Abstract
Schwann cell myelination depends on Krox-20/Egr2 and other promyelin transcription factors that are activated by axonal signals and control the generation of myelin-forming cells. Myelin-forming cells remain remarkably plastic and can revert to the immature phenotype, a process which is seen in injured nerves and demyelinating neuropathies. We report that c-Jun is an important regulator of this plasticity. At physiological levels, c-Jun inhibits myelin gene activation by Krox-20 or cyclic adenosine monophosphate. c-Jun also drives myelinating cells back to the immature state in transected nerves in vivo. Enforced c-Jun expression inhibits myelination in cocultures. Furthermore, c-Jun and Krox-20 show a cross-antagonistic functional relationship. c-Jun therefore negatively regulates the myelinating Schwann cell phenotype, representing a signal that functionally stands in opposition to the promyelin transcription factors. Negative regulation of myelination is likely to have significant implications for three areas of Schwann cell biology: the molecular analysis of plasticity, demyelinating pathologies, and the response of peripheral nerves to injury.
Introduction
The transition of immature Schwann cells to myelinating cells depends on promyelin gene regulatory proteins, including at least Krox-20, Nab1 and 2, Oct-6, Brn2, NFκB, and Sox-10 (Topilko et al., 1994; Bermingham et al., 1996; Jaegle et al., 2003; Nickols et al., 2003; Le et al., 2005a; Ghislain and Charnay, 2006). Myelinating cells readily lose their myelin and can dedifferentiate to a phenotype that resembles the immature state, and this transition, like myelination, involves a complex and orderly cellular transformation (Jessen and Mirsky, 2005). It is an important possibility that this reverse transition also requires distinct gene regulatory proteins that would function as negative regulators of the myelinated state and push myelinating cells back toward the immature phenotype. In this paper, we identify the basic leucine zipper protein c-Jun as a transcription factor that acts in this way in Schwann cells.
The ability of myelinating cells to dedifferentiate is seen when they are removed from axonal contact in injured nerves or in vitro and also in demyelinating neuropathies. Remarkably, dedifferentiated cells, even in adults, can remyelinate if they are allowed to associate with axons under appropriate conditions. Potentially, this choice between two alternative differentiation states is therefore open to Schwann cells throughout life. Studies of gene regulatory proteins that control the choice between two alternative fates have revealed the importance of cross-antagonistic signaling systems where a major role in fate choice is played by the balance between two sets of transcription factors that both specify alternative fates and inhibit the expression or the activity of each other. Examples of this include the GATA-1/c-Myb and GATA-1/PU.1 transcription factors in erythrocyte development and Scl(Tal1)/Olig2 in astrocyte development (Cantor and Orkin, 2002; Muroyama et al., 2005).
In Schwann cells, specification of myelin differentiation is dependent on the zinc finger transcription factor Krox-20 (Egr2). It is activated by the axonal signals that induce myelination. Krox-20−/− Schwann cells fail to myelinate, although they enter the earliest stage of myelination, the promyelin state. Enforced expression of Krox-20 in Schwann cells is sufficient to induce expression of myelin genes, remove cells from the cell cycle, and reduce susceptibility to apoptosis, all of which are developmental changes that normally take place when myelination begins. Expression of Krox-20 therefore sets in train and/or amplifies a set of changes associated with the adoption of a myelin fate (Nagarajan et al., 2001; Topilko and Meijer, 2001; Parkinson et al., 2004).
The transcription factor c-Jun, which is studied here, is a key component of the AP-1 transcription factor complex and forms, with JunB and JunD, the Jun protein family (Mechta-Grigoriou et al., 2001). Although the phosphorylation of c-Jun at NH2-terminal Ser-63 and -73 residues by JNK is important for many of its functions, other actions of c-Jun are independent of c-Jun phosphorylation but dependent on the presence of the protein (Raivich and Behrens, 2006). c-Jun is present in immature Schwann cells, but its expression is repressed by Krox-20 (Parkinson et al., 2004). Schwann cells therefore express low levels of c-Jun when they start myelinating.
In this paper, we report that c-Jun is an important regulator of Schwann cell differentiation because c-Jun acts as a potent suppressor of the myelin phenotype. This function is independent of its role in proliferation and death. c-Jun blocks myelination in neuron–Schwann cell cocultures, opposes the function of Krox-20 and cAMP-related myelin signals, and drives the dedifferentiation of early myelinating cells back to the immature Schwann cell phenotype. This effect is seen in injured nerves both in vivo and in vitro, using mice with conditional inactivation of c-Jun in Schwann cells. Some of these effects of c-Jun are possibly channeled through Sox-2, a transcription factor that inhibits myelination (Le et al., 2005b). We also show that c-Jun inhibits Krox-20 expression, suggesting that these proteins are components of a cross-inhibitory switch to control two alternative gene programs: while Krox-20 drives the myelination program, c-Jun is a negative regulator of myelination that promotes the opposite program of dedifferentiation.
Results
c-Jun and phosphorylated c-Jun are down-regulated during myelin differentiation and rapidly up-regulated as myelin breaks down in injured nerves
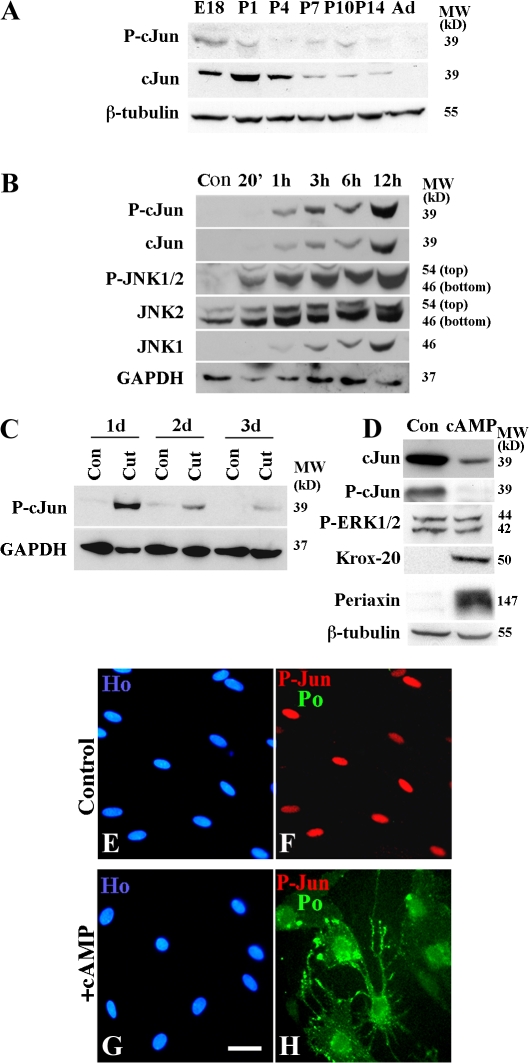
In early postnatal Schwann cells, c-Jun protein is down-regulated as proliferation decreases and myelination begins. When it is reexpressed when nerves are injured, Schwann cells reenter the cell cycle and dedifferentiate (De Felipe and Hunt, 1994; Stewart, 1995; Shy et al., 1996). We confirmed this for c-Jun, phospho–c-Jun, JNK 1 and 2, and phospho-JNK1/2. Up-regulation of these components after nerve injury, particularly of phospho-JNK1/2, was very rapid (Fig. 1, A–C). When purified cultures of primary Schwann cells were exposed to dibutyryl cAMP (db-cAMP) to induce myelin differentiation, c-Jun and phospho–c-Jun were suppressed (Fig. 1, D–H), which is in line with the down-regulation of c-Jun during myelination in vivo (Monuki et al., 1989; Stewart, 1995; Shy et al., 1996). Unlike in vivo, activation of myelin differentiation in these experiments did not involve cell cycle exit because the cells were quiescent before and after addition of db-cAMP (unpublished data).
Figure 1.
Components of the c-Jun pathway are regulated during development, Wallerian degeneration, and in vitro differentiation. A–D show Western blots. (A) Down-regulation of phospho–c-Jun and c-Jun proteins in sciatic nerve from embryonic day (E) 18 to adult. (B) Rapid up-regulation of JNK–c-Jun pathway components at 20 min (20′)–12 h after nerve cut when compared with contralateral control (Con). (C) Strong increase in phospho–c-Jun at 1, 2, and 3 d after transection (Cut) compared with the contralateral control (Con). (D) Activation of cAMP-related pathways (1 mM db-cAMP for 24 h) decreases c-Jun and phospho–c-Jun and increases the myelin proteins Krox-20 and periaxin, whereas phospho-ERK1/2 levels are unchanged. (E–H) Double immunolabeling of controls (E and F) and cells treated with 1 mM db-cAMP for 3 d (G and H), with phospho–c-Jun and P0 antibodies. Note that cAMP induces P0 and suppresses phospho–c-Jun levels in the P0-positive cells. Bar, 15 μm.
This regulatory profile of the JNK–c-Jun pathway in vivo, i.e., down-regulation as immature cells myelinate and up-regulation in the proliferating cells of injured nerves, shows that c-Jun is present when Schwann cells proliferate, which is in agreement with a role for c-Jun in Schwann cell division (Parkinson et al., 2004). However, when proliferating Schwann cells are removed from perinatal nerves and made quiescent in vitro, they continue to express c-Jun, showing that c-Jun expression is not sufficient for proliferation. Furthermore, when quiescent cells are induced by cAMP or enforced Krox-20 expression to adopt a myelin phenotype, c-Jun is down-regulated even when cell division is absent throughout the experiments (Parkinson et al., 2004; previous paragraph). Therefore, both in vivo and in vitro, c-Jun expression is suppressed as Schwann cells myelinate. But only in vivo is this correlated with exit from the cell cycle. These observations led us to examine whether down-regulation of c-Jun is important for the activation of the myelination program itself in addition to, and irrespective of, the role of this factor in cell cycle control.
c-Jun inhibits myelin gene expression
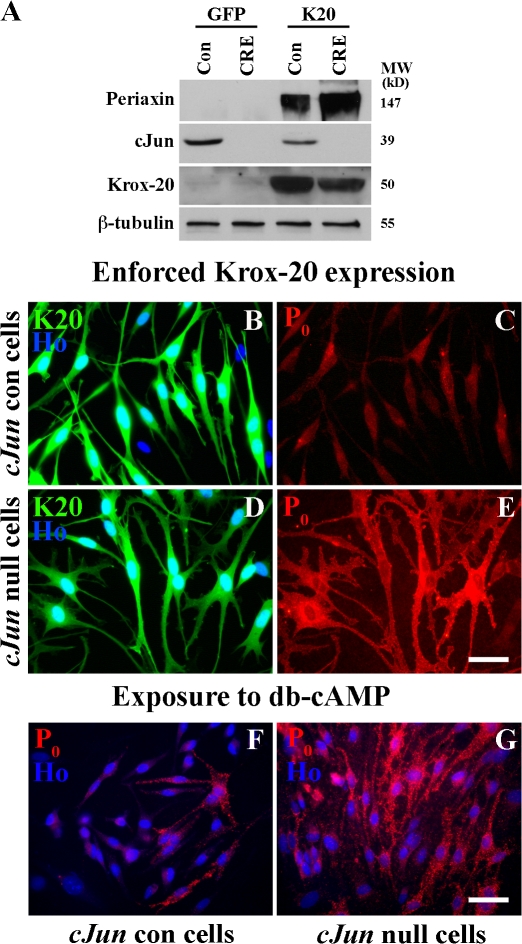
As mentioned in the previous section, c-Jun is expressed constitutively in quiescent cells maintained under basal conditions in vitro (De Felipe and Hunt, 1994; Stewart, 1995; Shy et al., 1996; Parkinson et al., 2004; unpublished data). We took advantage of this to test whether c-Jun inhibits myelin differentiation by comparing Krox-20–induced myelin gene expression (Parkinson et al., 2004) in normal c-Jun–expressing cells with that in c-Jun–null cells. Cells were prepared from mice carrying a floxed c-Jun allele (Junfl/fl) and infected in vitro with adenoviruses expressing either CRE recombinase to remove c-Jun or GFP as a control. Western blotting and immunolabeling confirmed that c-Jun was excised in essentially all cells after infection with CRE virus (Fig. 2 A). We found that Krox-20–induced expression of the myelin proteins protein zero (P0) and periaxin was strikingly facilitated in c-Jun–null cells (Fig. 2, B–E). DNA synthesis was essentially absent in both experimental conditions (unpublished data). Similar differences between c-Jun–null and control cells were obtained when quiescent cells cultured in defined medium (DM) were induced to express myelin genes by db-cAMP/β–neuregulin-1 (NRG-1; Fig. 2, F and G). Nevertheless, inhibition of the c-Jun pathway was not sufficient to trigger myelin differentiation. Without enforced Krox-20 expression or db-cAMP, neither genetic removal of c-Jun nor application of JNK blockers elevated periaxin or P0 (Fig. 2 A and not depicted).
Figure 2.
Genetic removal of c-Jun amplifies Krox-20 or cAMP-induced myelin protein expression. (A) Western blot showing that c-Jun is absent from Junfl/fl cells infected with CRE-expressing adenovirus. The blot also compares periaxin in control (Con) and c-Jun–null cells (CRE) infected with GFP control adenovirus (GFP) or a Krox-20/GFP virus (K20). Note high periaxin levels in Krox-20–infected c-Jun–null cells (CRE). (B–E) c-Jun control (cJun con) and c-Jun–null mouse Schwann cells 2 d after infection with Krox-20/GFP adenovirus. Note that Krox-20 induces much higher levels of P0 protein in c-Jun–null cells (D and E) than in control cells (B and C). The reason why P0 levels in the Krox-20–expressing control cells appear low in this picture (C) compared with other comparable experiments (e.g., Fig. 4 I) is that exposure had to be reduced (equally for C and E) to avoid overexposure in E. (F and G) P0 protein expression in control cells P0 (cJun con) and c-Jun–null mouse Schwann cells after 3 d of exposure to db-cAMP/NRG-1. Note that cAMP/NRG-1 induces substantially higher P0 levels in cells without c-Jun. Bars, 15 μm.
These experiments indicate that c-Jun is a negative regulator of myelin differentiation. They show that in normal cultured Schwann cells, constitutive c-Jun appears sufficient to act as a break on the transition of quiescent immature Schwann cells to the myelin phenotype. The inhibitory effect of c-Jun does not depend on enforced overexpression of c-Jun nor is it contingent on the role of c-Jun in Schwann cell proliferation.
c-Jun inhibits myelin genes without N-terminal phosphorylation
The experiments discussed in the previous section showed that genetic removal of c-Jun amplified Krox-20– or cAMP-induced myelin differentiation, despite the fact that both agents gradually suppress endogenous c-Jun and reduce it to very low levels by 2–3 d (Parkinson et al., 2004 and above). We then tested whether prevention of this down-regulation and maintenance of c-Jun expression would have the opposite effect and inhibit myelin gene activation because this would be the predicted outcome if c-Jun acts as a negative regulator of the myelin program.
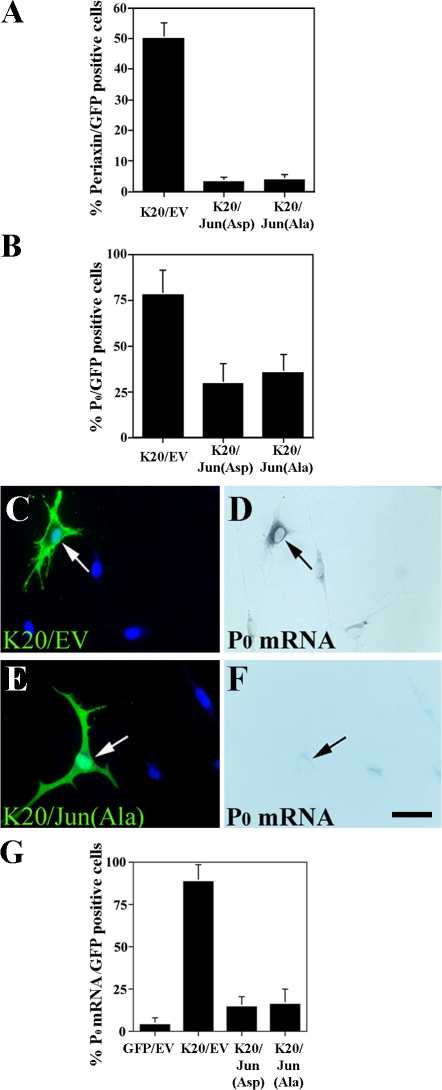
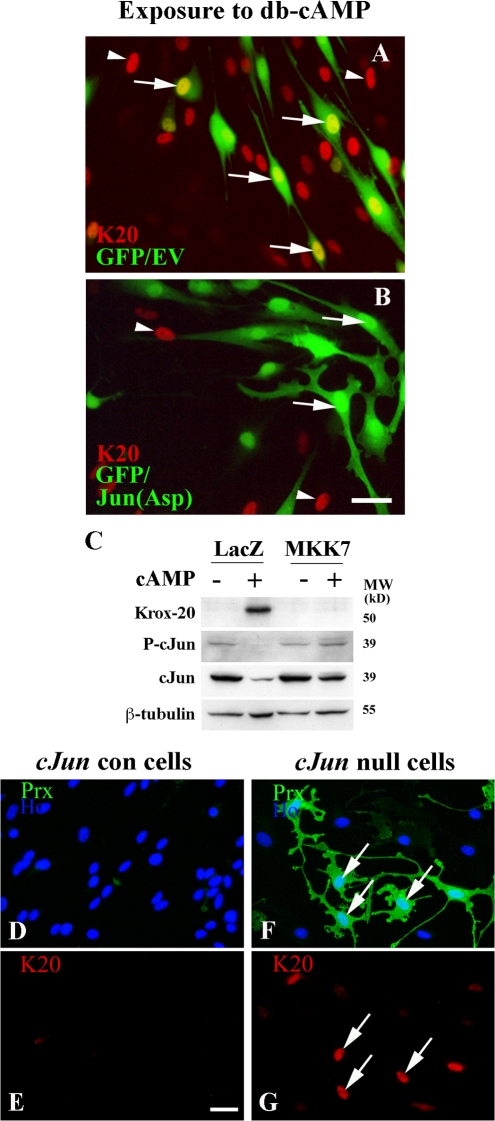
By gene transfection, we enforced expression of two different c-Jun molecules: Jun(Asp), in which all potential N-terminal phosphorylation sites have been mutated to aspartic acid, which mimics phosphorylated c-Jun; and Jun(Ala), in which all potential N-terminal phosphorylation sites are mutated to alanine and therefore cannot be phosphorylated by JNK (Papavassiliou et al., 1995; Watson et al., 1998). Using cotransfection, we showed that both Jun(Asp) and Jun(Ala) expression strongly (P < 0.001) inhibit the induction of both periaxin and P0 protein and mRNA by Krox-20 (Fig. 3, A–G; and not depicted). Expression of exogenous Jun proteins from both constructs was fully maintained in spite of cotransfection with Krox-20 (unpublished data).
Figure 3.
Persistent expression of c-Jun inhibits Krox-20–induced myelin protein expression. (A and B) Cotransfection of Krox-20/GFP with Jun(Asp) or with Jun(Ala) inhibits Krox-20–mediated induction of periaxin and P0. K20/EV represents cells cotransfected with Krox-20 and control vector. (C–F) P0 in situ experiment showing that cotransfection of Krox-20/GFP with Jun(Ala) inhibits Krox-20–mediated induction of P0 mRNA. C and D are controls, and the arrows show a cell coexpressing Krox-20 and a control vector where Krox-20 has induced P0 mRNA. Arrows in E and F show a cell coexpressing Krox-20 and Jun(Ala) where Jun(Ala) has inhibited Krox-induced P0 expression. Bar, 15 μm. (G) Percentages of GFP-positive cells that also express P0 mRNA in cells cotransfected with the constructs indicated. Error bars show one standard deviation of the mean.
This shows that effective induction of myelin genes by Krox-20 depends on Krox-20 simultaneously suppressing c-Jun. If this is prevented by enforcing sustained expression of Jun molecules, myelin gene expression is blocked or severely reduced. Notably, this inhibition of myelin genes does not appear to depend on phosphorylation of c-Jun.
MAPK kinase 7 (MKK7) inhibits myelin gene expression by elevating c-Jun
MKK7 specifically phosphorylates and activates JNK, which results in phosphorylation of c-Jun and sometimes in elevation of the levels of c-Jun protein. This cascade is thought to be an important activator of c-Jun signaling in several cell types. We therefore tested whether MKK7 would inhibit myelin differentiation.
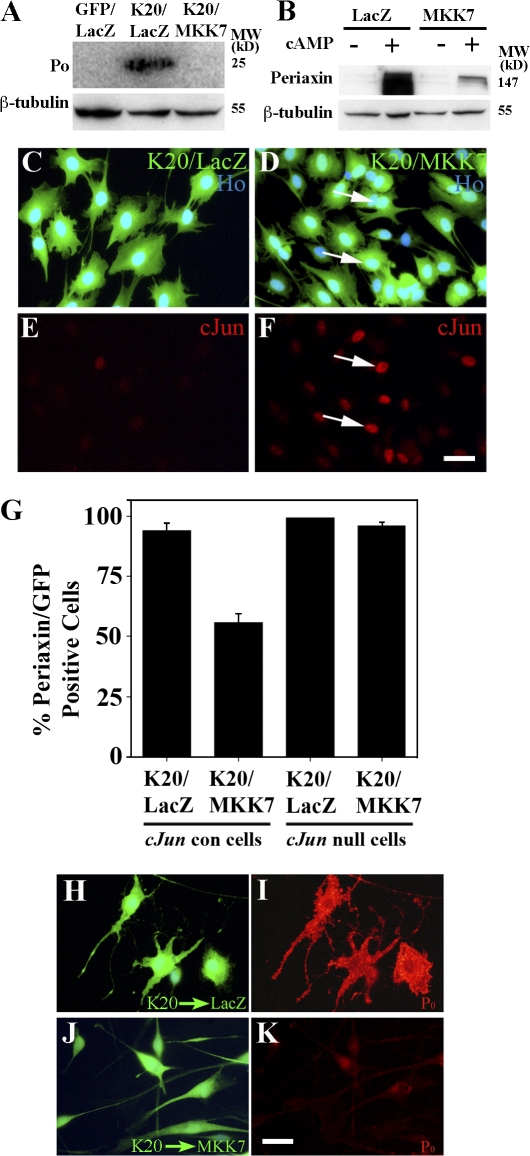
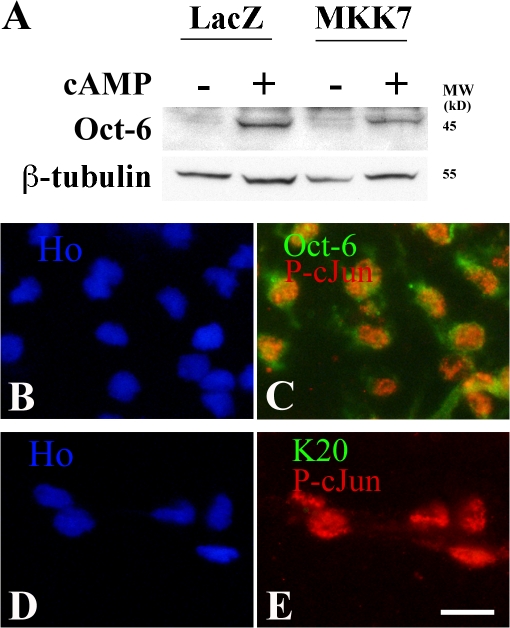
By infecting cells with an MKK7-expressing adenovirus, we found that MKK7 was a powerful inhibitor of Krox-20– or db-cAMP–induced myelin gene expression (Fig. 4, A and B). Enforced MKK7 expression also activated c-Jun in the Krox-20–expressing cells, in which c-Jun would normally be reduced to undetectable levels (Fig. 4, C–F). Similarly, MKK7 elevated c-Jun protein in cells in which c-Jun had been suppressed by db-cAMP (see Fig. 8 C). This suggested that MKK7 inhibited myelin genes by activating c-Jun. To demonstrate this, we compared the effect of MKK7 in c-Jun–null cells with that in control cells. As expected from the Western blots (Fig. 4, A and B), MKK7 significantly (P < 0.001) reduced the number of control cells that expressed periaxin in response to Krox-20 (Fig. 4 G). Importantly, this inhibitory effect was abolished in c-Jun–null cells (Fig. 4 G). Therefore, MKK7-mediated inhibition of myelin gene expression depends on c-Jun.
Figure 4.
MKK7 inhibits myelin gene expression in a c-Jun–dependent way. (A) Western blot showing that MKK7, presumably by activating JNK, inhibits Krox-20–induced myelin protein expression. The cells were coinfected with adenoviruses expressing the constructs indicated. (B) Western blot of cells infected with adenoviruses expressing control LacZ or activated MKK7 to activate JNK. Note that periaxin induced by 2 d of exposure to 1 mM of db-cAMP in LacZ control cells is inhibited by MKK7 expression. (C–F) MKK7 activates c-Jun even in the presence of Krox-20. C and E show that Krox-20 coinfected with a control LacZ-expressing adenovirus suppresses c-Jun levels. D and F show that when Krox-20 is coexpressed with MKK7, high c-Jun levels are maintained. (G) MKK7-mediated suppression of myelin gene expression depends on c-Jun. In normal cells (cJun con), Krox-20–induced periaxin expression (K20/LacZ) is suppressed by MKK7 (K20/MKK7). This suppression does not occur when this experiment is repeated in cells without c-Jun (cJun null). Error bars show one standard deviation of the mean. (H–K) Reactivation of JNK/c-Jun in Krox-20–expressing cells that already synthesize P0 abolishes P0 protein expression. Retrovirally infected cells already expressing Krox-20 and P0 were infected with either LacZ control (H and I) or MKK7-expressing (J and K) adenoviruses. Cells were labeled with either LacZ and P0 (H and I) or MKK7 and P0 (J and K) antibodies. Note down-regulation of P0 protein in Krox-20–expressing cells infected with MKK7 adenovirus. Bars, 15 μm.
Figure 8.
Cross-inhibitory relationship between c-Jun and Krox-20. (A and B) Cells cotransfected with empty GFP vector (to visualize transfected cells) and an empty control vector (EV; A) or a Jun(Asp) vector (B). Both cultures were then treated with 1 mM db-cAMP for 2 d to induce Krox-20 and were immunolabeled for Krox-20. In A, arrows point to induced Krox-20 in nuclei of GFP-positive control cells (yellow nuclei of Krox-20–positive GFP-positive cells). In B, no Krox-20 is induced (arrows) in cells containing Jun(Asp). Arrowheads in both panels indicate untransfected cells that have been induced to express Krox-20 by db-cAMP as controls for induction. (C) Activation of JNK inhibits induction of Krox-20. Western blot of cells infected with adenovirus expressing control LacZ or virus expressing activated MKK7 to activate JNK is shown. Note that the Krox-20 and periaxin induced by 2 d of exposure to 1 mM db-cAMP in LacZ control cells is inhibited by MKK7 expression. Note also that MKK7 elevates c-Jun in the presence of db-cAMP. (D–G) In c-Jun–null cells, loss of Krox-20 expression is significantly delayed. Double immunolabeling of c-Jun control cells (D and E) and c-Jun–null cells (F and G) for Krox-20 (red) and periaxin (green) after 2 d in culture in DM containing 20 ng/ml NRG-1 is shown. Note that Krox-20 has disappeared from the control cells, whereas many c-Jun–null cells still have Krox-20–positive nuclei (G, arrows). Note that c-Jun–null Krox-20–positive cells are also periaxin positive (F, arrows), whereas control cells have lost periaxin expression (D). Bars, 15 μm.
We also showed that delayed activation of c-Jun could reverse P0 expression, even in cells in which P0 expression was already established by retroviral expression of Krox-20. We added MKK7 adenovirus to activate c-Jun expression in cells that were already expressing high levels of P0. We found that, despite ongoing Krox-20 expression, MKK7 reversed the effects of Krox-20 and reduced P0 protein levels (Fig. 4, H–K; and see Materials and methods).
In the two previous sections, we showed that basal constitutive levels of c-Jun and enforced expression of Jun molecules suppress myelin genes. In this section, we revealed inhibition of myelin genes by MKK7/JNK-mediated activation of c-Jun. Therefore, all three ways of examining c-Jun signaling agree, showing that c-Jun has the potential to negatively regulate myelin differentiation.
c-Jun activation inhibits myelination in dorsal root ganglion (DRG)/Schwann cell cocultures
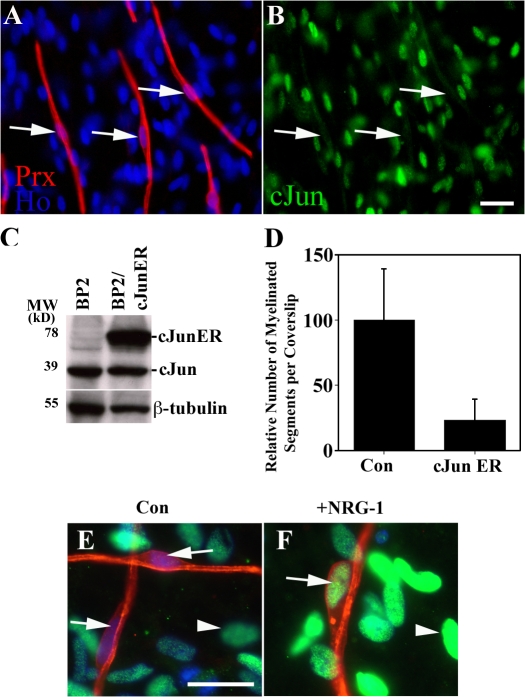
If c-Jun is a negative regulator of myelination, enforced activation of c-Jun in Schwann cells should inhibit myelination in DRG/Schwann cell cocultures. We verified that myelination in these cultures was associated with c-Jun down-regulation, as seen both in vivo and in response to Krox-20 expression. Double immunolabeling of myelinating Schwann cell/DRG cultures with antibodies to periaxin and c-Jun confirmed that ≥90% of the periaxin-positive myelinating cells no longer expressed c-Jun (Fig. 5, A–C). The remaining periaxin-positive cells showed weak c-Jun labeling, perhaps reflecting suboptimal conditions for myelination in vitro because c-Jun–positive nuclei are not seen in myelinating cells of normal nerves in vivo. To determine whether enforced c-Jun expression inhibits myelination, Schwann cells were retrovirally infected with either empty vector (BP2) or vector expressing a c-Jun–estrogen receptor fusion protein (BP2/cJunER), in which the transcriptional activity of c-Jun can be activated by the addition of 4-hydroxytamoxifen (Bossy-Wetzel et al., 1997). 2 wk after seeding purified neurons with either control (BP2) or cJunER-expressing (BP2/cJunER) cells, the cocultures were induced to myelinate by addition of ascorbate in the presence of 10−7 M 4-hydroxytamoxifen to activate the cJunER protein. 2 wk later, the cultures were immunolabeled for myelin basic protein (MBP) to identify myelinating cells, and the total number of myelinating Schwann cells per coverslip was counted. We found that activation of c-Jun significantly (P < 0.01) inhibited myelination (Fig. 5 D). In related experiments, we also induced c-Jun in DRG/c-JunER Schwann cell cocultures with tamoxifen, 2 wk after ascorbate had been added to induce myelination. In this case too there was a substantial reduction in the number of myelin segments relative to that seen in control cultures without tamoxifen, indicating that induction of c-Jun can exert effects both early and late in the myelination program (see Materials and methods).
Figure 5.
c-Jun expression inhibits myelination in Schwann cell/DRG neuron cocultures. (A and B) c-Jun is down-regulated in myelinating cells. Arrows show c-Jun–negative nuclei in periaxin-positive (red) myelinating cells. Numerous other cells that are not forming myelin remain c-Jun positive (green). (C) Western blot showing expression of endogenous c-Jun and cJunER fusion protein in purified Schwann cells retrovirally infected with either control empty vector (BP2) or vector expressing the cJunER fusion protein (BP2/cJunER). (D) The number of myelinating segments in cocultures is reduced by c-Jun expression (cJun ER). Error bars show one standard deviation of the mean. (E and F) In cocultures, c-Jun is reactivated in myelinating cells induced to demyelinate by high concentrations of NRG-1. (E) Control cultures with arrows showing c-Jun–negative nuclei associated with periaxin-positive (red) myelinating cells. Arrowhead shows c-Jun (green) in a nucleus not associated with myelinating cells. (F) Cultures exposed to 200 ng/ml NRG-1 for 3 d. Arrow shows activation of c-Jun in a degenerating myelin internode and arrowhead indicates c-Jun in a nucleus of a cell not engaged in myelin formation. Bars, 15 μm.
Exposure of DRG/Schwann cell cocultures to high concentrations of NRG-1 provides a demyelination signal and results in fragmentation of myelin internodes (Zanazzi et al., 2001). Using 200 ng/ml NRG-1 to confirm this, we found that the demyelination was accompanied by extensive increase in nuclear c-Jun expression, with ∼60% of identifiable myelin internodes now showing clear nuclear c-Jun expression (Fig. 5, E and F). This observation is consistent with c-Jun providing a signal that opposes myelin differentiation and promotes the immature Schwann cell phenotype. Collectively, these observations strengthen the conclusion that c-Jun is a negative regulator of the myelin program (Parkinson et al., 2004).
A central role for c-Jun in promoting dedifferentiation
When actively myelinating cells in developing nerves are removed from axonal contact, for example, by preparing dissociated cell cultures from neonatal nerves, not only is myelination arrested but cells revert to the immature phenotype: c-Jun reappears, Krox-20 levels fall, and cells stop expressing myelin genes and reactivate genes characteristic of immature Schwann cells (Jessen and Mirsky, 2005). Because c-Jun potentially blocks the forward transition toward myelination, we investigated whether c-Jun was also required for, or promoted, the reverse transition back to the immature phenotype. This possibility was also raised by two previous observations, namely the reactivation of c-Jun in demyelinating cocultures and the finding that delayed activation of c-Jun reversed myelin gene expression already established through enforced Krox-20 expression.
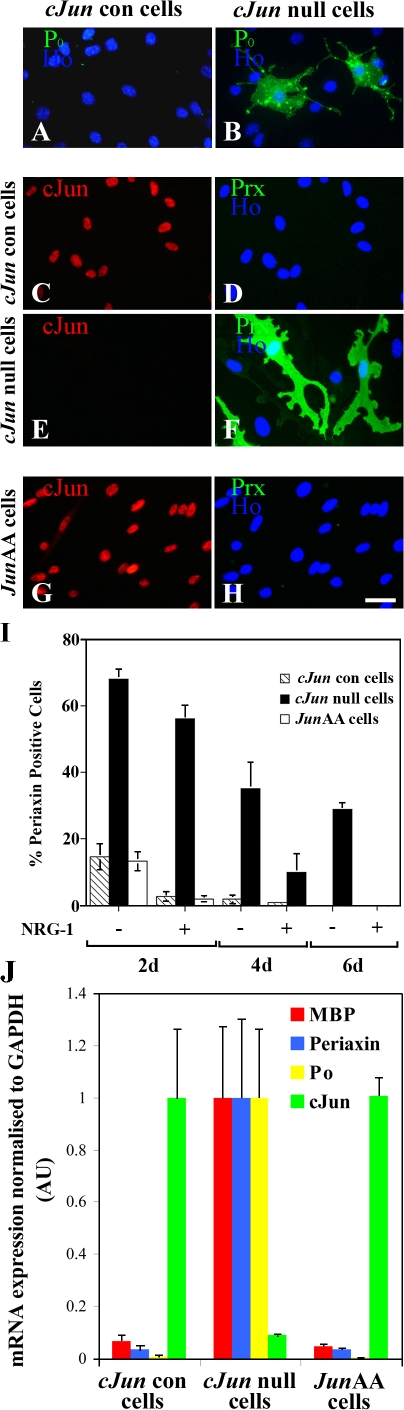
To test this, we examined the rate of dedifferentiation of neonatal myelinating cells in the absence of c-Jun. To obtain such cells, we used c-Jun–null Schwann cells from mice in which floxed c-Jun (Junfl/fl) had been excised in Schwann cells selectively by breeding with P0-CRE mice (see Materials and methods). No obvious morphological or immunohistochemical abnormalities were noted in these nerves by a morphological examination (and adult nerves contained the expected numbers of apparently normal myelinated and unmyelinated fibers; unpublished data). Actively myelinating cells were removed from axonal contact by dissociating neonatal nerves (postnatal day [P] 2–5) and plating the cells in culture. In wild-type cultures, c-Jun, which had been down-regulated in myelinating cells, was rapidly reexpressed after plating and seen in the large majority of myelinating cells by 6 h, whereas cultures from c-Jun–null animals were essentially c-Jun negative (unpublished data). In other cultures maintained for 5–7 d, immunolabeling showed that 100% of control cells (Junfl/fl; P0-CRE−) had c-Jun–positive nuclei, whereas ≤5% of cells from c-Jun–null mice (Junfl/fl; P0-CRE+) expressed c-Jun, indicating effective removal of c-Jun. NRG-1 was included in the medium in some experiments because NRG-1 signaling is up-regulated in cut nerves and has been implicated in the promotion of Schwann cell dedifferentiation after injury (Carroll et al., 1997; Kwon et al., 1997; Cheng et al., 1998; Zanazzi et al., 2001; Guertin et al., 2005). We found that the rate of loss of the myelin-associated proteins periaxin and P0 was strongly delayed in c-Jun–null cultures compared with c-Jun–positive controls (Fig. 6, A–F and I). A similar delay was seen when mRNA levels for periaxin, P0, and MBP were examined in these cultures by quantitative RT-PCR (Fig. 6 J). Therefore, when early myelinating cells are removed from axons, the rapid reactivation of c-Jun pushes the cells toward the immature phenotype.
Figure 6.
Neonatal myelinating cells dedifferentiate slowly in the absence of c-Jun. (A and B) Comparison of P0 expression in control cells from c-Junfl/fl/CRE−(cJun con) mice and cells without c-Jun from c-Junfl/fl/CRE+ (cJun null) mice prepared at P5 and cultured in DM containing 20 ng/ml NRG-1 for 4 d. (A) Cells with high myelination-related levels of P0 have largely disappeared from control cultures. (B) c-Jun–null cultures retain numerous cells with high P0, two of which are illustrated. (C–F) Similar delay in the loss of periaxin in cells prepared at P2 from nerves of cJun–null mice cultured for 2 d (see Materials and methods). (C and D) Control cultures are c-Jun positive and periaxin has largely disappeared. (E and F) c-Jun–null cultures are c-Jun negative and retain a large number of strongly periaxin-positive cells. (G and H) Similar experiments with cells from JunAA mice. These cells lose periaxin expression at the same rate as control cells, indicating that the major N-terminal phosphorylation sites of c-Jun are not needed for suppression of periaxin. Bar, 15 μm. (I) Quantification of the results illustrated in C–H. The graph shows the number of periaxin-positive cells (relative to the number 3 h after plating) in c-Jun control, c-Jun–null and JunAA cells after 2, 4, and 6 d in DM ± 20 ng/ml NRG-1. (J) mRNA for myelin genes disappears only slowly from c-Jun–null cells. Schwann cells purified from control, c-Jun–null, and JunAA P3 mice were plated for 48 h in DM before RNA was extracted and levels of myelin markers were analyzed by quantitative PCR. Relative expression levels of c-Jun, MBP, periaxin, and P0 (MPZ) were obtained by normalizing samples to GAPDH. The data were then expressed as a percentage of the maximum (1) for each of the four genes. The difference in MBP, P0, and periaxin mRNA levels between c-Jun control and null mice was significant (P < 0.01). The same applies to the differences between JunAA and null mice. The dataset for each gene was analyzed by one-way ANOVA and Tukey's Multiple Comparison test where appropriate (using GraphPad Prism Software). Error bars show one standard deviation of the mean. AU, arbitrary units.
N-terminal phosphorylation of c-Jun is not required for inhibition of myelin differentiation (Fig. 3). In agreement with this, Schwann cells from homozygous JunAA mice, in which the major N-terminal phosphorylation sites (serine-63 and -73) of both c-Jun alleles have been mutated to nonphosphorylatable alanine (Behrens et al., 1999), dedifferentiated at a similar rate to that of control cells (Fig. 6, G–I). This was confirmed at the mRNA level using quantitative RT-PCR (Fig. 6 J). Thus, the activity of the c-Jun protein drives myelinating cells toward the immature phenotype without requiring N-terminal phosphorylation.
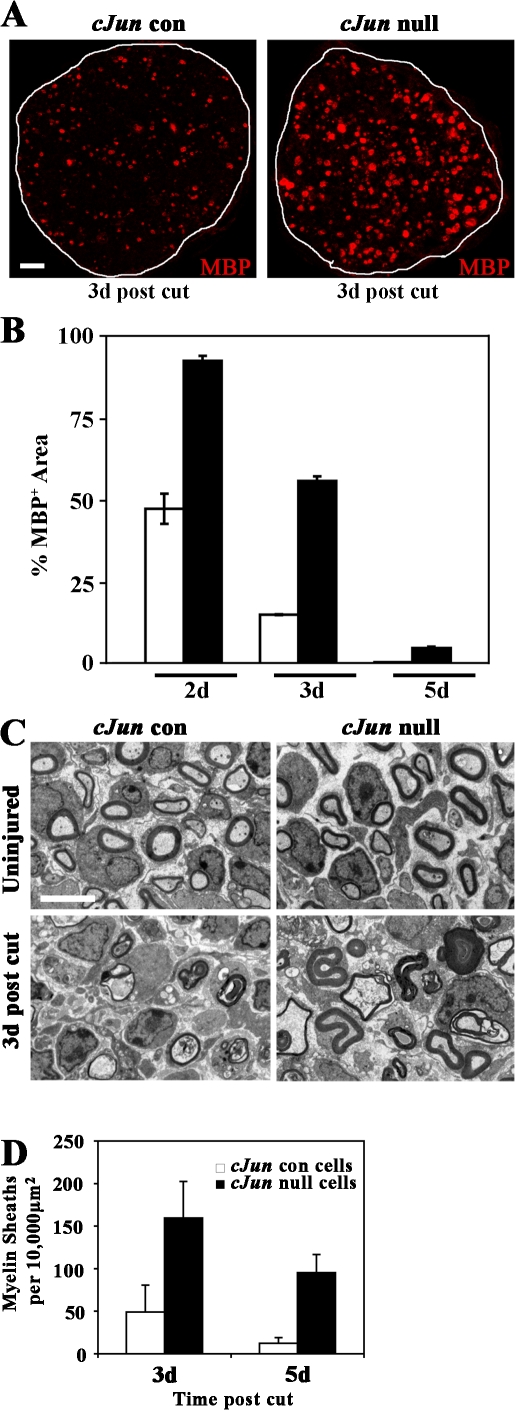
These experiments show that c-Jun has a role in sending actively myelinating cells back to the immature phenotype when the cells are removed from axonal contact in vitro. To test whether c-Jun also acted in this way in vivo, we cut the sciatic nerves of 5-d-old conditional c-Jun–null (Junfl/fl; P0-CRE+) and control (Junfl/fl; P0-CRE−) mice and compared the rate of myelin loss as a measure of dedifferentiation. Morphologically, this was done by counting the number of rounded or collapsed myelin sheaths that remained at 3 and 5 d after the operation. The results were confirmed by measuring the disappearance of MBP, using immunolabeling and image analysis of transverse nerve sections at 2, 3, and 5 d (Fig. 7; Perry et al., 1995). Both measures showed a marked delay of myelin loss in the c-Jun–null nerves. Thus, c-Jun, when reexpressed after injury, promotes dedifferentiation in vitro and in vivo.
Figure 7.
c-Jun drives dedifferentiation in vivo. (A) MBP immunolabeling of sciatic nerve sections showing delayed loss of myelin in c-Jun–null nerves compared with controls, 3 d after transection of nerves of 5-d-old mice. Bar, 10 μm. (B) Quantification of the delay in myelin disappearance by quantitative image analysis of MBP-immunolabeled sections (comparable to those shown in A) 2, 3, and 5 d after injury (expressed as percentage of MPB+ area in uncut P5 nerve). In every case, the difference between c-Jun–null and control nerves is significant (P < 0.01). (C) Electron micrographs showing c-Jun–null and control nerves from 5-d-old mice, intact and 3 d after injury as indicated. Note preservation of rounded or partially collapsed myelin sheaths in c-Jun–null nerves. Bar, 4 μm. (D) Counts of myelin sheaths (rounded or collapsed) in c-Jun–null and control nerves 3 and 5 d after injury (3 d, P < 0.05; 5 d, P < 0.01). Error bars show standard deviation of the mean.
A cross-inhibitory relationship between c-Jun and Krox-20
The fact that Krox-20 and c-Jun have opposing effects on myelination and that Krox-20 suppresses c-Jun raised the question of whether c-Jun, in turn, suppressed Krox-20 because cross-inhibitory relationships between transcription factors involved in fate determination have been described in other systems. We found that c-Jun suppressed Krox-20 in several types of experiment. First, enforced c-Jun expression in wild-type cells, using Jun(Asp) or Jun(Ala), completely blocked Krox-20 expression in response to db-cAMP elevation (Fig. 8, A and B; and not depicted). Second, db-cAMP–induced Krox-20 expression was blocked when c-Jun was activated by enforced expression of the upstream kinase MKK7 and the results were analyzed by Western blot (Fig. 8 C). Note that MKK7 also prevented the db-cAMP–induced down-regulation of c-Jun protein and phospho–c-Jun. Lastly, we examined the loss of Krox-20 that is seen when neonatal myelinating cells are removed from axonal contact and placed in culture (Fig. 8, D–G). When cells from control P2 nerves were plated in DM, Krox-20 protein expression disappeared within 24 h as expected. But when cells from c-Jun–null nerves were used, robust Krox-20 immunoreactivity remained in many cells at 2 d (Fig. 8 G), although Krox-20 immunolabeling decreased and eventually disappeared thereafter (not depicted). Therefore, when myelinating cells from normal neonatal nerves lose axonal contact, the resulting reactivation of c-Jun (above) contributes significantly to the rapid drop in Krox-20 that is seen under these circumstances.
Thus c-Jun suppresses Krox-20 expression in several situations. In contrast, we found that c-Jun did not suppress the myelin-related transcription factor Oct-6, which appears earlier than Krox-20 in developing nerves and controls the timing of Krox-20 activation, although its expression is not restricted to myelinating Schwann cells (Bermingham et al., 1996; Blanchard et al., 1996; Jaegle et al., 1996). Enforced expression of MKK7 to activate c-Jun did not significantly suppress db-cAMP–induced Oct-6 expression (Fig. 9 A). Also, phospho–c-Jun and Oct-6 were coexpressed by Schwann cells before myelination (Fig. 9, B–E). Coexpression of c-Jun and Krox-20 is seen neither in these cells nor later in development, when c-Jun is selectively lost from cells that activate Krox-20 and myelinate (Parkinson et al., 2004). Onset of myelination therefore involves a transition from immature cells coexpressing Oct-6 and c-Jun to myelinating cells that are c-Jun negative but coexpress Oct-6 and Krox-20, although Oct-6 disappears later from most myelinating cells.
Figure 9.
The JNK–c-Jun pathway does not suppress Oct-6. (A) Western blot of Schwann cells infected with adenoviruses expressing control LacZ or activated MKK7 to activate JNK. Note comparable induction of Oct-6 (by 1 mM db-cAMP for 24 h) in control cells and MKK7 cells. (B–E) Teased E17 sciatic nerve stained with HOECHST dye showing that Oct-6 and phospho–c-Jun are coexpressed in immature Schwann cells. (B and C) Double immunolabeling for phospho–c-Jun (P-cJun; red) and Oct-6 (green) in C shows coexpression in Schwann cell nuclei. (D and E) Double immunolabeling for phospho–c-Jun (red) and Krox-20 (green) in E shows phospho–c-Jun alone. Bar, 15 μm.
Krox-20 and c-Jun regulate Sox-2
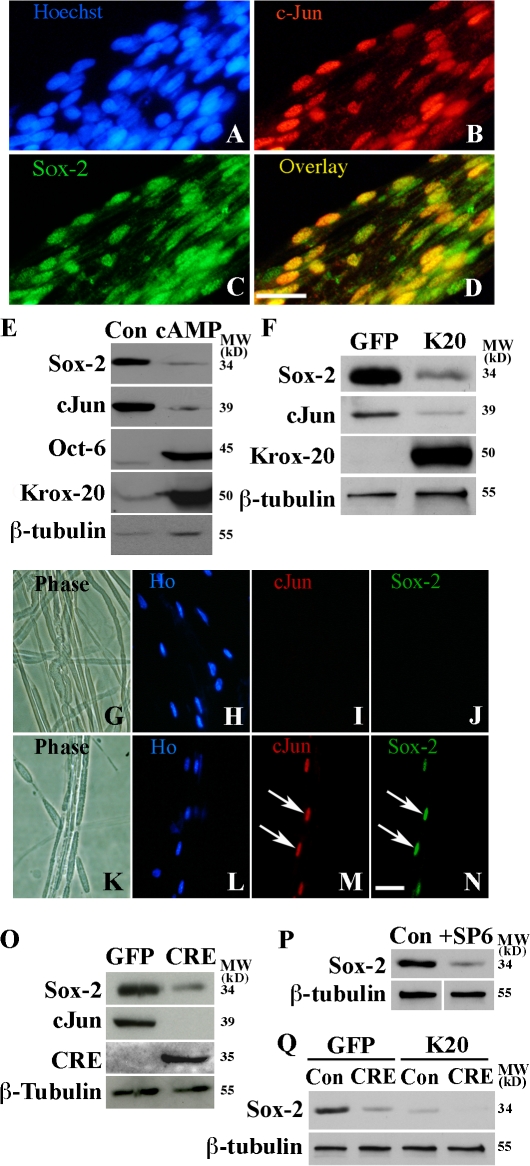
Because Sox-2 has been implicated in negative regulation of myelination (Le et al., 2005b), we examined whether Sox-2 and c-Jun were coexpressed and whether Sox-2 was suppressed by cAMP or Krox-20, as expected of a negative myelin regulator. We found that Sox-2 and c-Jun were coexpressed in the nuclei of immature Schwann cells that have not yet myelinated (Fig. 10, A–D) and were reactivated and coexpressed after injury (Fig. 10, G–N; and not depicted). In vitro, db-cAMP or enforced Krox-20 suppressed Sox-2 (Fig. 10, E and F). Thus, db-cAMP and Krox-20 suppress both c-Jun and Sox-2.
Figure 10.
Regulation of Sox-2 by Krox-20 and c-Jun. (A–D) Immunolabeling of teased newborn sciatic nerve. Nuclear c-Jun is in premyelinating cells (Parkinson et al., 2004), most of which also express Sox-2 (overlay). (E) db-cAMP down-regulates Sox-2 protein. Western blot of control untreated cells (Con) and cells treated with 1 mM db-cAMP for 3 d (cAMP) is shown. Note down-regulation of c-Jun and Sox-2 and increase in Oct-6 and Krox-20. (F) Krox-20 down-regulates Sox-2 and c-Jun. Western blot of cells 3 d after infection with either GFP control (GFP) or Krox-20 (K20) adenoviruses. (G–N) c-Jun and Sox-2 are coexpressed after nerve injury. Double immunolabeling of a teased uncut nerve (G–J) and of the distal stump of a nerve 3 d after transection (K–N). Note that neither c-Jun nor Sox-2 are expressed in intact nerve, whereas both factors are activated by injury and found in the same nuclei (arrows). Bars, 15 μm. (O) Sox-2 protein is reduced in c-Jun–null cells. Western blot of mouse Junfl/fl Schwann cells infected with either GFP control (GFP) or CRE-recombinase (CRE) adenoviruses. Note reduced levels of Sox-2 in CRE recombined cells. (P) Inhibition of JNK reduces Sox-2 protein levels. Western blot for Sox-2 in control cells (Con) and cells treated for 2 d with 30 μM SP600125 JNK inhibitor (+SP6). (Q) Western blot showing that enforced Krox-20 expression suppresses residual Sox-2 in c-Jun–null cells. Control cells (Con) and c-Jun–negative cells (CRE) were infected with control adenovirus (GFP) or adenovirus expressing Krox-20 (K20). Note the two-step reduction in Sox-2, first by removing c-Jun alone (GFP and CRE) and second by expressing Krox-20 from c-Jun–null cells (K20 and CRE).
In c-Jun–null cells generated by CRE expression in vitro, Sox-2 protein levels were reduced (Fig. 10 O). In accord with this, inhibition of JNK (which controls c-Jun levels; Figs. 4 and 8) by the inhibitor SP600125 or by adenoviral expression of the JNK binding domain of JIP-1 also reduced Sox-2 protein expression (Fig. 10 P and not depicted). Sox-2 remaining in cultures of c-Jun–null cells was suppressed to undetectable levels by enforced Krox-20 expression (Fig. 10 Q).
Collectively, these experiments show that c-Jun and Sox-2 are extensively coregulated. They are consistent with the possibility that c-Jun plays a role in regulating Sox-2 and that some of the inhibitory effects of c-Jun on myelination may be channeled through Sox-2.
Discussion
Much as Krox-20 promotes the transition from the immature Schwann cell phenotype toward myelination, we find that c-Jun acts in a reverse way to promote the immature Schwann cell state at the expense of myelination. c-Jun, at physiological expression levels and apparently without N-terminal phosphorylation, inhibits myelin gene activation by Krox-20 or cAMP elevation. Enforced c-Jun expression also inhibits myelination in cocultures. When early myelinating cells are released from axon-associated promyelin signals and placed in culture, the resulting c-Jun activation drives the cells back to the immature state. Also in vivo, reactivation of c-Jun after injury of neonatal nerves pushes early myelinating cells toward demyelination. In previous work, we also showed that c-Jun is important for events that characterize the immature but not myelinating state, namely cell death and proliferation (Parkinson et al., 2004).
The most striking abnormality in early postnatal nerves in conditional c-Jun–null mice is the failure of myelinating cells to dedifferentiate normally after injury. It is therefore likely that the negative influence of c-Jun on myelin differentiation that we have documented is, in vivo, more important for pushing the dedifferentiation program in injured or pathological nerves than for the regulation of myelination during normal development.
Consistent with the involvement of Krox-20 and c-Jun in antagonistic programs, expression of these factors is mutually exclusive: immature cells express relatively high levels of c-Jun and low levels of Krox-20, switching to high levels of Krox-20 and low levels of c-Jun in myelinating cells. In cut or crushed nerves, Krox-20 falls and c-Jun is reexpressed at high levels as cells transit back to the immature state. Furthermore, c-Jun and Krox-20 show a cross-antagonistic functional relationship because expression of Krox-20 suppresses c-Jun and enforced expression of c-Jun suppresses Krox-20.
c-Jun is therefore a transcription factor that negatively regulates the myelinating Schwann cell phenotype, representing a signal which functionally stands in opposition to the network of promyelin transcription factors, which includes Oct-6, Brn2, NFκB, Sox-10, and Nab1 and 2, in addition to the key role of Krox-20 (Topilko et al., 1994; Nagarajan et al., 2001; Jaegle et al., 2003; Le et al., 2005b; Ghislain and Charnay, 2006; LeBlanc et al., 2006). Negative regulation of myelin differentiation is likely to emerge as a major aspect of Schwann cell biology, with particular importance for plasticity, demyelinating pathology, and responses to injury and regeneration, in addition to developmental regulation. It is also likely that the molecular machinery of myelin suppression will turn out to be equally complex to that of its promyelin counterpart. Thus, inducible nitric oxide synthase, Sox-2, Erk1/2 activation, and Notch signaling have all been implicated in negative control of myelin differentiation (Harrisingh et al., 2004; Ogata et al., 2004; Le et al., 2005a; Agthong et al., 2006; Woodhoo, A., M. Duran, K.R. Jessen, and R. Mirsky. 2004. Differentiation. Abstr. 119). Similarly, the p38 MAPK signaling pathway, although important for events immediately before myelination, also accelerates demyelination (Fragoso et al., 2003; unpublished data), and delayed demyelination is seen in mice with genetic inactivation of Toll receptors, nitric oxide synthase, phospholipase A2, and matrix metalloprotease 9 (Levy et al., 2001; De et al., 2003; Shubayev et al., 2006; Boivin et al., 2007). In future work, it will be important to learn about the functional interactions between the different signaling systems that are emerging as potential myelin suppressors.
We find that Krox-20 represses both c-Jun and Sox-2 and that, in the absence of Krox-20, c-Jun is highly expressed in Schwann cells without the need for specific extrinsic signaling. Therefore, the loss of Krox-20 that follows nerve cut or crush is likely to allow the cells to resume their constitutive c-Jun expression. This will help push the cells toward dedifferentiation and promote proliferation as seen in Wallerian degeneration. A similar mechanism is likely to be operative during the dedifferentiation and proliferation that takes place when Krox-20 is genetically inactivated in adult nerves without axon transection (Decker et al., 2006). The way in which c-Jun and Sox-2 interact during these events remains to be examined.
Although we have shown previously that in Schwann cells, as in other cell types, c-Jun has a role in controlling proliferation (Parkinson et al., 2004), this effect can be clearly dissociated from the negative regulation of myelin differentiation. Several of the present experiments demonstrate this, including those in which genetic removal of c-Jun amplifies myelin gene expression in response to Krox-20 or cAMP and where myelin gene activation by these signals is inhibited by enforced expression of Jun or JNK activation by MKK7. It is also seen when clearance of Krox-20 and myelin proteins from early myelinating cells that have been removed from axons and placed in DM is accelerated by c-Jun or JunAA. In all these cases, Jun inhibits myelination or drives demyelination under conditions where Schwann cells are not dividing.
Suppression of myelination by c-Jun can also be dissociated from the classical N-terminal phosphorylation of c-Jun typically performed by JNK. Rather, suppression of the myelin phenotype appears to depend on the levels of the c-Jun protein itself. This can be seen from the fact that Jun(Ala), in which all potential N-terminal phosphorylation sites are absent, suppressed Krox-20–induced myelin gene expression as effectively as Jun(Asp), in which the N-terminal phosphorylation sites have been mutated to aspartic acid to mimic the active phosphorylated c-Jun. It is also evident from the observation that normal c-Jun and nonphosphorylatable c-Jun (in cells from JunAA mice) appear to be equally effective at driving down myelin gene expression in experiments where neonatal myelinating cells are removed from axonal contact and placed in culture. This is in line with results from other systems, where phosphorylation of c-Jun by JNK is not required for all its actions (Behrens et al., 1999; Raivich and Behrens, 2006). The JNK pathway may instead function to control c-Jun protein levels, in part by modulating the activity of several transcription factors that together regulate the c-jun promoter (Besirli et al., 2005), which is a situation that would explain the effects of the JNK activator MKK7 in our experiments.
In injured nerves, loss of myelin differentiation is a beneficial response that helps axonal regeneration and repair. But the loss of myelin differentiation in demyelinating neuropathies, such as Charcot-Marie-Tooth 1A and Guillain-Barré syndrome, is unhelpful and leads to disability. From a clinical standpoint, it will be important to determine whether c-Jun is a component of the mechanism that pushes myelinating cells toward demyelination in these pathological conditions because this would open new avenues for clinical intervention.
Materials and methods
HA-tagged Jun(Asp)- and Jun(Ala)-expressing plasmids were obtained from Dirk Bohmann (University of Rochester Medical Center, Rochester, NY; Papavassiliou et al., 1995). The cJunER fusion protein construct was obtained from Moshe Yaniv (Centre National de la Recherche Scientifique Institute Pasteur, Paris, France; Bossy-Wetzel et al., 1997). The JNK inhibitor SP600125 (Bennett et al., 2001) was obtained from BIOMOL International, L.P. NGF was obtained from Invitrogen. Oct-6 antibodies were obtained from Dies Meijer (Erasmus University Medical Center, Rotterdam, Holland) and John Bermingham (McLaughlin Research Institute, Great Falls, Montana) and periaxin antibodies were obtained from Peter Brophy and Diane Sherman (Centre for Neuroscience Research, Edinburgh, Scotland). Serine-63 phospho–c-Jun polyclonal antibody was obtained from Cell Signaling Technology. Polyclonal MKK7 antibodies and monoclonal antibodies to JNK1, JNK2, and serine-63 phospho–c-Jun were obtained from Santa Cruz Biotechnology, Inc. Polyclonal CRE recombinase and Sox-2 and lacZ antibodies were obtained from EMD and Millipore, respectively. 4-hydroxytamoxifen was obtained from Sigma-Aldrich. Monoclonal antibody to MBP was obtained from Sternberger Monoclonals. Polyclonal goat anti–rabbit Ig FITC was obtained from MP Biomedicals, donkey anti–mouse Ig Cy3 and streptavidin-Cy3 were obtained from Jackson ImmunoResearch Laboratories, and biotinylated sheep anti–mouse Ig was obtained from GE Healthcare. HOECHST dye was obtained from Sigma-Aldrich. Sources of other reagents are detailed elsewhere (Morgan et al., 1991, 1994; Archelos et al., 1993; Jessen et al., 1994; Dong et al., 1995; Stewart, 1995; Parkinson et al., 2001, 2003, 2004).
Transgenic mice
Animal experiments conformed to UK Home Office guidelines. To obtain Schwann cells lacking c-Jun, c-Junfl/fl mice (Behrens et al., 2002) were crossed with P0-CRE mice (Feltri et al., 2002; D'Antonio et al., 2006). Resulting P0-CRE+/c-Junfl/wt mice were crossed back to c-Junfl/fl animals. Schwann cells were prepared from P0-CRE+/c-Junfl/fl nerves, referred to as c-Jun–null cells, and from P0-CRE−/Junfl/fl littermates (controls). In some experiments, c-Jun was excised from cultured c-Junfl/fl cells using adenovirally expressed CRE-recombinase. Cells were also prepared from homozygous JunAA mice, in which serines 63 and 73 of c-Jun had been mutated to alanines (Behrens et al., 1999). Genotyping was performed as previously described (Behrens et al., 1999, 2002; Feltri et al., 2002). Immunolabeling of cells from c-Jun–null animals showed excision of c-Jun in almost all cells (Fig. 6 E).
Cell culture, transfection, infection, and protein analysis
Schwann cells were prepared from sciatic nerve and brachial plexus from newborn, P3, or P7 rats or from P3 or P5 mice as previously described (Morgan et al., 1991). Rat Schwann cells for myelin down-regulation experiments were prepared by negative immunopanning and used directly (Dong et al., 1999), whereas serum-purified cells were used elsewhere. Cells were cultured, unless otherwise stated, in serum-free supplemented medium (Jessen et al., 1994) containing 10−6 M insulin, referred to as DM. Unless otherwise stated, cell culture, gene transfer, immunohistochemistry, and Western blotting were performed as described previously (Parkinson et al., 2001, 2003, 2004).
Myelinating DRG/Schwann cell cocultures
Dissociated DRG neurons and myelinating cocultures from E15 rats were prepared essentially as described by Harrisingh et al. (2004). DRG neurons were seeded with either control infected (BP2) or cJunER (BP2/cJunER)-expressing Schwann cells and exposed to 10−7 M 4-hydroxytamoxifen to activate cJunER. For dissociated DRG cultures, DRG were dissected from E15 rats and dissociated by trypsin/collagenase digestion. Cells were plated on PDL/laminin-coated glass coverslips in MEM/10% FCS supplemented with 10−3 M insulin, 0.3% glucose, and 50 ng/ml NGF. Cultures were pulsed twice for 72 h with 10−5 M cytosine arabinoside to remove endogenous fibroblasts and Schwann cells. Approximately 2 wk after plating, the pure DRG neurons were seeded with either control infected (BP2) or cJunER (BP2/cJunER)-expressing Schwann cells. Cultures were maintained for a further 2 wk to allow Schwann cell proliferation and association with axons before treatment for a further 2 wk with medium containing 50 ng/ml ascorbate, to induce basal lamina and myelination, and 10−7 M 4-hydroxytamoxifen. The number of myelin segments per coverslip was determined after immunolabeling with MBP antibodies. Separate cultures were maintained without seeding exogenous Schwann cells to ensure that there were no endogenous Schwann cells contributing to myelination. Each experiment was performed in triplicate, and data are from four independent experiments.
In other experiments, we added tamoxifen 10–14 d after c-JunER Schwann cells had been induced to myelinate by addition of ascorbate. The tamoxifen was added for 4–6 d before fixation and immunolabeling for MBP. In this case also there was a substantial reduction in the number of myelin segments relative to control cultures, indicating that induction of c-Jun is able to exert effects both early and late in the myelination program. In two experiments, with a total of 10 experimental and 10 control coverslips, the mean reduction in the total number of myelin segments was 92.1% in cultures to which tamoxifen had been added. In related experiments, we also showed that delayed activation of c-Jun could reverse Krox-20–induced P0 expression in cultured c-JunER Schwann cells in which P0 expression was already established by adenoviral expression of Krox-20. Cells expressing cJunER were infected with Krox-20 adenovirus and c-Jun was activated 3 d later by addition of tamoxifen for 3 d before fixation and immunolabeling with P0 antibodies. In two experiments with triplicate coverslips, we found that despite ongoing Krox-20 expression, tamoxifen induction of c-Jun lowered already induced P0 protein levels from 64.7 to 34.8%.
For DRG explant cultures, DRG were dissected from rat E15 embryos and placed on PDL/laminin-coated coverslips in DM supplemented with 0.5% FCS and 50 ng/ml NGF. Cultures were maintained for 2 wk before the addition of 50 ng/ml ascorbate and for a further 2 wk to induce basal lamina formation and myelination. After this time, the cultures were fixed and immunolabeled with antibodies against P0 or MBP.
Myelin protein down-regulation assays
Schwann cells from c-Junfl/fl/CRE− (cJun con) mice and cells without c-Jun from c-Junfl/fl/CRE+ (cJun null) mice, from animals aged between P2–5, were plated on PDL/laminin-coated coverslips. 3 h after plating, time zero coverslips were fixed and immunolabeled. Sister cultures were topped up with DM alone or DM supplemented with 20 ng/ml NRG-1. Coverslips were then fixed and immunolabeled at the time points stated in the text. For quantitative evaluation of the number of periaxin-positive cells in c-Jun control and c-Jun–null cells at various time points after culturing, the number of periaxin-positive cells per coverslip was counted and expressed as a percentage of the number at time zero.
Quantitative RT-PCR
Schwann cells from P0-CRE+/Junfl/fl and P0-CRE−/Junfl/fl nerves were dissociated and plated on to PDL/laminin-coated 35-mm dishes as described in the previous section. At 3 h after plating, some dishes were used for RNA extraction (time zero control). Experimental cells were taken for RNA extraction at 48 h after plating. RNA was purified using the RNeasy plus kit (QIAGEN) and 1.5 μg RNA was converted to cDNA using the Superscript II first-strand synthesis system (Invitrogen). Real-time PCR was performed using the DyNAmo SYBR Green qPCR kit (Finnzymes) and the Opticon 2 DNA Engine (MJ Research). Relative expression values were obtained by normalizing to GAPDH for each data point.
Primer sequences for genotyping and quantitative RT-PCR
c-Junfl mice.
Forward, CCGCTAGCACTCACGTTGGTAGGC; and reverse, CTCATACCAGTTCGCACAGGCGGC.
P0-CRE mice.
Forward, GCTGGCCCAAATGTTGCTGG; and reverse, CCACCACCTCTCCATTGCAC.
Quantitative RT-PCR.
GAPDH forward, TGCACCACCAACTGCTTAG; GAPDH reverse, GGATGCAGGGATGATGTTC; KROX20 forward, AGCTGCCTGACAGCCTCTAC; KROX20 reverse, GTTTCTAGGCGCAGAGATGG; MBP forward, ACACAAGAACTACCCACTACGG; MBP reverse, GGGTGTACGAGGTGTCACAA; Periaxin forward, GACTCACCGGCAGCTAAGAG; Periaxin reverse, GCCCTTCATCTCGTATCCAG; cjun forward, ACCCCCACTCAGTTCTTGTG; cjun reverse, AGTTGCTGAGGTTGGCGTAG; P0 forward, CTGGTCCAGTGAATGGGTCT; and P0 reverse CATGTGAAAGTGCCGTTGTC.
Imaging
Immunolabeled coverslips were analyzed at room temperature with microscopes (Eclipse E8000 or Optiphot-2; Nikon) or using Plan Fluor 20× 0.5, 40× 0.75, 40× 1.3, or 40× 1.3 NA oil objectives. Images were acquired using a digital camera (DZM 1200; Nikon) and Act-1 acquisition software (Nikon). Images were processed using Photoshop (version 7.0 or 9.0; Adobe). A confocal microscope (Multi-photon UV; Leica) with constituent software and a Plan-Apochromat 40× oil objective was used at room temperature to acquire images of MBP-immunopositive areas in 5-μm wax-embedded transverse sections of degenerating nerve segments. The quantitative analysis of MBP-positive fluorescent areas was then performed using Image J image analysis software (National Institutes of Health-).
Distal stumps of transected sciatic nerves from 5-d-old mice were processed for electron microscopy, as described previously (Sharghi-Namini et al., 2006), and viewed in an electron microscope (1010; JEOL) operating at 80 KV. Photographic images of 10–15 representative areas of the whole sciatic nerve were acquired at a magnification of 5,000 using a camera (Orius SC10001; Gatan) and digital micrograph software (version 3.11.2; Gatan). Images were imported into Photoshop (version 7.0), and the total number of rounded or collapsed myelin sheaths per image was counted. Image J was used to calculate the total nerve area and the area represented by the images. Counts were expressed as the number of myelin sheaths per 10,000 μm2.
Acknowledgments
We thank Dirk Bohmann and Moshe Yaniv for gifts of reagents, Peter Brophy, Diane Sherman, Dies Meijer, and John Bermingham Jr. for antibodies, and Deborah Bartram for editorial assistance.
This work was supported by a Wellcome Trust Program Grant to K.R. Jessen, R. Mirsky, and D.B. Parkinson. L. Feltri and L. Wrabetz were supported by Telethon Italy and the National Institutes of Health, and A.C. Lloyd and L.A. Noon were supported by a Cancer Research United Kingdom Program Grant.
D.B. Parkinson, A. Bhaskaran, and P. Arthur-Farraj contributed equally to this paper.
D.B. Parkinson's present address is Peninsula Medical School, Tamar Science Park, Plymouth, Devon PL6 8BU, UK.
Abbreviations used in this paper: db-cAMP, dibutyryl cAMP; DM, defined medium; DRG, dorsal root ganglion; E, embryonic day; MBP, myelin basic protein; MKK7, MAPK kinase 7; NRG-1, β–neuregulin-1; P, postnatal day; P0, protein zero.
References
- Agthong, S., A. Kaewsema, N. Tanomsridejchai, and V. Chentanez. 2006. Activation of MAPK ERK in peripheral nerve after injury. BMC Neurosci. 7:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archelos, J.J., K. Roggenbuck, J. Schneider-Schaulies, C. Linington, K.V. Toyka, and H.P. Hartung. 1993. Production and characterization of monoclonal antibodies to the extracellular domain of P0. J. Neurosci. Res. 35:46–53. [DOI] [PubMed] [Google Scholar]
- Behrens, A., M. Sibilia, and E.F. Wagner. 1999. Amino-terminal phosphorylation of c-Jun regulates stress-induced apoptosis and cellular proliferation. Nat. Genet. 21:326–329. [DOI] [PubMed] [Google Scholar]
- Behrens, A., M. Sibilia, J.P. David, U. Mohle-Steinlein, F. Tronche, G. Schutz, and E.F. Wagner. 2002. Impaired postnatal hepatocyte proliferation and liver regeneration in mice lacking c-jun in the liver. EMBO J. 21:1782–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett, B.L., D.T. Sasaki, B.W. Murray, E.C. O'Leary, S.T. Sakata, W. Xu, J.C. Leisten, A. Motiwala, S. Pierce, Y. Satoh, et al. 2001. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc. Natl. Acad. Sci. USA. 98:13681–13686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermingham, J.R. Jr., S.S. Scherer, S. O'Connell, E. Arroyo, K.A. Kalla, F.L. Powell, and M.G. Rosenfeld. 1996. Tst-1/Oct-6/SCIP regulates a unique step in peripheral myelination and is required for normal respiration. Genes Dev. 10:1751–1762. [DOI] [PubMed] [Google Scholar]
- Besirli, C.G., E.F. Wagner, and E.M. Johnson Jr. 2005. The limited role of NH2-terminal c-Jun phosphorylation in neuronal apoptosis: identification of the nuclear pore complex as a potential target of the JNK pathway. J. Cell Biol. 170:401–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard, A.D., A. Sinanan, E. Parmantier, R. Zwart, L. Broos, D. Meijer, C. Meier, K.R. Jessen, and R. Mirsky. 1996. Oct-6 (SCIP/Tst-1) is expressed in Schwann cell precursors, embryonic Schwann cells, and postnatal myelinating Schwann cells: comparison with Oct-1, Krox-20, and Pax-3. J. Neurosci. Res. 46:630–640. [DOI] [PubMed] [Google Scholar]
- Boivin, A., I. Pineau, B. Barrette, M. Filali, N. Vallières, S. Rivest, and S. Lacroix. 2007. Toll-like receptor signaling is critical for Wallerian degeneration and functional recovery after peripheral nerve injury. J. Neurosci. 27:12565–12576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossy-Wetzel, E., L. Bakiri, and M. Yaniv. 1997. Induction of apoptosis by the transcription factor c-Jun. EMBO J. 16:1695–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor, A.B., and S.H. Orkin. 2002. Transcriptional regulation of erythropoiesis: an affair involving multiple partners. Oncogene. 21:3368–3376. [DOI] [PubMed] [Google Scholar]
- Carroll, S.L., M.L. Miller, P.W. Frohnert, S.S. Kim, and J.A. Corbett. 1997. Expression of neuregulins and their putative receptors, ErbB2 and ErbB3, is induced during Wallerian degeneration. J. Neurosci. 17:1642–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, L., F.S. Esch, M.A. Marchionni, and A.W. Mudge. 1998. Control of Schwann cell survival and proliferation: autocrine factors and neuregulins. Mol. Cell. Neurosci. 12:141–156. [DOI] [PubMed] [Google Scholar]
- D'Antonio, M., A. Droggiti, M.L. Feltri, J. Roes, L. Wrabetz, R. Mirsky, and K.R. Jessen. 2006. TGFbeta type II receptor signaling controls Schwann cell death and proliferation in developing nerves. J. Neurosci. 26:8417–8427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De, S., M.A. Trigueros, A. Kalyvas, and S. David. 2003. Phospholipase A2 plays an important role in myelin breakdown and phagocytosis during Wallerian degeneration. Mol. Cell. Neurosci. 24:753–765. [DOI] [PubMed] [Google Scholar]
- De Felipe, C., and S.P. Hunt. 1994. The differential control of c-jun expression in regenerating sensory neurons and their associated glial cells. J. Neurosci. 14:2911–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker, L., C. Desmarquet-Trin-Dinh, E. Taillebourg, J. Ghislain, J.M. Vallat, and P. Charnay. 2006. Peripheral myelin maintenance is a dynamic process requiring constant Krox20 expression. J. Neurosci. 26:9771–9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, Z., A. Brennan, N. Liu, Y. Yarden, G. Lefkowitz, R. Mirsky, and K.R. Jessen. 1995. NDF is a neuron-glia signal and regulates survival, proliferation, and maturation of rat Schwann cell precursors. Neuron. 15:585–596. [DOI] [PubMed] [Google Scholar]
- Dong, Z., A. Sinanan, D. Parkinson, E. Parmantier, R. Mirsky, and K.R. Jessen. 1999. Schwann cell development in embryonic mouse nerves. J. Neurosci. Res. 56:334–348. [DOI] [PubMed] [Google Scholar]
- Feltri, M.L., D. Graus Porta, S.C. Previtali, A. Nodari, B. Migliavacca, A. Cassetti, A. Littlewood-Evans, L.F. Reichardt, A. Messing, A. Quattrini, et al. 2002. Conditional disruption of β1 integrin in Schwann cells impedes interactions with axons. J. Cell Biol. 156:199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragoso, G., J. Robertson, E. Athlan, E. Tam, G. Almazan, and W.E. Mushynski. 2003. Inhibition of p38 mitogen-activated protein kinase interferes with cell shape changes and gene expression associated with Schwann cell myelination. Exp. Neurol. 183:34–46. [DOI] [PubMed] [Google Scholar]
- Ghislain, J., and P. Charnay. 2006. Control of myelination in Schwann cells: a Krox20 cis-regulatory element integrates Oct6, Brn2 and Sox10 activities. EMBO Rep. 7:52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guertin, A.D., D.P. Zhang, K.S. Mak, J.A. Alberta, and H.A. Kim. 2005. Microanatomy of axon/glial signaling during Wallerian degeneration. J. Neurosci. 25:3478–3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrisingh, M.C., E. Perez-Nadales, D.B. Parkinson, D.S. Malcolm, A.W. Mudge, and A.C. Lloyd. 2004. The Ras/Raf/ERK signalling pathway drives Schwann cell dedifferentiation. EMBO J. 23:3061–3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaegle, M., W. Mandemakers, L. Broos, R. Zwart, A. Karis, P. Visser, F. Grosveld, and D. Meijer. 1996. The POU factor Oct-6 and Schwann cell differentiation. Science. 273:507–510. [DOI] [PubMed] [Google Scholar]
- Jaegle, M., M. Ghazvini, W. Mandemakers, M. Piirsoo, S. Driegen, F. Levavasseur, S. Raghoenath, F. Grosveld, and D. Meijer. 2003. The POU proteins Brn-2 and Oct-6 share important functions in Schwann cell development. Genes Dev. 17:1380–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen, K.R., and R. Mirsky. 2005. The origin and development of glial cells in peripheral nerves. Nat. Rev. Neurosci. 6:671–682. [DOI] [PubMed] [Google Scholar]
- Jessen, K.R., A. Brennan, L. Morgan, R. Mirsky, A. Kent, Y. Hashimoto, and J. Gavrilovic. 1994. The Schwann cell precursor and its fate: A study of cell death and differentiation during gliogenesis in rat embryonic nerves. Neuron. 12:509–527. [DOI] [PubMed] [Google Scholar]
- Kwon, Y.K., A. Bhattacharyya, J.A. Alberta, W.V. Giannobile, K. Cheon, C.D. Stiles, and S.L. Pomeroy. 1997. Activation of ErbB2 during wallerian degeneration of sciatic nerve. J. Neurosci. 17:8293–8299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le, N., R. Nagarajan, J.Y. Wang, J. Svaren, C. LaPash, T. Araki, R.E. Schmidt, and J. Milbrandt. 2005. a. Nab proteins are essential for peripheral nervous system myelination. Nat. Neurosci. 8:932–940. [DOI] [PubMed] [Google Scholar]
- Le, N., R. Nagarajan, J.Y. Wang, T. Araki, R.E. Schmidt, and J. Milbrandt. 2005. b. Analysis of congenital hypomyelinating Egr2Lo/Lo nerves identifies Sox2 as an inhibitor of Schwann cell differentiation and myelination. Proc. Natl. Acad. Sci. USA. 102:2596–2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc, S.E., S.W. Jang, R.M. Ward, L. Wrabetz, and J. Svaren. 2006. Direct regulation of myelin protein zero expression by the Egr2 transactivator. J. Biol. Chem. 281:5453–5460. [DOI] [PubMed] [Google Scholar]
- Levy, D., P. Kubes, and D.W. Zochodne. 2001. Delayed peripheral nerve degeneration, regeneration, and pain in mice lacking inducible nitric oxide synthase. J. Neuropathol. Exp. Neurol. 60:411–421. [DOI] [PubMed] [Google Scholar]
- Mechta-Grigoriou, F., D. Gerald, and M. Yaniv. 2001. The mammalian Jun proteins: redundancy and specificity. Oncogene. 20:2378–2389. [DOI] [PubMed] [Google Scholar]
- Monuki, E.S., G. Weinmaster, R. Kuhn, and G. Lemke. 1989. SCIP: a glial POU domain gene regulated by cyclic AMP. Neuron. 3:783–793. [DOI] [PubMed] [Google Scholar]
- Morgan, L., K.R. Jessen, and R. Mirsky. 1991. The effects of cAMP on differentiation of cultured Schwann cells: progression from an early phenotype (04+) to a myelin phenotype (P0+, GFAP−, N-CAM−, NGF-receptor−) depends on growth inhibition. J. Cell Biol. 112:457–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan, L., K.R. Jessen, and R. Mirsky. 1994. Negative regulation of the P0 gene in Schwann cells: suppression of P0 mRNA and protein induction in cultured Schwann cells by FGF2 and TGFß1, TGFß2 and TGFß3. Development. 120:1399–1409. [DOI] [PubMed] [Google Scholar]
- Muroyama, Y., Y. Fujiwara, S.H. Orkin, and D.H. Rowitch. 2005. Specification of astrocytes by bHLH protein SCL in a restricted region of the neural tube. Nature. 438:360–363. [DOI] [PubMed] [Google Scholar]
- Nagarajan, R., J. Svaren, N. Le, T. Araki, M. Watson, and J. Milbrandt. 2001. EGR2 mutations in inherited neuropathies dominant-negatively inhibit myelin gene expression. Neuron. 30:355–368. [DOI] [PubMed] [Google Scholar]
- Nickols, J.C., W. Valentine, S. Kanwal, and B.D. Carter. 2003. Activation of the transcription factor NF-kappaB in Schwann cells is required for peripheral myelin formation. Nat. Neurosci. 6:161–167. [DOI] [PubMed] [Google Scholar]
- Ogata, T., S. Iijima, S. Hoshikawa, T. Miura, S. Yamamoto, H. Oda, K. Nakamura, and S. Tanaka. 2004. Opposing extracellular signal-regulated kinase and Akt pathways control Schwann cell myelination. J. Neurosci. 24:6724–6732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papavassiliou, A.G., M. Treier, and D. Bohmann. 1995. Intramolecular signal transduction in cJun. EMBO J. 14:2014–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson, D.B., Z. Dong, H. Bunting, J. Whitfield, C. Meier, H. Marie, R. Mirsky, and K.R. Jessen. 2001. Transforming growth factor β (TGFß) mediates Schwann cell death in vitro and in vivo: examination of c-Jun activation, interactions with survival signals, and the relationship of TGFß-mediated death to Schwann cell differentiation. J. Neurosci. 21:8572–8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson, D.B., S. Dickinson, A. Bhaskaran, M.T. Kinsella, P.J. Brophy, D.L. Sherman, S. Sharghi-Namini, M.B. Duran Alonso, R. Mirsky, and K.R. Jessen. 2003. Regulation of the myelin gene periaxin provides evidence for Krox-20-independent myelin-related signalling in Schwann cells. Mol. Cell. Neurosci. 23:13–27. [DOI] [PubMed] [Google Scholar]
- Parkinson, D.B., A. Bhaskaran, A. Droggiti, S. Dickinson, M. D'Antonio, R. Mirsky, and K.R. Jessen. 2004. Krox-20 inhibits Jun-NH2-terminal kinase/c-Jun to control Schwann cell proliferation and death. J. Cell Biol. 164:385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry, V.H., J.W. Tsao, S. Fearn, and M.C. Brown. 1995. Radiation-induced reductions in macrophage recruitment have only slight effects on myelin degeneration in sectioned peripheral nerves of mice. Eur. J. Neurosci. 7:271–280. [DOI] [PubMed] [Google Scholar]
- Raivich, G., and A. Behrens. 2006. Role of the AP-1 transcription factor c-Jun in developing, adult and injured brain. Prog. Neurobiol. 78:347–363. [DOI] [PubMed] [Google Scholar]
- Sharghi-Namini, S., M. Turmaine, C. Meier, V. Sahni, F. Umehara, K.R. Jessen, and R. Mirsky. 2006. The structural and functional integrity of peripheral nerves depends on the glial-derived signal desert hedgehog. J. Neurosci. 26:6364–6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shubayev, V.I., M. Angert, J. Dolkas, W.M. Campana, K. Palenscar, and R.R. Myers. 2006. TNFalpha-induced MMP-9 promotes macrophage recruitment into injured peripheral nerve. Mol. Cell. Neurosci. 31:407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shy, M.E., Y. Shi, L. Wrabetz, J. Kamholz, and S.S. Scherer. 1996. Axon-Schwann cell interactions regulate the expression of c-jun in Schwann cells. J. Neurosci. Res. 43:511–525. [DOI] [PubMed] [Google Scholar]
- Stewart, H.J. 1995. Expression of c-Jun, Jun B, Jun D and cAMP response element binding protein by Schwann cells and their precursors in vivo and in vitro.Eur. J. Neurosci. 7:1366–1375. [DOI] [PubMed] [Google Scholar]
- Topilko, P., and D. Meijer. 2001. Transcription factors that control Schwann cell development and myelination. In Glial Cell Development, 2nd Edition. K.R. Jessen and W.D. Richardson, editors. Oxford University Press, Oxford. 223-244.
- Topilko, P., S. Schneider-Maunoury, G. Levi, A. Baron-Van Evercooren, A.B. Chennoufi, T. Seitanidou, C. Babinet, and P. Charnay. 1994. Krox-20 controls myelination in the peripheral nervous system. Nature. 371:796–799. [DOI] [PubMed] [Google Scholar]
- Watson, A., A. Eilers, D. Lallemand, J. Kyriakis, L.L. Rubin, and J. Ham. 1998. Phosphorylation of c-Jun is necessary for apoptosis induced by survival signal withdrawal in cerebellar granule neurons. J. Neurosci. 18:751–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanazzi, G., S. Einheber, R. Westreich, M.J. Hannocks, D. Bedell-Hogan, M.A. Marchionni, and J.L. Salzer. 2001. Glial growth factor/neuregulin inhibits Schwann cell myelination and induces demyelination. J. Cell Biol. 152:1289–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]