Abstract
Central nervous system myelination requires the synthesis of large amounts of myelin basic protein (MBP) at the axon–glia contact site. MBP messenger RNA (mRNA) is transported in RNA granules to oligodendroglial processes in a translationally silenced state. This process is regulated by the trans-acting factor heterogeneous nuclear ribonucleoprotein (hnRNP) A2 binding to the cis-acting A2 response element (A2RE). Release of this repression of MBP mRNA translation is thus essential for myelination. Mice deficient in the Src family tyrosine kinase Fyn are hypomyelinated and contain reduced levels of MBP. Here, we identify hnRNP A2 as a target of activated Fyn in oligodendrocytes. We show that active Fyn phosphorylates hnRNP A2 and stimulates translation of an MBP A2RE–containing reporter construct. Neuronal adhesion molecule L1 binding to oligodendrocytes results in Fyn activation, which leads to an increase in hnRNP A2 phosphorylation. These results suggest that Fyn kinase activation results in the localized translation of MBP mRNA at sites of axon–glia contact and myelin deposition.
Introduction
During central nervous system (CNS) myelination, oligodendrocytes extend membrane processes toward an axonal contact site followed by ensheathment, resulting in a compacted multilamellar myelin sheath (Hartline and Colman, 2007). This axon–glia unit facilitates rapid saltatory propagation of action potentials along the axon. The protein content of myelin is dominated by proteolipid protein and myelin basic protein (MBP). The establishment and maintenance of myelin thus requires the specific delivery of large amounts of proteolipid protein and MBP to the axon–glia contact site, which is precisely regulated in time and space (Simons and Trotter, 2007). MBP protein is involved in compaction of the myelin sheath: the importance of MBP is exemplified by the naturally occurring mouse mutant shiverer, in which a large part of the MBP gene is deleted, resulting in a failure to synthesize MBP protein. This culminates in an inability to myelinate the CNS, although oligodendrocyte processes contact and start to wrap axons. The mutant phenotype can be rescued by introduction of transgenic MBP (Popko et al., 1987). Interestingly, the level of MBP expression correlates with the amount of myelin synthesized. Similarly, the Long Evans shaker (les) rat is severely dysmyelinated in the CNS, which results from a lack of MBP expression due to a large insertion in the MBP gene causing aberrant MBP mRNA processing (O'Connor et al., 1999). Glial signals initiating myelination are still largely unknown, but mice deficient in the Src family nonreceptor tyrosine kinase Fyn also exhibit severe hypomyelination in the forebrain (Sperber et al., 2001). Fyn kinase is up-regulated in oligodendrocytes concomitantly with active myelination (Krämer et al., 1999) and is involved in the regulation of process outgrowth in cultured oligodendrocytes by binding to microtubules and the microtubule-associated protein tau (Osterhout et al., 1999; Klein et al., 2002).
Ribosomal subunits and MBP mRNA were isolated from purified myelin (Colman et al., 1982), which suggests the localized translation of MBP mRNA in the myelin compartment. It was later shown that MBP mRNA is transported in a translationally silenced state in RNA granules to the distal processes of cultured oligodendrocytes. These granules contain RNA-binding proteins including heterogeneous nuclear ribonucleoprotein A2 (hnRNP A2). HnRNP A2 binds to a cis-acting element in the MBP mRNA 3′ untranslated region (UTR) termed the A2 response element (A2RE; Ainger et al., 1997; Munro et al., 1999). HnRNP A2 is thought to bind to MBP mRNA in the nucleus, and the complex is then exported to the cytoplasm, where granules are formed and transported in a microtubule-dependent manner along the oligodendrocyte processes (Carson and Barbarese, 2005). The formation of MBP-RNA–containing polysomes is prevented, resulting in translational inhibition that must be relieved when the granule reaches its destination (Kosturko et al., 2006). The signals that result in the induction of localized translation of MBP mRNA are unknown, but because the timing of myelination is precisely regulated concordant with axonal maturation, it is likely that a signaling cascade originating from the neuronal compartment of the axon–glia unit initiates the termination of translational repression of MBP mRNA.
Antibody-induced cross-linking of F3 expressed by oligodendrocytes results in an activation of Fyn kinase in lipid rafts (Krämer et al., 1999). We proposed that in vivo, a neuronal interaction partner binds to oligodendroglial F3, activating Fyn and initiating the myelination process at this axon–glial contact site. Here, we show that activated Fyn in oligodendrocytes leads to tyrosine phosphorylation of hnRNP A2 and translation of an A2RE-containing reporter. We show that L1 binding to oligodendrocytes results in activation of Fyn kinase and the Fyn-dependent phosphorylation of hnRNP A2. We demonstrate that oligodendroglial F3 is a binding partner for neuronal L1. Fyn activation may thus be a critical regulatory element of the myelination process by triggering hnRNP A2 phosphorylation and local activation of MBP mRNA translation at sites of glia–neuronal contact.
Results and discussion
hnRNP A2 is phosphorylated by activated Fyn
To identify downstream targets of F3-triggered signaling events mediated by Fyn activation, we activated Fyn kinase by antibody-mediated cross-linking of oligodendroglial F3 (Krämer et al., 1999) followed by purification of tyrosine-phosphorylated proteins by immunoaffinity chromatography and their identification by mass spectrometry. HnRNP A2 was one of these proteins (Fig. S1, available at http://www.jcb.org/cgi/content/full/jcb.200706164/DC1).
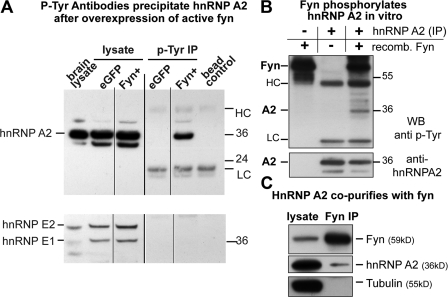
We generated expression vectors coding for the wild type (FynWT) and a constitutively active Fyn mutant (Fyn+; Sette et al., 2002) by site-directed mutagenesis. The oligodendroglial progenitor cell line Oli-neu (Jung et al., 1995; Trajkovic et al., 2006) was transfected with control EGFP or Fyn+ constructs, and tyrosine-phosphorylated proteins were purified from cell lysates by immunoprecipitation with phosphotyrosine-specific antibody-coupled beads. HnRNP A2 was immunoprecipitated in the presence of Fyn+ (Fig. 1 A), which suggests that hnRNP A2 is either phosphorylated directly by Fyn or binds to another protein that is tyrosine phosphorylated in response to activated Fyn. In contrast, hnRNP E1, a binding partner of hnRNP A2 (Kosturko et al., 2006), which is not a target of Src family kinases (Ostareck-Lederer et al., 2002), was not coimmunoprecipitated. To demonstrate that hnRNP A2 is a direct target of Fyn kinase, we performed in vitro kinase assays with purified proteins. Incubation of endogenous hnRNP A2 immunoprecipitated from Oli-neu cells with purified recombinant Fyn kinase in vitro resulted in tyrosine phosphorylation of hnRNP A2 (Fig. 1 B). HnRNP A2 is not phosphorylated in the absence of recombinant Fyn, excluding the possibility that a copurifying tyrosine kinase phosphorylates hnRNP A2.
Figure 1.
hnRNP A2 is phosphorylated by and immunoprecipitates with Fyn kinase. (A) Oli-neu cells were transfected with control EGFP or a constitutively active Fyn (Fyn+) construct. Tyrosine-phosphorylated proteins were immunoprecipitated (P-Tyr IP) from cell lysates (lysate) and analyzed for hnRNP A2 and hnRNP E1/E2. HnRNP A2 immunoprecipitates from cells transfected with active Fyn, whereas hnRNP E1/E2 is absent. Mouse brain lysate and antibody beads alone served as blotting controls. Note that the hnRNP A2 antibody also recognizes the splice variants hnRNP B1 and B0a. HC and LC, heavy and light chain of mouse anti-phosphotyrosine antibody used in the immunopreciptiation. Black lines indicate that intervening lines have been spliced out. (B) Endogenous hnRNP A2 was immunoprecipitated from Oli-neu cells and subjected to an in vitro kinase assay using purified recombinant Fyn kinase. The top shows a phosphotyrosine blot and a band at 36 kD only in the presence of recombinant Fyn. This was identified as hnRNP A2 by reblotting with an hnRNP A2 antibody (bottom). Numbers to the right of the gel blots indicate molecular mass in kD. (C) HnRNP A2 coimmunoprecipitates with Fyn from Oli-neu cells transfected with wild-type Fyn, whereas a control protein (α-tubulin) does not.
We then analyzed whether hnRNP A2 and Fyn interact in oligodendrocytes. Oli-neu cells were transfected with a FynWT plasmid and Fyn was immunoprecipitated with polyclonal antibodies. Western blots with monoclonal antibodies recognizing Fyn or hnRNP A2 showed that hnRNP A2 coimmunoprecipitates with Fyn (Fig. 1 C). These results demonstrate that Fyn binds and phosphorylates hnRNP A2 directly.
Activated Fyn enhances translation of an A2RE-containing luciferase reporter in the oligodendroglial cell line Oli-neu
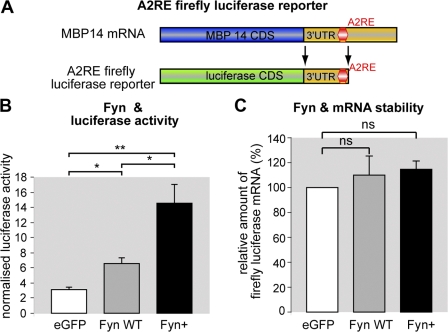
In a variety of cells, including oligodendrocytes, distinct mRNAs are transported in a translationally silenced state in RNA–protein complexes termed RNA granules until they reach their destination, where a specific signal triggers local translation (Czaplinski and Singer, 2006; Kiebler and Bassell, 2006). Src family kinases have been shown to phosphorylate RNA-binding proteins in these granules, leading to the liberation of the mRNA and triggering local protein synthesis (Ostareck-Lederer et al., 2002; Huttelmaier et al., 2005). HnRNP A2 has been shown to bind to an 11-nucleotide–containing region in the 3′ UTR of distinct mRNAs including MBP (Munro et al., 1999). This cis-acting element is termed A2RE, and in oligodendrocytes, the binding of the trans-acting factor hnRNP A2 to the A2RE of MBP mediates localization of MBP mRNA to the distal tips of oligodendroglial processes. It has been further suggested that translational repression is dependent on the recruitment of hnRNP E1 by hnRNP A2 to A2RE-containing mRNAs in granules (Kosturko et al., 2006). To assess if Fyn affects translation of MBP mRNA, a region of the 3′ UTR of MBP including the A2RE was cloned downstream of a firefly luciferase reporter (Fig. 2 A; Huttelmaier et al., 2005). This translational reporter was cotransfected with EGFP (control), FynWT, or Fyn+ into Oli-neu cells, and a DualGlo luciferase assay was performed to quantify the amount of translated luciferase reporter. The activities of the A2RE firefly reporter and renilla luciferase were normalized to compensate for potential effects of Fyn on general cellular translation processes or transfection variations. Compared with EGFP-transfected control cells, the overexpression of FynWT significantly increases the amount of normalized A2RE luciferase reporter activity. Furthermore, overexpression of Fyn+ further augments reporter activity (Fig. 2 B). To verify that Fyn overexpression does not affect the mRNA stablility of the A2RE luciferase reporter, total RNA was isolated from the transfected Oli-neu cells used in the luciferase assays and analyzed by quantitative RT-PCR (qRT-PCR). As illustrated in Fig. 2 C, the amount of normalized firefly luciferase A2RE reporter mRNA does not differ significantly in FynWT- and Fyn+-transfected cells compared with EGFP control cells. These experiments thus show that Fyn activity stimulates translation of the A2RE-containing region of MBP mRNA. Intriguingly, it has been reported that Fyn activity leads to enhanced MBP gene transcription, which is mediated by a distinct region in the MBP promoter that was not included in our construct (Umemori et al., 1999). As Fyn kinase activation is crucial for myelination, it is likely that Fyn activity affects both synthesis and translation of MBP mRNA. The observed reduction of MBP protein levels in the Fyn knockout mouse (Umemori et al., 1999; Sperber et al., 2001) can be explained by the observed effect on MBP transcription and, furthermore, by the role of Fyn activation in stimulating MBP mRNA translation reported here.
Figure 2.
Fyn kinase enhances translation of A2RE containing mRNA. (A) An A2RE-containing region of the 3′ UTR of MBP14 mRNA was cloned downstream of the firefly luciferase coding sequence (CDS) and used as a translational reporter in the DualGlo luciferase assay. (B) Oli-neu cells were nucleofected with an A2RE containing firefly luciferase reporter, a renilla luciferase control, and either wild-type Fyn (Fyn WT), constitutively active Fyn (Fyn+), or EGFP. Firefly was normalized to renilla luciferase activity for every measurement in all experiments (n = 3). (C) Total RNA was isolated from the cells used in the luciferase assay shown in B. qRT-PCR was performed to compare A2RE firefly luciferase mRNA from Fyn (WT and Fyn+)-transfected cells with EGFP-transfected cells (renilla was used for normalization). Error bars indicate SEM. Significance was assessed with t tests: *, P < 0.05; **, P < 0.01; ns, not significant.
L1 binds to oligodendroglial F3/contactin
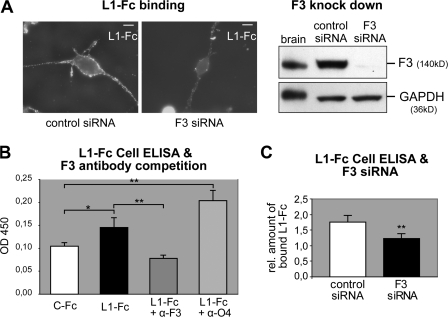
F3 is expressed by neurons (Ranscht, 1988; Gennarini et al., 1989) and, additionally, by oligodendrocytes (Einheber et al., 1997; Koch et al., 1997). Stimulation of Fyn kinase activity by antibody-mediated cross-linking of oligodendroglial F3 may mimic the binding of a neuronal ligand such as L1 cell adhesion molecule (L1-CAM; Krämer et al., 1999), and L1-F3 binding in trans has been demonstrated previously (Brümmendorf et al., 1993; Perrin et al., 2001). We expressed a recombinant L1-Fc fusion protein (the extracellular domain of mouse L1-CAM fused to the Fc region of human IgG (Oleszewski et al., 1999) in Cos 7 cells and purified L1-Fc by affinity chromatography (Fig. S2, available at http://www.jcb.org/cgi/content/full/jcb.200706164/DC1). Living Oli-neu cells were incubated with L1-Fc, and bound L1-Fc was visualized with Cy2-coupled anti–human antibodies. The binding to Oli-neu cells was considerably reduced by suppressing expression of F3 protein with siRNA (Fig. 3 A). Binding of L1-Fc measured by cell enzyme-linked immunosorbent assay (ELISA) is significantly reduced in the presence of monoclonal F3 antibodies or F3 siRNA (Fig. 3, B and C), providing evidence that the interaction with F3 contributes significantly to the L1 binding. However, it is likely that L1 binds additional surface molecules on oligodendrocytes such as β1 integrins (Oleszewski et al., 1999), which are expressed by these cells (ffrench-Constant and Colognato, 2004).
Figure 3.
L1-Fc binds to oligodendroglial F3. (A) L1-Fc was bound to Oli-neu cells in the presence of control or F3 siRNA and detected with an anti–human Fc Cy2 antibody (left). F3 knockdown was analyzed by Western blotting of the cells used for L1-Fc binding. Mouse brain lysate was used as control and GAPDH served as a loading control (right). Bars, 10 μm. (B) Differentiated Oli-neu cells were incubated with 75 nM of control Fc (human IgG, C-Fc) or L1-Fc and 75 nM L1-Fc in the presence of monoclonal F3 or O4 (control) antibodies. Binding was quantified by cell ELISA (see Materials and methods; n = 6). (C) Oli-neu cells were treated with 0 or 25 nM L1-Fc after treatment with control or F3 siRNA. Binding was quantified by cell ELISA and the ratio of 25 nM Li-Fc–treated cells/0 nM L1-Fc–treated cells was plotted to express the relative amount of bound L1-Fc in control and F3 siRNA-treated cells. Note that because of a different experimental setup, the reduction of F3 protein levels was not as efficient as in the experiment shown in A (n = 9). Error bars indicate SEM. Significance was assessed by t tests: *, P < 0.02; **, P < 0.01.
L1 binding activates Fyn kinase and leads to Fyn-dependent phosphorylation of hnRNP A2
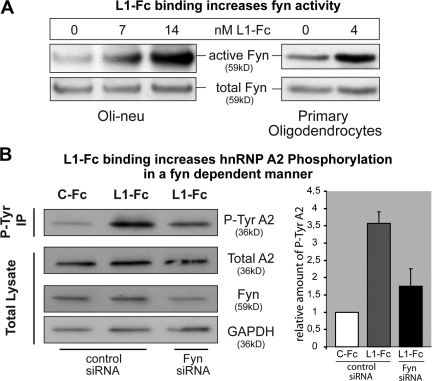
Differentiated Oli-neu cells or primary oligodendrocytes were incubated with L1-Fc, and total cell lysates were analyzed by Western blotting using antibodies recognizing either activated Src family kinases, including Fyn, or total Fyn protein. L1-Fc binding stimulated oligodendroglial Fyn (Fig. 4 A). Binding of a control human immunoglobulin did not induce such enhanced activation of Fyn (Fig. S3 A, available at http://www.jcb.org/cgi/content/full/jcb.200706164/DC1). Furthermore, L1-Fc binding stimulated phosphorylation of hnRNP A2 in primary oligodendrocytes (Fig. S3 B) and Oli-neu cells (Fig. 4 B). This increase in phosphorylation of hnRNP A2 was strongly reduced by Fyn siRNA (Fig. 4 B), validating the fact that Fyn mediates the L1-Fc–triggered A2 phosphorylation.
Figure 4.
L1-Fc binding activates Fyn kinase and leads to Fyn-dependent hnRNP A2 phosphorylation. (A) Differentiated Oli-neu cells or primary oligodendrocytes were treated with the indicated concentrations of L1-Fc. Equal amounts of cell lysates were analyzed by Western blotting using the indicated antibodies. Numbers on top indicate L1-Fc concentration in nM. (B, left) Oli-neu cells were incubated with control Fc (C-Fc, human IgG) or L1-Fc in the presence of control or Fyn siRNA. Tyrosine-phosphorylated proteins were immunoprecipitated (P-Tyr IP) and analyzed by Western blotting for hnRNP A2 (P-Tyr A2). Levels of tyrosine-phosphorylated A2 are strongly increased in L1-Fc–treated cells compared with control Fc–treated cells, and this effect is reduced in cells treated with Fyn siRNA. Total lysates (before IP) were analyzed by Western blotting with hnRNP A2, Fyn, and GAPDH antibodies and demonstrated unchanged levels of total hnRNP A2 and a reduction of Fyn protein by Fyn siRNA. GAPDH served as a loading control. (B, right) The diagram represents the data from three such experiments. Protein bands of tyrosine-phosphorylated hnRNP A2 were densitometrically quantified and the values of control Fc–treated cells were set to 1. The relative increase of tyrosine-phosphorylated hnRNP A2 in L1-Fc–treated cells in the presence of control and Fyn siRNA was plotted. Error bars indicate SEM; n = 3.
We thus demonstrated that L1-Fc binds to oligodendrocytes; F3 is one of the partners and L1-Fc binding leads to an activation of Fyn kinase in a similar fashion to antibody-mediated ligation of oligodendroglial F3. Moreover, L1-Fc binding stimulates Fyn-dependent phosphorylation of the hnRNP A2 protein. However, Fyn activation may additionally occur by alternative pathways as shown in Fig. 5 C, including ligation of integrins (Colognato et al., 2004; Liang et al., 2004) or immunoglobulin γFc receptors (Nakahara et al., 2003). Fyn activity is increased in oligodendrocytes grown on laminin 2, a ligand for α6β1 integrins (Colognato et al., 2004); however, β1 integrin knockout mice and L1 knockout mice myelinate normally (Benninger et al., 2006). In contrast, F3 knockout mice show myelination abnormalities in the CNS (Fernandes, F., U. Bergstrom, and B. Ranscht. 2007. Society for Neuroscience, Neuroscience Meeting. Abstr. 459). The cis association of F3 in oligodendrocytes with another transmembrane molecule such as Caspr or protein tyrosine phosphatase α, as shown in neurons, may aid in F3-mediated signal transduction (Peles et al., 1997; Zeng et al., 1999).
Figure 5.
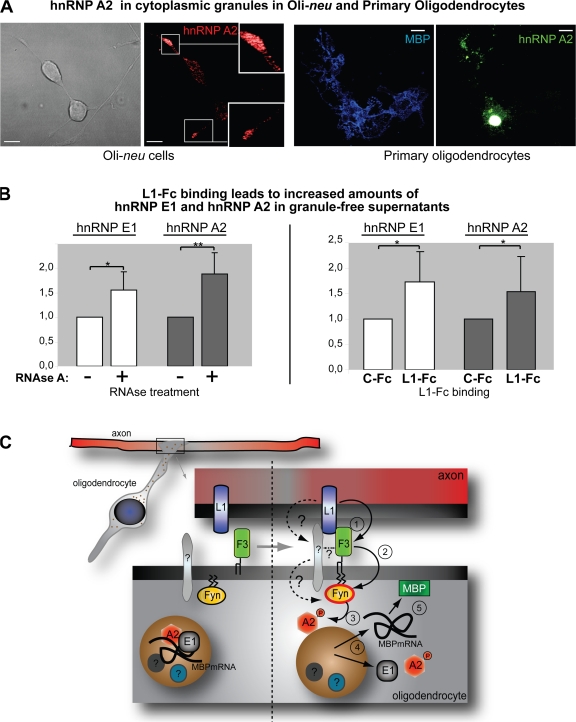
HnRNP E1 and A2 are released from granules. (A) Oli-neu cells and primary oligodendrocytes were transfected with full-length hnRNP A2 and immunostained for hnRNP A2 or MBP. Images were acquired by confocal microscopy, and either a single slice (Oli-neu) or the complete stack (primary oligodendrocytes) is depicted. HnRNP A2–containing granules are present in the processes of Oli-neu cells as well as primary oligodendrocytes. Insets show an enlarged view of the boxed sections. Bars, 10 μm. (B) A granule-free supernatant was analyzed by Western blotting for hnRNP E1 and A2 proteins after RNase A treatment or L1-Fc binding. Western blot bands were analyzed densitometrically from 8 and 15 experiments for RNase treatment and L1-Fc binding, respectively. The control values (RNase A and C-Fc) were set to 1 and the mean relative increase of hnRNP E1 and A2 in the granule-free fraction was plotted in response to RNase A treatment or L1-Fc binding. Error bars indicate SEM; significance was tested with t tests: *, P ≤ 0.05; **, P ≤ 0.01. n = 8 (RNase A) and n = 15 (L1-Fc). (C) The model illustrates the proposed events: During initial axon–glial contacts, neuronal L1 binds glial F3 (1), leading to an activation of Fyn (2), which phosphorylates hnRNP A2 (3). This leads to a release of hnRNP A2 and E1 from the granule and liberation of MBP mRNA (4) at the axon–glial contact site, allowing localized synthesis of the MBP protein (5) required for generation of the myelin sheath. The dotted lines illustrate potential alternative activation pathways of Fyn kinase mediated by L1 binding.
L1-Fc binding triggers the release of hnRNP E1 and hnRNP A2 from RNA granules
HnRNP A2–containing RNA granules can be detected in processes of Oli-neu cells as well as in primary mouse oligodendrocytes (Fig. 5 A). In oligodendrocytes, hnRNP E1 has been shown to be associated with granules, and it has been suggested that release of this protein from the granules may be critical for releasing translational repression of MBP mRNA (Carson et al., 2006). This release of hnRNP E1 would result in an increase of soluble hnRNP E1 in the cytoplasm. We treated a postnuclear supernatant of an Oli-neu cell lysate with RNase A to degrade the RNA and dismantle these ribonucleoprotein complexes. High-speed ultracentrifugation was used to pellet the remaining RNA granules and other cellular organelles, yielding a granule-free cytosolic fraction containing soluble proteins. RNase A treatment followed by high-speed ultracentrifugation leads to increased levels of hnRNP A2 and E1 in the supernatant (Fig. 5 B). Furthermore, an increase of hnRNP E1 and hnRNP A2 could be detected in the granule-free supernatants of Oli-neu cells in response to L1-Fc binding, which was not seen after incubation with human IgG (Fig. 5 B).
As depicted (Fig. 5 C), we propose that oligodendroglial Fyn is activated by the binding of neuronal L1 to glial receptors including F3. L1 is down-regulated on myelinated axons in culture (Coman et al., 2005), which suggests that there is a functional role of L1 in the early phases of myelination. Activated Fyn phosphorylates hnRNP A2 in granules that have reached the axon–glia contact site. HnRNP E1 and hnRNP A2 are then released from the transport granules, resulting in termination of repression of MBP mRNA, which is then translated locally at the site of axon–glia contact. Synthesis of MBP protein at the site of myelin formation is thus ensured. Because of the basic nature of MBP and the large amounts required for myelin biogenesis, MBP translation at other locations could result in the association and compaction of intracellular membranes and dysfunction. We proposed that Fyn stabilizes and recruits microtubules toward the axon–glia contact site by binding tau and tubulin (Klein et al., 2002). RNA granules are transported on microtubules, and thus local activation of Fyn at sites of axon–glia contact could recruit microtubules that direct MBP mRNA in RNA granules to this location. Localized MBP synthesis could then be initiated by phosphorylation of hnRNP A2. This is in agreement with the proposed role of Fyn in early myelinogenesis (Krämer et al., 1999; Osterhout et al., 1999; Sperber et al., 2001) and the interplay of L1 and Fyn we report here. Our findings present a functional link between axon–glia signaling and myelinogenesis and suggest the involvement of translational control of myelin proteins in site-specific myelin deposition.
Materials and methods
Antibodies
Polyclonal antibodies against Fyn, hnRNP E1 (Santa Cruz Biotechnology, Inc.), and Src-pY418, which recognizes activated Fyn (Invitrogen), were used. Monoclonal antibodies against hnRNP A2 (EF67; provided by W. Rigby, Dartmouth Medical School, Lebanon, NH), α-tubulin DM1A (Sigma-Aldrich), Fyn (BD Biosciences), F3 (clone 11-111), and L1 (clone 555; provided by F. Rathjen, Max Delbrück Center for Molecular Medicine, Berlin, Germany) were used. Anti-phosphotyrosine (clone 4G10) antibody and 4G10-conjugated agarose beads (Millipore) were also used.
Plasmid construction and RNAi
Wild-type Fyn cDNA (provided by B. Schraven, University of Magdeburg, Magdeburg, Germany) was cloned into the NheI–EcoRI site of pEGFP-C3 (Clontech Laboratories, Inc.), thereby replacing EGFP. Constitutively active Fyn (Fyn+) was obtained by site-directed mutagenesis (Quikchange II; Stratagene) by changing tyrosine 528 to phenylalanine (Sette et al., 2002). Full-length hnRNP A2 cDNA was obtained by RT-PCR from total Oli-neu cell RNA and cloned downstream of a FLAG tag in pcDNA3.1 (Invitrogen) as described previously (Huttelmaier et al., 2005). A 378-nucleotide-long portion of the 3′ UTR of MBP mRNA containing the A2RE was amplified by PCR from MBP14 3′ UTR containing template cDNA (provided by M. Simons, Max-Planck Institute of Experimental Medicine, Göttingen, Germany) using two primers (5′-CGAATTCCTCAGCCTTCCCGAATCC-3′ and 5′-GGCTCGAGATGCTCTCTGGCTCCTT-3′), and we fused the 3′ end of the coding sequence of firefly luciferase into the EcoRI and XhoI sites of the pcDNA 3.1 vector (Huttelmaier et al., 2005). Plasmids were transfected either by electroporation (Genepulser; Bio-Rad Laboratories) or by nucleofection (Amaxa Biosystems).
F3- and Fyn-directed synthetic siRNA (target sequences: 5′-CAGGTCTTTCATAGTACTCAA-3′ and 5′-CTCGTTGTTTCTGGAGAAGAA-3′, respectively) and nonsilencing control siRNA (target sequence 5′-AATTCTCCGAACGTGTCACGT-3′) were obtained from QIAGEN and transfected using Amaxa Biosystems technology.
Expression and purification of L1-Fc
L1-Fc (provided by P. Altevogt, German Cancer Center, Heidelberg, Germany; Oleszewski et al., 1999) was expressed in Cos 7 cells. The secreted fusion protein was purified from the medium using a HighTrap protein A column (GE Healthcare) and dialyzed in PBS.
L1-Fc binding
Oli-neu cells were transfected with F3 or nonsilencing control siRNA and plated into culture dishes containing coverslips. Coverslips were incubated with L1-Fc (150 μg/ml in PBS containing 0.3% [vol/vol] normal goat serum [NGS]) on ice for 60 min, washed, fixed with PFA, and blocked with 10% (vol/vol) NGS in PBS. L1-Fc was detected with a goat anti–human secondary antibody coupled to Cy2. The remaining cells in the dish were lysed and proteins were analyzed by SDS-PAGE and Western blotting.
L1-Fc cell ELISA
Oli-neu cells were transfected with siRNA and grown in the presence of 1 mM dibutyryl-cAMP to induce differentiation. Cells were incubated in Sato medium containing 1% horse serum with 75 nM C-Fc (human IgG, control) or L1-Fc for 1 h at 4°C, washed, and fixed with 4% PFA. After blocking (30 min with PBS and 10% FCS at RT), HRP-coupled anti–human Fc antibody was incubated for 60 min and the cells were washed. Tetramethylbenzidine (Thermo Fisher Scientific) was used to detect bound HRP according to the manufacturer's instructions. Color development was measured in an ELISA reader and the ratio of L1-Fc treated and untreated control cells was plotted for control siRNA– and F3 siRNA–treated cells.
For antibody blocking experiments, untransfected differentiated Oli-neu cells were treated as stated above, but F3 or O4 (control) antibodies were added during the incubation with 75 nM L1-Fc.
Luciferase assay and RNA stability
Oli-neu cells (106) were nucleofected with 250 ng A2RE firefly luciferase reporter construct, 100 ng renilla luciferase, 650 ng pEGFP C3 (Clontech Laboratories, Inc.), and 1 μg of either wild-type Fyn (FynWT), constitutively active Fyn (Fyn+), or pEGFP-C3 plasmids. After 2 d, cells were scraped off in PBS and a luciferase assay was performed according the manufacturer's instructions (Dualglo; Promega). Firefly luciferase activities were normalized with renilla luciferase activities. Total RNA was isolated from remaining cells and analyzed by qRT-PCR for firefly and renilla luciferase mRNA. The amount of normalized firefly luciferase in FynWT- and Fyn+-transfected cells was related to EGFP-transfected cells.
Kinase assay
Immunoprecipitated hnRNP A2 from Oli-neu cells was incubated on protein G beads in kinase buffer (40 mM Pipes, pH 7.02, 10 mM MgCl2, and 50 μM ATP) either with or without 40 ng of recombinant human FynT kinase (Invitrogen) for 20 min at 30°C. The reaction was stopped and proteins were eluted by boiling the beads in sample buffer.
Cell culture, immunofluorescence, and immunoprecipitation
Primary oligodendrocytes were obtained from E14-16 mice as described previously (Krämer et al., 1999). Oli-neu cells (Jung et al., 1995) were cultured in Sato medium containing 1% horse serum and differentiated by the addition of 1 mM dibutyryl-cAMP if applicable. Cells were fixed with 4% PFA and immunofluorescence staining was performed as described previously (Krämer-Albers et al., 2006). Cells were mounted in moviol and confocal images were acquired with a microscope (TCS SP5) using a 63× 1.4 NA oil lens and LAS AF software (all from Leica). Nonconfocal images were acquired with a microscope (DMLB) with a 100× 1.3 NA oil objective lens connected to a digital camera (DFC 350F) using Application Suite 2.5.0 software (all from Leica). Images were adjusted using Photoshop (Adobe).
Tyrosine-phosphorylated proteins were purified by immunoprecipitation using 4G10 antibody–coupled agarose according to the manufacturer's instructions (Millipore).
Fyn activation by L1-Fc
Differentiated Oli-neu cells were treated with different concentrations of L1-Fc in 3% (wt/vol) BSA for 1 h at 4°C, washed with PBS, and incubated for 5 min at 37°C with anti–human Fc antibody (1:100 in 3% [wt/vol] BSA). Control cells were treated with anti–human Fc antibody alone or human IgG control Fc and anti–human Fc (Fig. S3). Cells were washed twice with PBS and lysed, and equal amounts of postnuclear supernatants were analyzed by Western blotting.
Online supplemental material
Fig. S1 shows the purification of tyrosine-phosphorylated proteins after antibody-mediated F3 stimulation, and hnRNP A2 peptides identified by mass spectrometry. Fig. S2 demonstrates the quality of purified recombinant L1-Fc protein by Coomassie staining and Western blot analysis. Fig. S3 shows that the L1 portion of the L1-Fc fusion protein leads to Fyn activation and increased hnRNP A2 phosphorylation. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200706164/DC1.
Supplementary Material
Acknowledgments
We thank W. Rigby (hnRNP A2 antibody), F. Rathjen (F3 and L1 antibodies), P. Altevogt (L1-Fc expression vector), M. Simons (MBP14 cDNA), and B. Schraven (Fyn cDNA) for generous gifts and K. Bruns for help with mass spectrometry. We also would like to thank U. Stapf and L. Niedens for excellent technical help.
We thank the Deutsche Forschungsgemeinschaft (Neuro-Graduate School scholarship to R. White, priority program SPP “Cell Polarity” to J. Trotter) and the European Union Sixth Framework Programme (“Signalling and Traffic” to J. Trotter) for financial support.
Abbreviations used in this paper: A2RE, A2 response element; CNS, central nervous system; ELISA, enzyme-linked immunosorbent assay; hnRNP, heterogeneous nuclear ribonucleoprotein; MBP, myelin basic protein; qRT-PCR, quantitative RT-PCR; UTR, untranslated region.
References
- Ainger, K., D. Avossa, A.S. Diana, C. Barry, E. Barbarese, and J.H. Carson. 1997. Transport and localization elements in myelin basic protein mRNA. J. Cell Biol. 138:1077–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benninger, Y., H. Colognato, T. Thurnherr, R.J. Franklin, D.P. Leone, S. Atanasoski, K.A. Nave, C. Ffrench-Constant, U. Suter, and J.B. Relvas. 2006. Beta1-integrin signaling mediates premyelinating oligodendrocyte survival but is not required for CNS myelination and remyelination. J. Neurosci. 26:7665–7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brümmendorf, T., M. Hubert, U. Treubert, R. Leuschner, A. Tarnok, and F.G. Rathjen. 1993. The axonal recognition molecule F11 is a multifunctional protein: specific domains mediate interactions with Ng-CAM and restrictin. Neuron. 10:711–727. [DOI] [PubMed] [Google Scholar]
- Carson, J.H., and E. Barbarese. 2005. Systems analysis of RNA trafficking in neural cells. Biol. Cell. 97:51–62. [DOI] [PubMed] [Google Scholar]
- Carson, J.H., N. Blondin, and G. Korza. 2006. Rules of engagement promote polarity in RNA trafficking. BMC Neurosci. 7:S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman, D.R., G. Kreibich, A.B. Frey, and D.D. Sabatini. 1982. Synthesis and incorporation of myelin polypeptides into CNS myelin. J. Cell Biol. 95:598–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colognato, H., S. Ramachandrappa, I.M. Olsen, and C. ffrench-Constant. 2004. Integrins direct Src family kinases to regulate distinct phases of oligodendrocyte development. J. Cell Biol. 167:365–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coman, I., G. Barbin, P. Charles, B. Zalc, and C. Lubetzki. 2005. Axonal signals in central nervous system myelination, demyelination and remyelination. J. Neurol. Sci. 233:67–71. [DOI] [PubMed] [Google Scholar]
- Czaplinski, K., and R.H. Singer. 2006. Pathways for mRNA localization in the cytoplasm. Trends Biochem. Sci. 31:687–693. [DOI] [PubMed] [Google Scholar]
- Einheber, S., G. Zanazzi, W. Ching, S. Scherer, T.A. Milner, E. Peles, and J.L. Salzer. 1997. The axonal membrane protein Caspr, a homologue of neurexin IV, is a component of the septate-like paranodal junctions that assemble during myelination. J. Cell Biol. 139:1495–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ffrench-Constant, C., and H. Colognato. 2004. Integrins: versatile integrators of extracellular signals. Trends Cell Biol. 14:678–686. [DOI] [PubMed] [Google Scholar]
- Gennarini, G., G. Cibelli, G. Rougon, M.G. Mattei, and C. Goridis. 1989. The mouse neuronal cell surface protein F3: a phosphatidylinositol-anchored member of the immunoglobulin superfamily related to chicken contactin. J. Cell Biol. 109:775–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartline, D.K., and D.R. Colman. 2007. Rapid conduction and the evolution of giant axons and myelinated fibers. Curr. Biol. 17:R29–R35. [DOI] [PubMed] [Google Scholar]
- Huttelmaier, S., D. Zenklusen, M. Lederer, J. Dictenberg, M. Lorenz, X. Meng, G.J. Bassell, J. Condeelis, and R.H. Singer. 2005. Spatial regulation of beta-actin translation by Src-dependent phosphorylation of ZBP1. Nature. 438:512–515. [DOI] [PubMed] [Google Scholar]
- Jung, M., E. Kramer, M. Grzenkowski, K. Tang, W. Blakemore, A. Aguzzi, K. Khazaie, K. Chlichlia, G. von Blankenfeld, H. Kettenmann, et al. 1995. Lines of murine oligodendroglial precursor cells immortalized by an activated neu tyrosine kinase show distinct degrees of interaction with axons in vitro and in vivo. Eur. J. Neurosci. 7:1245–1265. [DOI] [PubMed] [Google Scholar]
- Kiebler, M.A., and G.J. Bassell. 2006. Neuronal RNA granules: movers and makers. Neuron. 51:685–690. [DOI] [PubMed] [Google Scholar]
- Klein, C., E.M. Kramer, A.M. Cardine, B. Schraven, R. Brandt, and J. Trotter. 2002. Process outgrowth of oligodendrocytes is promoted by interaction of fyn kinase with the cytoskeletal protein tau. J. Neurosci. 22:698–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch, T., T. Brugger, A. Bach, G. Gennarini, and J. Trotter. 1997. Expression of the immunoglobulin superfamily cell adhesion molecule F3 by oligodendrocyte-lineage cells. Glia. 19:199–212. [DOI] [PubMed] [Google Scholar]
- Kosturko, L.D., M.J. Maggipinto, G. Korza, J.W. Lee, J.H. Carson, and E. Barbarese. 2006. Heterogeneous nuclear ribonucleoprotein (hnRNP) E1 binds to hnRNP A2 and inhibits translation of A2 response element mRNAs. Mol. Biol. Cell. 17:3521–3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krämer, E.M., C. Klein, T. Koch, M. Boytinck, and J. Trotter. 1999. Compartmentation of Fyn kinase with glycosylphosphatidylinositol-anchored molecules in oligodendrocytes facilitates kinase activation during myelination. J. Biol. Chem. 274:29042–29049. [DOI] [PubMed] [Google Scholar]
- Krämer-Albers, E.M., K. Gehrig-Burger, C. Thiele, J. Trotter, and K.A. Nave. 2006. Perturbed interactions of mutant proteolipid protein/DM20 with cholesterol and lipid rafts in oligodendroglia: implications for dysmyelination in spastic paraplegia. J. Neurosci. 26:11743–11752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, X., N.A. Draghi, and M.D. Resh. 2004. Signaling from integrins to Fyn to Rho family GTPases regulates morphologic differentiation of oligodendrocytes. J. Neurosci. 24:7140–7149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro, T.P., R.J. Magee, G.J. Kidd, J.H. Carson, E. Barbarese, L.M. Smith, and R. Smith. 1999. Mutational analysis of a heterogeneous nuclear ribonucleoprotein A2 response element for RNA trafficking. J. Biol. Chem. 274:34389–34395. [DOI] [PubMed] [Google Scholar]
- Nakahara, J., K. Tan-Takeuchi, C. Seiwa, M. Gotoh, T. Kaifu, A. Ujike, M. Inui, T. Yagi, M. Ogawa, S. Aiso, et al. 2003. Signaling via immunoglobulin Fc receptors induces oligodendrocyte precursor cell differentiation. Dev. Cell. 4:841–852. [DOI] [PubMed] [Google Scholar]
- O'Connor, L.T., B.D. Goetz, J.M. Kwiecien, K.H. Delaney, A.L. Fletch, and I.D. Duncan. 1999. Insertion of a retrotransposon in Mbp disrupts mRNA splicing and myelination in a new mutant rat. J. Neurosci. 19:3404–3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleszewski, M., S. Beer, S. Katich, C. Geiger, Y. Zeller, U. Rauch, and P. Altevogt. 1999. Integrin and neurocan binding to L1 involves distinct Ig domains. J. Biol. Chem. 274:24602–24610. [DOI] [PubMed] [Google Scholar]
- Ostareck-Lederer, A., D.H. Ostareck, C. Cans, G. Neubauer, K. Bomsztyk, G. Superti-Furga, and M.W. Hentze. 2002. c-Src-mediated phosphorylation of hnRNP K drives translational activation of specifically silenced mRNAs. Mol. Cell. Biol. 22:4535–4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterhout, D.J., A. Wolven, R.M. Wolf, M.D. Resh, and M.V. Chao. 1999. Morphological differentiation of oligodendrocytes requires activation of Fyn tyrosine kinase. J. Cell Biol. 145:1209–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peles, E., M. Nativ, M. Lustig, M. Grumet, J. Schilling, R. Martinez, G.D. Plowman, and J. Schlessinger. 1997. Identification of a novel contactin-associated transmembrane receptor with multiple domains implicated in protein-protein interactions. EMBO J. 16:978–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin, F.E., F.G. Rathjen, and E.T. Stoeckli. 2001. Distinct subpopulations of sensory afferents require F11 or axonin-1 for growth to their target layers within the spinal cord of the chick. Neuron. 30:707–723. [DOI] [PubMed] [Google Scholar]
- Popko, B., C. Puckett, E. Lai, H.D. Shine, C. Readhead, N. Takahashi, S.W. Hunt III, R.L. Sidman, and L. Hood. 1987. Myelin deficient mice: expression of myelin basic protein and generation of mice with varying levels of myelin. Cell. 48:713–721. [DOI] [PubMed] [Google Scholar]
- Ranscht, B. 1988. Sequence of contactin, a 130-kD glycoprotein concentrated in areas of interneuronal contact, defines a new member of the immunoglobulin supergene family in the nervous system. J. Cell Biol. 107:1561–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sette, C., M.P. Paronetto, M. Barchi, A. Bevilacqua, R. Geremia, and P. Rossi. 2002. Tr-kit-induced resumption of the cell cycle in mouse eggs requires activation of a Src-like kinase. EMBO J. 21:5386–5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons, M., and J. Trotter. 2007. Wrapping it up: the cell biology of myelination. Curr. Opin. Neurobiol. 17:533–540. [DOI] [PubMed] [Google Scholar]
- Sperber, B.R., E.A. Boyle-Walsh, M.J. Engleka, P. Gadue, A.C. Peterson, P.L. Stein, S.S. Scherer, and F.A. McMorris. 2001. A unique role for Fyn in CNS myelination. J. Neurosci. 21:2039–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trajkovic, K., A.S. Dhaunchak, J.T. Goncalves, D. Wenzel, A. Schneider, G. Bunt, K.A. Nave, and M. Simons. 2006. Neuron to glia signaling triggers myelin membrane exocytosis from endosomal storage sites. J. Cell Biol. 172:937–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemori, H., Y. Kadowaki, K. Hirosawa, Y. Yoshida, K. Hironaka, H. Okano, and T. Yamamoto. 1999. Stimulation of myelin basic protein gene transcription by Fyn tyrosine kinase for myelination. J. Neurosci. 19:1393–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng, L., L. D'Alessandri, M.B. Kalousek, L. Vaughan, and C.J. Pallen. 1999. Protein tyrosine phosphatase α (PTPα) and contactin form a novel neuronal receptor complex linked to the intracellular tyrosine kinase fyn. J. Cell Biol. 147:707–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.