactivation of Krox-20 in the adult was shown to result in demyelination, indicating that Krox-20 is also required to maintain the myelin sheath long after it has formed (Decker et al., 2006).
Parkinson et al. (2008) have now examined the role of c-Jun, a basic leucine zipper transcription factor and an important component of the AP-1 complex, in Schwann cell myelination. In previous studies, they reported that c-Jun is expressed by and promotes Schwann cell proliferation before myelination and that c-Jun's expression is then down-regulated at the onset of myelination by Krox-20 (Parkinson et al., 2004). In the current study, they demonstrate that c-Jun can potently inhibit myelination. Thus, forced expression of c-Jun inhibits myelin gene expression in cultured Schwann cells in response to promyelinating factors and also blocks myelination in neuron–Schwann cell cocultures. Loss of c-Jun by conditional inactivation of a floxed allele enhanced myelin gene expression in cultured Schwann cells. These results suggest that c-Jun expression may regulate the onset of myelination during development, although its precise role during development remains to be determined.
It is of note that c-Jun is rapidly up-regulated in Schwann cells after injury. Explanting Schwann cells from nerves into culture (which removes them from contact with axons), treating myelinating cocultures with high doses of a growth factor to induce demyelination, or transecting nerves to initiate Wallerian degeneration all up-regulate c-Jun expression. This up-regulation appears to play an important role in promoting dedifferentiation. Conditional inactivation of c-Jun markedly delayed Schwann cell dedifferentiation under the aforementioned conditions, including, strikingly, during Wallerian degeneration of neonatal nerves. In the latter case, myelin sheaths remained intact even several days after axon transection. These findings indicate c-Jun activation has an important involvement in promoting dedifferentiation of Schwann cells after injury. Although dedifferentiation and myelin sheath breakdown were significantly delayed, they still occurred, suggesting that other pathways may be involved (see subsequent paragraphs).
How does c-Jun promote dedifferentiation? Parkinson et al. (2008) provide strong evidence that c-Jun functions by antagonizing the expression of Krox-20. Forced expression of c-Jun suppresses Krox-20, but not Oct-6, induction. Conversely, inactivation of c-Jun delays the down-regulation of Krox-20 that normally occurs after injury. As loss of Krox-20 by itself leads to myelin breakdown (Decker et al., 2006), these findings suggest that the induction of c-Jun during injury triggers myelin breakdown by inhibiting Krox-20. Interestingly, c-Jun may act synergistically with another transcription factor, Sox-2, which was previously shown to also inhibit myelination (Le et al., 2005). Expression of c-Jun and Sox-2 appear to be coregulated. Both are expressed early in the Schwann cell lineage, down-regulated with myelination, and rapidly reexpressed upon injury, which suggests that they may cooperate in their dedifferentiative effects.
c-Jun is a downstream target of JNK, a serine-threonine kinase that directly phosphorylates c-Jun, enhancing its activity and expression (Johnson and Nakamura, 2007). JNK is phosphorylated and activated by MAPK kinases (MKK), including MKK7. Parkinson et al. (2008) detected robust phospho–c-Jun expression shortly after nerve injury, which implicates the JNK pathway in Schwann cell dedifferentiation after injury. In agreement with this, forced expression of MKK7 in Schwann cells phenocopied the inhibitory effects of c-Jun on myelination. Whether this rapid increase in c-Jun expression and JNK activity reflects the loss of promyelinating signals provided by the axon, the expression of injury signals from the transected nerve (Scherer and Salzer, 2001), or both is not yet known. Interestingly, c-Jun also inhibits myelin gene expression independently of the N-terminal phosphorylation classically mediated by JNK, which suggests that the main involvement of JNK may be to control c-Jun levels.
The JNK pathway is one of the three canonical MAPK signaling pathways, which also include the MAPK and p38 kinases. Each of these kinase pathways has been implicated in regulating Schwann cell differentiation. The Ras–MAPK pathway, like the JNK pathway, is a negative regulator of Schwann cell differentiation and myelination (Harrisingh et al., 2004; Ogata et al., 2004). In contrast, p38 kinase (Haines et al., 2008), as well as phosphatidylinositol 3-kinase (Maurel and Salzer, 2000), is promyelinating. These findings suggest that a dynamic balance between promyelination and dedifferentiation signaling pathways operates during development and injury. This balance between promyelination and dedifferentiation may be reflected in the dynamic switch between Krox-20 versus c-Jun and Sox-2, respectively.
Finally, these studies have important implications for the adaptive changes in Schwann cell differentiation that occur during development and after injury but likely in pathological settings as well. Many demyelinating neuropathies are characterized by Schwann cell dedifferentiation and proliferation (Feldman et al., 2008), and an emerging literature suggests that aberrant MAPK signaling may be involved (Cavaletti et al., 2007). As such, these pathways, including the JNK signaling pathway highlighted by the studies of Parkinson et al. (2008), may be attractive candidates for targeted therapeutic intervention.
Abbreviation used in this paper: MKK, MAPK kinase.
References
- Cavaletti, G., M. Miloso, G. Nicolini, A. Scuteri, and G. Tredici. 2007. Emerging role of mitogen-activated protein kinases in peripheral neuropathies. J. Peripher. Nerv. Syst. 12:175–194. [DOI] [PubMed] [Google Scholar]
- Decker, L., C. Desmarquet-Trin-Dinh, E. Taillebourg, J. Ghislain, J.M. Vallat, and P. Charnay. 2006. Peripheral myelin maintenance is a dynamic process requiring constant Krox20 expression. J. Neurosci. 26:9771–9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman, E.L., D.R. Cornblath, J. Porter, R. Dworkin, and S. Scherer. 2008. National Institute of Neurological Disorders and Stroke (NINDS): advances in understanding and treating neuropathy, 24-25 October 2006; Bethesda, Maryland. J. Peripher. Nerv. Syst. 13:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghislain, J., and P. Charnay. 2006. Control of myelination in Schwann cells: a Krox20 cis-regulatory element integrates Oct6, Brn2 and Sox10 activities. EMBO Rep. 7:52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines, J.D., G. Fragoso, S. Hossain, W.E. Mushynski, and G. Almazan. 2008. p38 mitogen-activated protein kinase regulates myelination. J. Mol. Neurosci. 35:23–33. [DOI] [PubMed] [Google Scholar]
- Harrisingh, M.C., E. Perez-Nadales, D.B. Parkinson, D.S. Malcolm, A.W. Mudge, and A.C. Lloyd. 2004. The Ras/Raf/ERK signalling pathway drives Schwann cell dedifferentiation. EMBO J. 23:3061–3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen, K.R., and R. Mirsky. 2005. The origin and development of glial cells in peripheral nerves. Nat. Rev. Neurosci. 6:671–682. [DOI] [PubMed] [Google Scholar]
- Johnson, G.L., and K. Nakamura. 2007. The c-jun kinase/stress-activated pathway: regulation, function and role in human disease. Biochim. Biophys. Acta. 1773:1341–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le, N., R. Nagarajan, J.Y. Wang, T. Araki, R.E. Schmidt, and J. Milbrandt. 2005. Analysis of congenital hypomyelinating Egr2Lo/Lo nerves identifies Sox2 as an inhibitor of Schwann cell differentiation and myelination. Proc. Natl. Acad. Sci. USA. 102:2596–2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel, P., and J.L. Salzer. 2000. Axonal regulation of Schwann cell proliferation and survival and the initial events of myelination requires PI 3-kinase activity. J. Neurosci. 20:4635–4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nave, K.A., and J.L. Salzer. 2006. Axonal regulation of myelination by neuregulin 1. Curr. Opin. Neurobiol. 16:492–500. [DOI] [PubMed] [Google Scholar]
- Ogata, T., S. Iijima, S. Hoshikawa, T. Miura, S. Yamamoto, H. Oda, K. Nakamura, and S. Tanaka. 2004. Opposing extracellular signal-regulated kinase and Akt pathways control Schwann cell myelination. J. Neurosci. 24:6724–6732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson, D.B., A. Bhaskaran, P. Arthur-Farraj, L.A. Noon, A. Woodhoo, A.C. Lloyd, M.L. Feltri, L. Wrabetz, A. Behrens, R. Mirsky, and K.R. Jessen. 2008. c-Jun is a negative regulator of myelination. J. Cell Biol. 181:625–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson, D.B., A. Bhaskaran, A. Droggiti, S. Dickinson, M. D'Antonio, R. Mirsky, and K.R. Jessen. 2004. Krox-20 inhibits Jun-NH2-terminal kinase/c-Jun to control Schwann cell proliferation and death. J. Cell Biol. 164:385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer, S.S., and J.L. Salzer. 2001. Axon-Schwann cell interactions during peripheral nerve degeneration and regeneration. In Glial Cell Development. 2nd Edition. K.R. Jessen and W.D. Richardson, editors. Oxford University Press, London. 299–330.