Abstract
During anaphase, the nonkinetochore microtubules in the spindle midzone become compacted into the central spindle, a structure which is required to both initiate and complete cytokinesis. We show that Tektin 2 (Tek2) associates with the spindle poles throughout mitosis, organizes the spindle midzone microtubules during anaphase, and assembles into the midbody matrix surrounding the compacted midzone microtubules during cytokinesis. Tek2 small interfering RNA (siRNA) disrupts central spindle organization and proper localization of MKLP1, PRC1, and Aurora B to the midzone and prevents the formation of a midbody matrix. Video microscopy revealed that loss of Tek2 results in binucleate cell formation by aberrant fusion of daughter cells after cytokinesis. Although a myosin II inhibitor, blebbistatin, prevents actin-myosin contractility, the microtubules of the central spindle are compacted. Strikingly, Tek2 siRNA abolishes this actin-myosin–independent midzone microtubule compaction. Thus, Tek2-dependent organization of the central spindle during anaphase is essential for proper midbody formation and the segregation of daughter cells after cytokinesis.
Introduction
During mitosis, the bipolar spindle serves to segregate sister chromatids into the two daughter cells. As the chromosomes move toward the spindle poles at anaphase, the overlapping nonkinetochore microtubules in the midzone bundle into a structure known as the central spindle (Straight and Field, 2000; Glotzer, 2005), which serves to stimulate the initiation of the cleavage furrow and ensure completion of cytokinesis (Wheatley, and Wang, 1996; for reviews see Oegema and Mitchison, 1997; McCollum, 2004). As the cleavage furrow constricts the central spindle during cytokinesis, the midzone microtubules become highly compacted into a transient structure known as the midbody, which connects the two daughter cells after division and is later abscised (for review see Otegui et al., 2005). At the center of the midbody, the overlapping microtubules are surrounded by an electron-dense structure known as the midbody matrix or Flemming body (Mullins and Biesele, 1977). The microtubules in the midbody matrix are remarkably stable to depolymerization, suggesting that the midbody matrix imparts stability to the overlapping plus ends (Salmon et al., 1976; Mullins and McIntosh, 1982).
Several key mitotic regulators have been shown to redistribute to the central spindle during anaphase and then become highly organized into the midbody during cytokinesis (Skop et al., 2004; for review see Otegui et al., 2005). These include the centralspindlin complex, Polo-like kinase, the microtubule bundling protein PRC1, and the chromosome passenger complex, containing INCENP, survivin, borealin, and Aurora B kinase (for reviews see Adams et al., 2001; Vader et al., 2006). Disruption of these midzone components results in the lack of proper midbody assembly and failure of cytokinesis (Mishima et al., 2002; Mollinari et al., 2002; Matuliene, and Kuriyama, 2002).
Tektin 2 (the mammalian homologue of the sea urchin tektin B protein) was previously proposed to be a component of the mitotic spindle matrix (Pickett-Heaps et al., 1984; Steffen, and Linck, 1992), and yet little is known about its function in somatic cells (Setter et al., 2006). Tektins were originally identified as structural components of axonemal doublet microtubules in cilia and flagella (Linck, 1976; Linck, and Langevin, 1982). Tektins represent a family of elongated proteins that assemble into extended filaments, which are similar in structure to intermediate filaments but tektins are distinct from intermediate filaments proteins (Setter et al., 2006). Previous work revealed that one member of the tektin family, tektin B (i.e., tektin 2), associated with the centrosomes and the spindle remnant after Ca2+-induced microtubule depolymerization (Steffen, and Linck, 1992; Steffen et al., 1994). However, little is known about the function of tektin proteins in mammalian somatic cells, particularly during mitosis.
Results and discussion
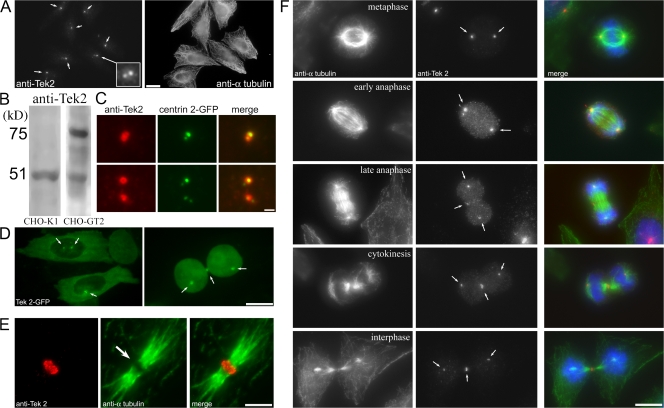
To test whether tektins play a role in mitosis, we generated antibodies against mouse tektin 2 (anti-Tek2). Immunofluorescence microscopy showed that Tek2 is centrosomal during interphase. It localized to two foci surrounding the position of the centrioles, as judged by colocalization with the centriole marker centrin 2-GFP (Fig. 1, A–C). This centrosomal localization was confirmed in a CHO cell line expressing Tek2-GFP (CHO-GT2 cells), where Tek2-GFP localized to the two centrosomes during both interphase and mitosis (Fig. 1 D). In addition, Tek2-GFP localized to the midbody region during cytokinesis (Fig. 1 D, arrows). High-magnification imaging of the midbody revealed that anti-Tek2 decorates a region surrounding the central midbody matrix (Fig. 1 E). Although anti-Tek2 is excluded from the matrix, Tek2-GFP is found in the matrix, suggesting that it forms a disc-like structure at the midbody (Fig. 1 D).
Figure 1.
Tek2 localizes to the centrosome and midbody in CHO cells. (A) Immunofluorescence localization of Tek2 in interphase CHO cells. Tek2 decorates the centrosomes (arrows). (B) Anti-Tek2 decorates a single polypeptide in CHO cells and two polypeptides in CHO cells stably transfected with GFP-Tek2. (C) Tek2 colocalizes with centrioles, indicated by centrin 2–GFP–expressing cells. (D) Tek2-GFP decorates the centrosomes during interphase and spindle poles/midbody during mitosis. Arrows show tektin 2–GFP localization to the centrosomes. (E) Anti-Tek2 decorates the center of the midbody region surrounding the midbody matrix. (F) Tek2 concentrates (arrows) at the midzone beginning in late anaphase. Bars: (A) 20 μm; (C and E) 1 μm; (D and F) 10 μm.
Imaging of mitotic cells revealed that Tek2 concentrated at spindle poles during metaphase, at the central spindle region in late anaphase, and at the midbody during cytokinesis (Fig. 1 F). In isolated CHO mitotic spindles sedimented onto coverslips, anti-Tek2 labeled the poles at all stages of mitosis (Fig. S1, available at http://www.jcb.org/cgi/content/full/jcb.200711160/DC1). Although no obvious Tek2 organization was seen in metaphase cells other than at the spindle poles, there was fibrous Tek2 staining in early/late anaphase, suggesting that Tek2 interacts with microtubules before becoming concentrated at the midbody.
To determine whether Tek2 played a role during spindle assembly/cytokinesis, we knocked down Tek2 expression with siRNAs. Depletion of Tek2 protein by >90% did not prevent bipolar mitotic spindles from forming but led to an increase in binucleate interphase cells (Fig. 2). In cells transfected with siRNA constructs unique to either the untranslated region (UTR) or the ORF of mouse Tek2, the number of binucleate cells increased to 18 and 29%, respectively, compared with 8% in cells transfected with scrambled (SCR) Tek2 siRNA and 5% in untransfected cells (Fig. 2 C).
Figure 2.
Tek2 siRNA blocks the formation of the midbody and completion of cytokinesis. (A) Frames from video microscopy sequence of CHO cells expressing a Tek2 siRNA construct targeting the ORF. Two cells enter and exit mitosis, initiate cytokinesis, and then fail cytokinesis and fuse together (arrows). Time is in h:min. (B) siRNA of Tek2 depletes both Tek2 protein and Tek2 mRNA. (C) Tek2 siRNA induces binucleate CHO cells. The mean percentage of binucleate cells for untransfected cells and cells transfected with siRNA constructs targeting the ORF, UTR, or SCR ORF is shown. 100 cells per experiment; n = 3. Error bars are SEM. (D) Tek2 siRNA prevents the formation of the midbody matrix. Cells are linked by overlapping microtubules that lack the central Flemming body (insets). Overlapping microtubules lack Tek2 label; however, Tek2 still decorates the spindle poles, presumably because Tek2 at the centrosome is resistant to degradation. Tek2 forms around the midbody in SCR-expressing cells. Arrows represent Tektin localization. The bottom right inset shows the central midbody (left arrow). Bars, 10 μm.
Next, time-lapse video microscopy was used to monitor mitosis in CHO cells constitutively expressing a Tek2 ORF siRNA construct (Tek2 knockdown [KD] cells). 60 individual CHO Tek2-KD cells were imaged as they exited mitosis. The loss of Tek2 did not prevent cells from undergoing anaphase, exiting mitosis, or undergoing cytokinesis (Fig. 2 A), and 43 cells (72%) divided into two distinct daughters. However, after cytokinesis, 17 cells (28%) fused back together, resulting in a multinucleate cell. Tek2-KD cells often lacked a midbody matrix and, in some cases, these microtubules connected the two daughters (Fig. 2 D, inset). There was no exclusion of anti–α-tubulin at the midbody region. In other cases, there was no pronounced connection between the two daughter cells before refusion (Fig. 2 A). This suggests that the lack of Tek2 allowed the microtubule plus ends of each daughter to interact beyond the midzone overlap, leading to the fusion of daughter cells.
We next examined the localization of the previously characterized central spindle proteins Aur B, MKLP1, and PRC1. Imaging of cells transfected with the SCR Tek2 siRNA construct (SCR control cells) revealed that the localization of these proteins to the central spindle was not affected by the transfection/selection process (Fig. 3, A, D, and G) compared with untransfected controls (Fig. S2, available at http://www.jcb.org/cgi/content/full/jcb.200711160/DC1).
Figure 3.
Loss of Tek2 disrupts the localization of midzone components Aur B, MKLP1, and PRC1 to the central spindle and midbody. (A) Localization of Aur B to SCR siRNA-transfected CHO cells. Three cells in early and late anaphase and in cytokinesis are shown. Aur B localizes to the overlapping midzone microtubules in anaphase (arrows) and to either side of the midbody matrix in cytokinesis (inset). (B) Effect of Tek2 siRNA on Aur B localization and central spindle organization during anaphase. The central spindle lacks the compaction seen in controls, and Aur B is widely distributed across the overlapping microtubules. Inset shows detail of mislocalized Aur B (broad staining). (C) Tek2 siRNA prevents the formation of a midbody. Aur B fails to become organized. The overlapping microtubules that connect the two cells do not form a Flemming body (insets). (D) Localization of MKLP1 to SCR siRNA-transfected CHO cells. Two cells in late anaphase and cytokinesis are shown. MKLP1 localizes to the overlapping midzone microtubules in anaphase and to either side of the midbody matrix in cytokinesis (arrows). (E and F) Effect of Tek2 siRNA on MKLP1 localization and central spindle organization during anaphase. The central spindle lacks the compaction seen in controls, and MKLP1 is partially diminished and broadly distributed across the overlapping microtubules. Insets show mislocalization of MKLP-1 (broad staining). (G) Localization of PRC1 to SCR siRNA-transfected CHO cells. PRC1 decorates the central region of the overlapping midzone microtubules in anaphase (arrow). (H and I) Effect of Tek2 siRNA on PRC1 localization and central spindle organization during anaphase. The midzone microtubules are diffuse and unbundled. PRC1 is distributed broadly across the midzone (arrows) and also decorates nonmidzone microtubules (arrowheads). Bar: (A and D) 20 μm; (B, C, and E–I) 10 μm.
Depletion of Tek2 disrupted the localization of Aur B to the central spindle and midbody region. During anaphase in Tek2-KD cells, the microtubules in the central spindle were poorly organized and Aur B staining was diffuse throughout the midzone region (Fig. 3 B, inset). During cytokinesis, depletion of Tek2 prevented the midbody from forming at all, and anti–Aur B decorated a broad region of the overlapping microtubules that formed between the daughter cells. No midbody matrix was observed (Fig. 3 C, insets). MKLP1 localization to the midzone was also disrupted by KD of Tek2 (Fig. 3, E and F). Not only was the localization pattern diffuse but the level of MKLP1 labeling was diminished compared with the SCR controls. The localization of PRC1 to the midzone in Tek2 siRNA cells was dramatically disrupted (Fig. 3, H and I). Instead of the tight concentration to the central midbody seen in wild-type and SCR-treated cells (Fig. 3 G, arrow), PRC1 was found to be broadly distributed across the midzone (Fig. 3, H and I, arrows). Anti-PRC1 labeling also extended along microtubules and microtubule bundles toward the polar regions (Fig. 3, H and I, arrowheads). This localization pattern is drastically different from that seen during siRNA KD of Kif-4, the chromokinesin responsible for localization of PRC1 to the midzone during anaphase (Kurasawa et al., 2004), where loss of Kif-4 resulted in a broadening of the localization pattern of PRC1 to the central midzone but did not result in disruption of the midzone microtubules or localization of PRC1 outside of the midzone. In this case, the loss of Tek2 expression resulted in a lack of midzone microtubule bundling, and the midzone components Aur B, MKLP1, and PRC1 were not restricted to the narrow band at the center of the midzone during anaphase.
To further examine the role of Tek2 in midbody organization, we used blebbistatin, a small molecule inhibitor that is specific for nonmuscle myosin II (Straight et al., 2003). In blebbistatin, cells enter mitosis, proceed through anaphase, and build a normal central spindle. However, because they lack actin/myosin contractility, the cleavage furrow does not contract (Straight et al., 2003). These cells eventually exit mitosis and reform interphase nuclei without cleaving. The two daughter cells remain transiently interconnected by a central spindle. This allows for the observation of postanaphase events of central spindle microtubule organization without the contraction of the cleavage furrow, which obscures the morphological features of the midzone structure.
Pairs of interconnected daughter cells with decondensed chromosomes were identified. These cells had exited mitosis without cleaving, but the pair of interphase daughter cells remained connected by an intact central spindle (Fig. 4, A and B). Interestingly, the central spindle in these pairs of SCR control cells maintained their characteristic compact shape and also contained the presumptive midbody region, which is indicated by a central area that excludes anti-tubulin staining, even in the absence of a cleavage furrow (Fig. 4, A and B, arrows). In these cells, Tek2 was concentrated in this presumptive midbody region, as was Aur B, which was used as a marker for midzone organization (Fig. 4, A and B, arrows). This suggests that during normal anaphase (elaborated by blebbistatin treatment), there is an activity that functions to bundle and compact the central spindle independently of actin-myosin contractility induced by the cleavage furrow, which is similar to that previously observed (Martineau et al., 1995; Straight et al., 2003; Yüce et al., 2005).
Figure 4.
Blebbistatin reveals that the compaction of the midzone microtubules occurs in the absence of actin/myosin contractility. (A) Blebbistatin prevents actin-myosin contractility during cytokinesis. Tek2 localizes to the dark microtubule overlap region (the presumptive midbody) in SCR siRNA-treated cells. Small arrows, Tektin staining at spindle poles; large arrows, position of spindle midzone overlap. (B) Aur B also localizes to the presumptive midbody in blebbistatin-treated SCR siRNA cells (arrows). The central spindle region is compacted in the absence of a contractile ring. (C and D) Tek2 siRNA with an ORF construct prevents the compaction of the central spindle in blebbistatin-treated cells. Tek2 is missing from the midzone, there is no presumptive midbody (arrows), and Aur B is broadly distributed across the midzone. Bars, 10 μm.
Immunofluorescence of postmitotic Tek2-KD cells treated with blebbistatin revealed that in the absence of Tek2, the midzone microtubules were not bundled (Fig. 4, C and D; and Fig. S3, available at http://www.jcb.org/cgi/content/full/jcb.200711160/DC1). There was no organized central spindle, and Aur B distribution was diffuse across the overlapping microtubules. There was no presumptive midbody in the Tek2 siRNA cells. Instead, there were widely overlapping disorganized microtubules (Fig. 4, C and D, large arrows). This indicates that Tek2 plays a key role in compacting the midzone microtubules during anaphase at a time well before it becomes concentrated at the midbody. This suggests the possibility that Tek2 provides the structural cues to organize the midzone microtubules necessary to generate the boundaries of the central spindle microtubules before cytokinesis, perhaps via direct interactions with microtubules, as is seen for tektins and associated proteins in axonemes (Nojima et al., 1995; Hinchcliffe and Linck, 1998).
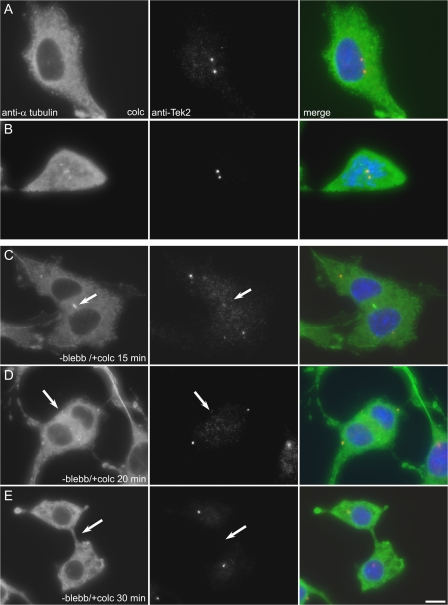
To determine if Tek2 localization to the midbody matrix required intact microtubules, CHO cells were treated with blebbistatin to elaborate postanaphase cells. The blebbistatin was then washed out in the presence of colcemid to depolymerize the postanaphase microtubule network (Fig. 5). Colcemid treatment of control cells in either interphase or mitosis did not disrupt Tek2 localization to the centrosome (Fig. 5, A and B). In blebbistatin-released anaphase cells, microtubule depolymerization with colcemid induced the loss of Tek2 to the midzone/midbody region (Fig. 5, C–E). These observations reveal that Tek2 localization to the midzone and midbody is dependent on intact microtubules. Interestingly, in some cells the midzone microtubule depolymerized, and a cleavage furrow formed as the blebbistatin was washed out in the presence of colcemid (Fig. 5 E). Midzone microtubules are thought to be highly resistant to drug-induced depolymerization, although they can depolymerize in the presence of cold and colcemid or nocodazole (Uetake and Sluder, 2007), and their continued presence is required to allow ingression of the cleavage furrow (Wheatley and Wang, 1996). Perhaps the phenomenon we observed here is related to the timing of washout. The presence of the midzone during prolonged anaphase may obviate the need for intact midzone microtubules, as is seen for cell washed out of cytochalasin (Martineau et al., 1995).
Figure 5.
Tek2 localization to the midbody requires microtubules. Immunofluorescence of CHO cells labeled with anti–a-tubulin and anti-Tek2.(A and B) Control CHO cells treated with colcemid. The microtubule network is depolymerized, and Tek2 remains localized to the interphase centrosomes and mitotic spindle poles. (C–E) Anaphase cells after washout of blebbistatin into colcemid. Loss of microtubules in postanaphase cells does not disrupt Tek2 localization to the spindle poles. As the microtubule network depolymerizes, anti-Tek2 labeling at the midzone/midbody is lost (arrows). At 30 min after blebbistatin washout, the cells assemble a cleavage furrow (E). Bar, 10 μm.
Our finding that Tek2 relocated from the spindle poles to the midbody during late anaphase is reminiscent of Centriolin and Cep55, proteins that are centrosomal during metaphase/anaphase and then relocalize to the midbody during cytokinesis (Gromley et al., 2003; Fabbro et al., 2005; Zhao et al., 2006). However, Centriolin and Cep55 are not involved in central spindle organization. Their loss does not disrupt the central spindle or midbody nor cause fusion of daughter cells. Instead, these proteins are involved in recruiting new membrane to the cleavage furrow, resulting in midbody abscission (Gromley et al., 2005; Zhao et al., 2006; for reviews see Hinchcliffe, 2003; Doxsey, 2005). Loss of Tek2 appears to give a very different phenotype. Instead of preventing midbody abscission, loss of Tek2 appears to disrupt the normal organization of the midzone microtubules. These poorly bundled microtubules may have an indirect effect on the completion of cytokinesis by physically preventing the proper and coordinated membrane fusion to the nascent cleavage furrow.
Tektins are evolutionarily conserved from Chlamydomonas reinhardtii to human, including both Caenorhabditis elegans and Drosophila melanogaster, and are essential for many developmental processes, such as fertilization and neuronal development, which are dependent upon cilia and flagella (Norrander et al., 1998; Keller et al., 2005; Setter et al., 2006). During axoneme/basal body assembly, tektins directly interact with microtubules, imparting the intricate arrangement of doublet/triplet microtubules necessary for these organelles (Nojima et al., 1995; Hinchcliffe and Linck, 1998). In this paper, we show that in somatic cells, Tek2 plays a similar structural role in building the central spindle, and our results suggest that tektin may contribute to providing the complex morphology and inherent stability to the midzone microtubules necessary during cytokinesis.
Midzone microtubules form a characteristic overlap and, as anaphase proceeds, the length of the plus-end overlap decreases (Saxton and McIntosh, 1987; Shelden and Wadsworth, 1990; Mastronarde et al., 1993). In Tek2-KD cells, the microtubule overlap does not appear well defined, resulting in diffuse midzone microtubules with ill-defined boundaries (Fig. 3). This observation, combined with the broad diffuse localization of Aur B, MKLP1, and, in particular, PRC1, suggests that Tek2 serves to stabilize the plus-end boundaries of microtubules in the midzone. This phenotype is also evident in images of Tek2-KD cells arrested with blebbistatin, where the boundaries of microtubule overlap appear particularly ill defined (Fig. 4). During the assembly of ciliary and flagellar axonemes, tektins presumably interact with the growing plus end of doublet microtubules as they grow out, and tektins also serve to limit the length of the axoneme (Norrander et al., 1995; Linck, and Stephens, 2007). At present, we support the model where Tek2 functions to delineate the plus-end boundaries of the midzone microtubules, perhaps by stabilizing them via direct interaction. Alternatively, association of Tek2 with the midzone overlap provides structural cues that contribute to the recruitment of key midzone components, such as PRC1, centralspindlin, and the chromosome passenger complex, which themselves interconnect and stabilize the microtubule overlap. Although further work is needed to understand the interactions between tektins and midzone microtubules, this study has provided insight into the complex events that lead to the formation, bundling, and organization of the midzone microtubules in the central spindle.
Materials and methods
Unless otherwise noted, all reagents were obtained from Sigma-Aldrich.
Cell culture and treatments
CHO-K1 cells (American Type Culture Collection) were cultured in Ham's F12 media containing 10% FCS and 1 mg/ml pen-strep (Invitrogen).
Anti-Tek2 polyclonal antibodies
Full-length mouse tektin 2 (Open Biosystems; accession no. NM_011902) was sub-cloned into the pET14 vector (EMD) and transformed into lBL21 bacteria (DE3; Invitrogen), and protein expression was induced by IPTG. Recombinant Tek2 was separated on a 7.5% SDS-PAGE curtain gel and transblotted onto nitrocellulose. The tektin 2 band was cut out as a strip used to immunize rabbits, and antisera was affinity purified as previously described (Hinchcliffe and Linck, 1998).
GFP–tektin 2 construct and stable cell line creation
Full-length mouse tektin 2 was subcloned into pBluescript II containing the GFP gene, and the GFP–tektin 2 fragment was subcloned into the pCI-Neo expression vector (Promega)
CHO-K1 cells stably expressing Tek2-GFP were generated as previously described (Durcan et al., 2008). Cells were transfected with Fugene 6 (Roche), and the day after transfection, Hepes-buffered F12 media containing 2 mg/ml G418 was added to cells. After 3 d in G418, cells were passaged, small groups of cells were seeded into 24-well dishes, and the concentration of G418 was lowered to 50 μg/ml. CHO–tektin 2 (CHO-GT2) cells were screened by fluorescence microscopy. Tertiary clones were identified with maximal numbers of cells showing optimal GFP–tektin 2 expression and aliquots were frozen down.
Mitotic spindle isolation
Mitotic spindles and midbodies were isolated as previously described (Sellitto and Kuriyama, 1988). Once the extraction was complete, as monitored by phase microscopy, the spindles or midbodies were spun onto coverslips at 14,000 g for 15 min at 4°C.
Microscopy
Cells on coverslips were fixed in ice-cold methanol. Immunofluorescence was performed using α-tubulin (mouse D1a clone; Sigma-Aldrich), and either anti-Tek2 (this study) anti–Aur B, anti-MKLP1, or anti-PRC1 (all obtained from Santa Cruz Biotechnology, Inc.). Secondary antibodies used were Alexa-594 goat anti–rabbit (Invitrogen) and Alexa-488 goat anti–mouse. Fixed cells were imaged at room temperature with a 63× 1.4 NA Apo oil immersion lens on an upright microscope (DM RXA2; Leica). Images were acquired with Simple PCI software (Compix Media) using a cooled charge-coupled device camera (ORCA-ER; Hamamatsu Photonics). Images were acquired as a Z stack at 0.2-μm intervals and compiled as maximum projections, and image overlays were created with Photoshop 6.0 (Adobe).
For time-lapse imaging, CHO-GT2 cells were plated onto biocleaned glass coverslips in imaging media (Ham's F-12 without phenol red; PromoCell GmbH) containing 12 mM Hepes, pH 7.2, and 10% FBS and assembled onto aluminium support slides, as previously described (Hinchcliffe et al., 2001). Time-lapse images were captured using a microscope stand (DM RXA2; Leica) equipped with fluorescence and differential interference optics and enclosed in a custom-made Plexiglas box maintained at 37°C. Live-cell differential interference contrast image sequences were captured with a 40× 0.7 NA Apo dry objective. Live-cell fluorescence images were captured using a spinning disk confocal head (CSU-10; Yokagawa), as modified by McBain Industries, using a 488-nm 200-mW Sapphire continuous-wave optically pumped solid-state laser (CW-OPSL; Coherent), connected through a fiber optic cable into the excitation port of the spinning disk confocal head, and shuttered via a TTL pulse through a shutter controller (MAC5000; Ludl). Confocal fluorescent images were taken through a Plan Apo 63× 1.3 NA 37°C glycerol immersion objective (Leica). The detector on the confocal microscope was a digital charge-coupled device camera (ORCA-AG; Hamamatsu Photonics), and images were captured using Simple PCI imaging software.
Tektin 2 siRNA KD
To transiently knock down levels of tektin 2 in CHO-K1 cells, oligos were created to target sequences within the mouse tektin 2 genes. For transient transfections, these oligos were cloned into the pSHAG vector (G. Hannon, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY; Paddison et al., 2004). Oligos were created to target sequences in both the 5′ UTR and the ORF of Tek2. An SCR ORF oligo was created as a control siRNA oligo that would not knock down tektin 2 (T2 ORF, AACATGACAATAGGACCCGCC; T2 SCR, agacaacacgcactaatgcgc; T2 UTR, AAGACAGACCACTGAGGAAATACAG).
To transiently knock down tektin 2 levels, CHO cells were transiently transfected with the pSHAG constructs described in the previous paragraph in a 4:1 ratio with pBluescriptII-RFP to allow identification of transfected cells. To generate CHO cells stably expressing either the Tek2 ORF or SCR constructs, oligos were cloned behind a U6 promoter in a pBluescriptII vector containing the neomycin gene. Cells were transfected and selected with G418, and tektin 2 KD was verified by both RT-PCR and Western blot. Only low passage number cells were used in experiments, as cells transfected with the siRNA construct became multinucleate over time.
RT-PCR
Total RNA was harvested from cells using Qiashredder and the RNeasy mini kit (QIAGEN). For a reverse transcription reaction in an RNase free environment, 2 μg mRNA was added to an RNase-free PCR tube (Ambion). The mRNA was amplified using 2 μM of random hexamers, creating first-strand cDNA. After reverse transcription, the RT mix was incubated in the presence of either tektin 2, 18s ribosomal RNA, or both sets of PCR primers. The PCR reactions were run at standard temperatures on a 2% TBE agarose gel for 37 cycles, with a melting temperature of 54°C (1 min).
Blebbistatin treatment
Blebbistatin (− active enantiomer; EMD) was added at a final concentration of 100 μM to unsynchronized CHO cells expressing the Tek2 ORF siRNA, the Tek2 SCR siRNA, or no transfected for two hours. Cells were then fixed and immunolabeled for α-tubulin and either anti-Tek2 or anti–Aur B.
Online supplemental material
Fig. S1 shows that Tek2 localizes to isolated CHO mitotic spindles. Fig. S2 shows localization of Aur B, MKLP1, and PRC1 in control CHO cells. Fig. S3 shows that Tek2 compacts the central spindle microtubules. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200711160/DC1.
Supplementary Material
Acknowledgments
We thank both Walter Steffen and Richard Linck for instilling our initial/continuous love for tektin proteins, the Cytokinetic Mafia for its continued underground encouragement, and Kevin Vaughan and Holly Goodson for critically reading this manuscript.
This research was sponsored by National Institutes of Health grant R01 GM072754 to E.H. Hinchcliffe. E.H. Hinchcliffe is a Research Scholar of the American Cancer Society.
T.M. Durcan and E.S. Halpin contributed equally to this paper.
T.M. Durcan's present address is Montreal Neurological Institute, McGill University, Montreal, Quebec, Canada H3A 2B4.
T. Rao's present address is Dept. of Biochemistry, McGill University, Montreal, Quebec, Canada H3G 1V6.
N.S. Collins' present address is Program in Genetic Counseling, University of Arkansas at Little Rock, Little Rock, AR 72204.
E.K. Tribble's present address is Dept. of Cell and Developmental Biology, University of North Carolina, Chapel Hill, NC 27599.
Abbreviations used in this paper: KD, knockdown; SCR, scrambled; UTR, untranslated region.
References
- Adams, R.R., M. Carmena, and W.C. Earnshaw. 2001. Chromosomal passengers and the (aurora) ABCs of mitosis. Trends Cell Biol. 11:49–54. [DOI] [PubMed] [Google Scholar]
- Doxsey, S.J. 2005. Molecular links between centrosome and midbody. Mol. Cell. 20:170–172. [DOI] [PubMed] [Google Scholar]
- Durcan, T.M., E.S. Halpin, L. Casaletti, K.T. Vaughan, M. Pierson, S. Woods, and E.H. Hinchcliffe. 2008. Centrosome duplication proceeds during mimosine-induced G1 cell cycle arrest. J. Cell. Physiol. 215:182–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbro, M., B.B. Zhou, M. Takahashi, B. Sarcevic, P. Lal, M.E. Graham, B.G. Gabrielli, P.J. Robinson, E.A. Nigg, Y. Ono, and K.K. Khanna. 2005. Cdk1/Erk2- and Plk1-dependent phosphorylation of a centrosome protein, Cep55, is required for its recruitment to midbody and cytokinesis. Dev. Cell. 9:477–488. [DOI] [PubMed] [Google Scholar]
- Glotzer, M. 2005. The molecular requirements for cytokinesis. Science. 307:1735–1739. [DOI] [PubMed] [Google Scholar]
- Gromley, A., A. Jurczyk, J. Sillibourne, E. Halilovic, M. Mogensen, I. Groisman, M. Blomberg, and S. Doxsey. 2003. A novel human protein of the maternal centriole is required for the final stages of cytokinesis and entry into S phase. J. Cell Biol. 161:535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromley, A., C. Yeaman, J. Rosa, S. Redick, C.T. Chen, S. Mirabelle, M. Guha, J. Sillibourne, and S.J. Doxsey. 2005. Centriolin anchoring of exocyst and SNARE complexes at the midbody is required for secretory-vesicle-mediated abscission. Cell. 123:75–87. [DOI] [PubMed] [Google Scholar]
- Hinchcliffe, E.H. 2003. Cell cycle: seeking permission from the mother centriole. Curr. Biol. 13:R646–R648. [DOI] [PubMed] [Google Scholar]
- Hinchcliffe, E.H., and R.W. Linck. 1998. Two proteins isolated from sea urchin sperm flagella: structural components common to the stable microtubules of axonemes and centrioles. J. Cell Sci. 111:585–595. [DOI] [PubMed] [Google Scholar]
- Hinchcliffe, E.H., F.J. Miller, M. Cham, A. Khodjakov, and G. Sluder. 2001. Requirement of a centrosomal activity for cell cycle progression through G1 into S phase. Science. 291:1547–1550. [DOI] [PubMed] [Google Scholar]
- Keller, L.C., E.P. Romijn, I. Zamora, J.R. Yates, and W.F. Marshall. 2005. Proteomic analysis of isolated chlamydomonas centrioles reveals orthologs of ciliary-disease genes. Curr. Biol. 15:1090–1098. [DOI] [PubMed] [Google Scholar]
- Kurasawa, Y., W.C. Earnshaw, Y. Mochizuki, N. Dohmae, and K. Todokoro. 2004. Essential roles for KIF4 and its binding partner PRC1 in organized central spindle midzone formation. EMBO J. 23:3237–3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linck, R.W. 1976. Flagellar doublet microtubules: fractionation of minor components and alpha-tubulin from specific regions of the A-tubule. J. Cell Sci. 20:405–439. [DOI] [PubMed] [Google Scholar]
- Linck, R.W., and G.L. Langevin. 1982. Structure and chemical composition of insoluble filamentous components of sperm flagellar microtubules. J. Cell Sci. 58:1–22. [DOI] [PubMed] [Google Scholar]
- Linck, R.W., and R.E. Stephens. 2007. Functional protofilament numbering of ciliary, flagellar, and centriolar microtubules. Cell Motil. Cytoskeleton. 64:489–495. [DOI] [PubMed] [Google Scholar]
- Martineau, S.N., P.R. Andreassen, and R.L. Margolis. 1995. Delay of HeLa cell cleavage into interphase using dihydrocytochalasin B: retention of a postmitotic spindle and telophase disc correlates with synchronous cleavage recovery. J. Cell Biol. 131:191–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuliene, J., and R. Kuriyama. 2002. Kinesin-like protein CHO1 is required for the formation of midbody matrix and the completion of cytokinesis in mammalian cells. Mol. Biol. Cell. 13:1832–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastronarde, D.N., K.L. McDonald, R. Ding, and J.R. McIntosh. 1993. Interpolar microtubules in PTK cells. J. Cell Biol. 123:1475–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCollum, D. 2004. Cytokinesis: the central spindle takes center stage. Curr. Biol. 14:R953–R955. [DOI] [PubMed] [Google Scholar]
- Mishima, M., S. Kaitna, and M. Glotzer. 2002. Central spindle assembly and cytokinesis require a kinesin-like protein/RhoGAP complex with microtubule bundling activity. Dev. Cell. 2:41–54. [DOI] [PubMed] [Google Scholar]
- Mollinari, C., J.P. Kleman, W. Jiang, G. Schoehn, T. Hunter, and R.L. Margolis. 2002. PRC1 is a microtubule binding and bundling protein essential to maintain the mitotic spindle midzone. J. Cell Biol. 157:1175–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins, J.M., and J.J. Biesele. 1977. Terminal phase of cytokinesis in D-98s cells. J. Cell Biol. 73:672–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins, J.M., and J.R. McIntosh. 1982. Isolation and initial characterization of the mammalian midbody. J. Cell Biol. 94:654–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nojima, D., R.W. Linck, and E.H. Egelman. 1995. At least one of the protofilaments in flagellar microtubules is not composed of tubulin. Curr. Biol. 5:158–167. [DOI] [PubMed] [Google Scholar]
- Norrander, J.M., R.W. Linck, and R.E. Stephens. 1995. Transcriptional control of tektin A mRNA correlates with cilia development and length determination during sea urchin embryogenesis. Development. 121:1615–1623. [DOI] [PubMed] [Google Scholar]
- Norrander, J., M. Larson, S. Ståhl, C. Höög, and R. Linck. 1998. Expression of ciliary tektins in brain and sensory development. J. Neurosci. 18:8912–8918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oegema, K., and T.J. Mitchison. 1997. Rappaport rules: cleavage furrow induction in animal cells. Proc. Natl. Acad. Sci. USA. 94:4817–4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otegui, M.S., K.J. Verbrugghe, and A.R. Skop. 2005. Midbodies and phragmoplasts: analogous structures involved in cytokinesis. Trends Cell Biol. 15:404–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddison, P.J., M. Cleary, J.M. Silva, C.K. Hang, N. Sheth, R. Sachidanandam, and G.J. Hannon. 2004. Cloning of short hairpin RNAs for gene knockdown in mammalian cells. Nat. Methods. 1:163–167. [DOI] [PubMed] [Google Scholar]
- Pickett-Heaps, J., T. Spurck, and D. Tippit. 1984. Chromosome motion and the spindle matrix. J. Cell Biol. 99:137s–143s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton, W.M., and J.R. McIntosh. 1987. Interzone microtubule behavior in late anaphase and telophase spindles. J. Cell Biol. 105:875–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon, E.D., D. Goode, T.M. Maugel, and D.B. Bonar. 1976. Pressure-induced depolymerization of spindle microtubules. III. Differential stability in HeLa cells. J. Cell Biol. 69:443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellitto, C., and R. Kuriyama. 1988. Distribution of a matrix component of the midbody during the cell cycle in Chinese hamster ovary cells. J. Cell Biol. 106:431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setter, P.W., E. Malvey-Dorn, W. Steffen, R.E. Stephens, and R.W. Linck. 2006. Tektin interactions and a model for molecular functions. Exp. Cell Res. 312:2880–2896. [DOI] [PubMed] [Google Scholar]
- Shelden, E., and P. Wadsworth. 1990. Interzonal microtubules are dynamic during spindle elongation. J. Cell Sci. 97:273–281. [DOI] [PubMed] [Google Scholar]
- Skop, A.R., H. Liu, J. Yates, B.J. Meyer, and R. Heald. 2004. Dissection of the mammalian midbody proteome reveals conserved cytokinesis mechanisms. Science. 305:61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen, W., and R.W. Linck. 1992. Evidence for a non-tubulin spindle matrix and for spindle components immunologically related to tektin filaments. J. Cell Sci. 101:809–822. [DOI] [PubMed] [Google Scholar]
- Steffen, W., E.A. Fajer, and R.W. Linck. 1994. Centrosomal components immunologically related to tektins from ciliary and flagellar microtubules. J. Cell Sci. 107:2095–2105. [DOI] [PubMed] [Google Scholar]
- Straight, A.F., and C.M. Field. 2000. Microtubules, membranes and cytokinesis. Curr. Biol. 10:R760–R770. [DOI] [PubMed] [Google Scholar]
- Straight, A.F., A. Cheung, J. Limouze, I. Chen, N.J. Westwood, J.R. Sellers, and T.J. Mitchison. 2003. Dissecting temporal and spatial control of cytokinesis with a myosin II Inhibitor. Science. 299:1743–1747. [DOI] [PubMed] [Google Scholar]
- Uetake, Y., and G. Sluder. 2007. Cell cycle progression without an intact microtubule cytoskeleton. Curr. Biol. 17:2081–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vader, G., R.H. Medema, and S.M. Lens. 2006. The chromosomal passenger complex: guiding Aurora-B through mitosis. J. Cell Biol. 173:833–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatley, S.P., and Y. Wang. 1996. Midzone microtubule bundles are continuously required for cytokinesis in cultured epithelial cells. J. Cell Biol. 135:981–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yüce, O., A. Piekny, and M. Glotzer. 2005. An ECT2–centralspindlin complex regulates the localization and function of RhoA. J. Cell Biol. 170:571–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, W.M., A. Seki, and G. Fang. 2006. Cep55, a microtubule-bundling protein, associates with centralspindlin to control the midbody integrity and cell abscission during cytokinesis. Mol. Biol. Cell. 17:3881–3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.