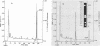Abstract
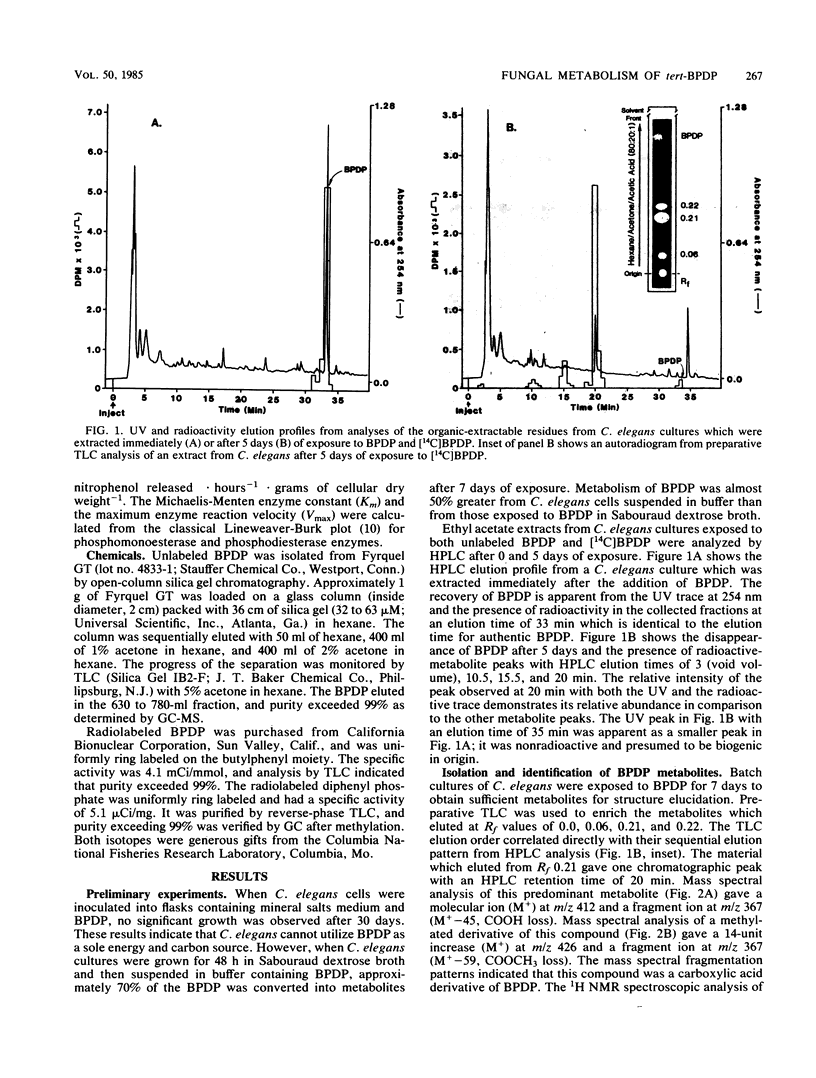
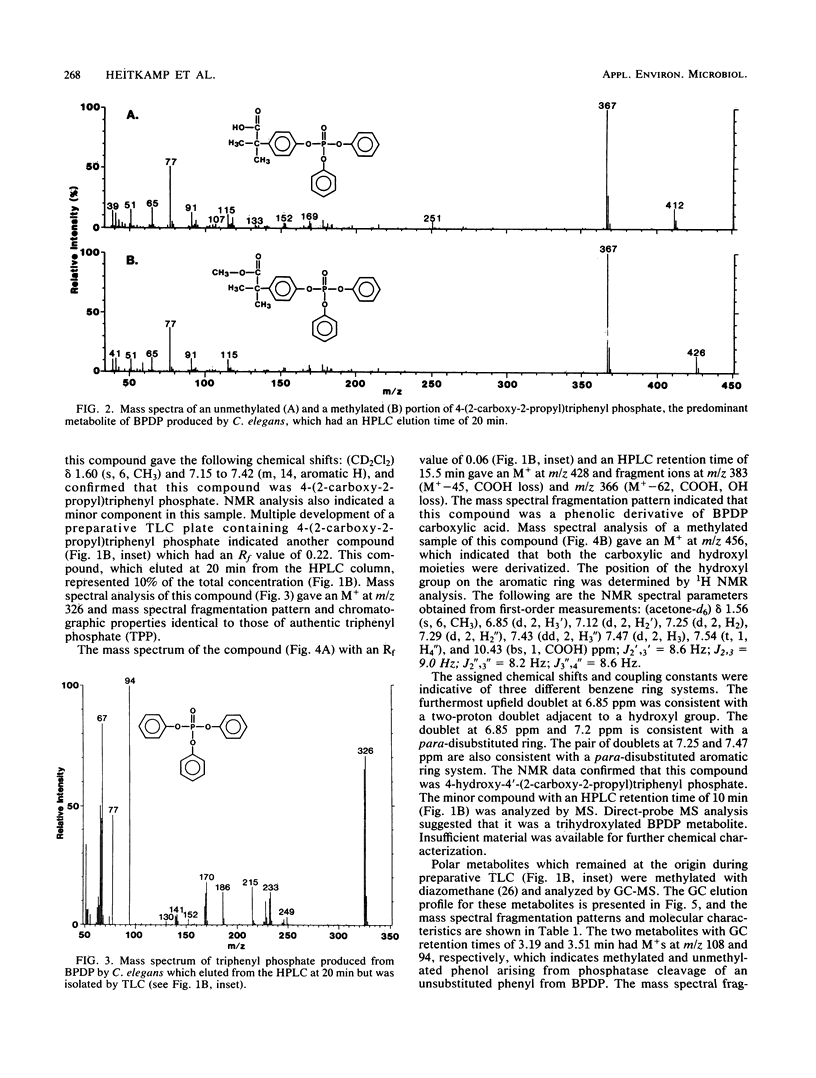
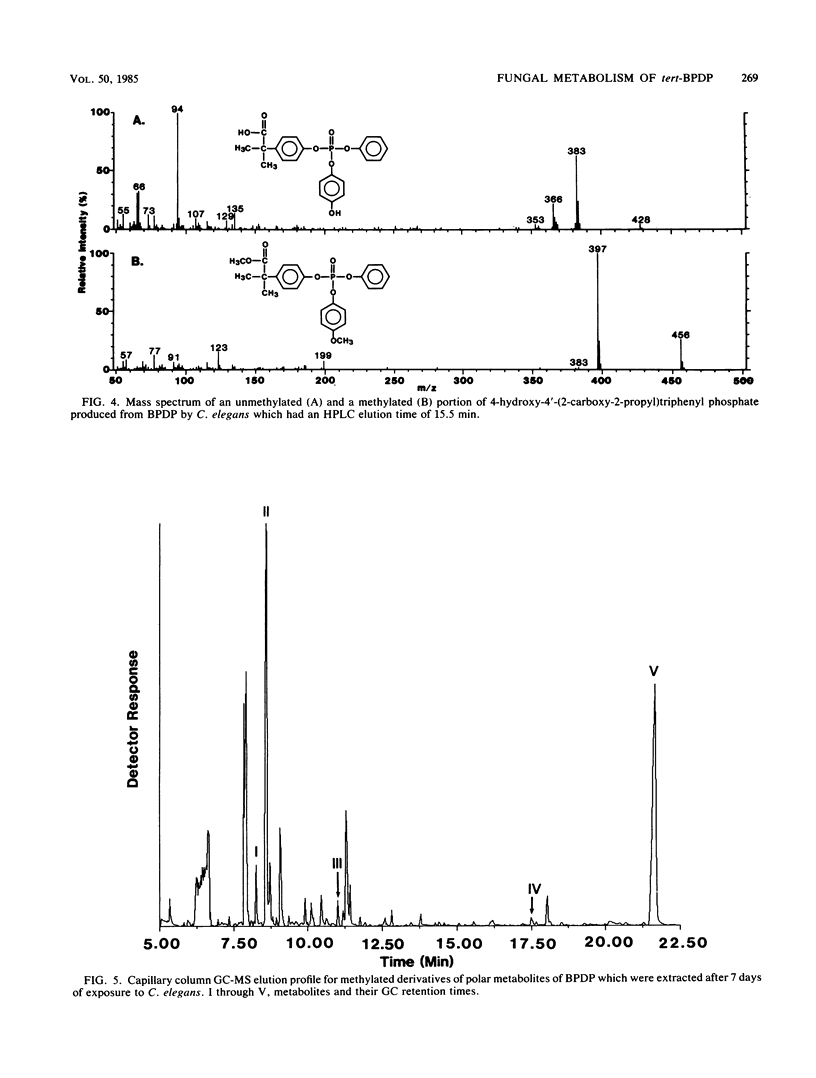
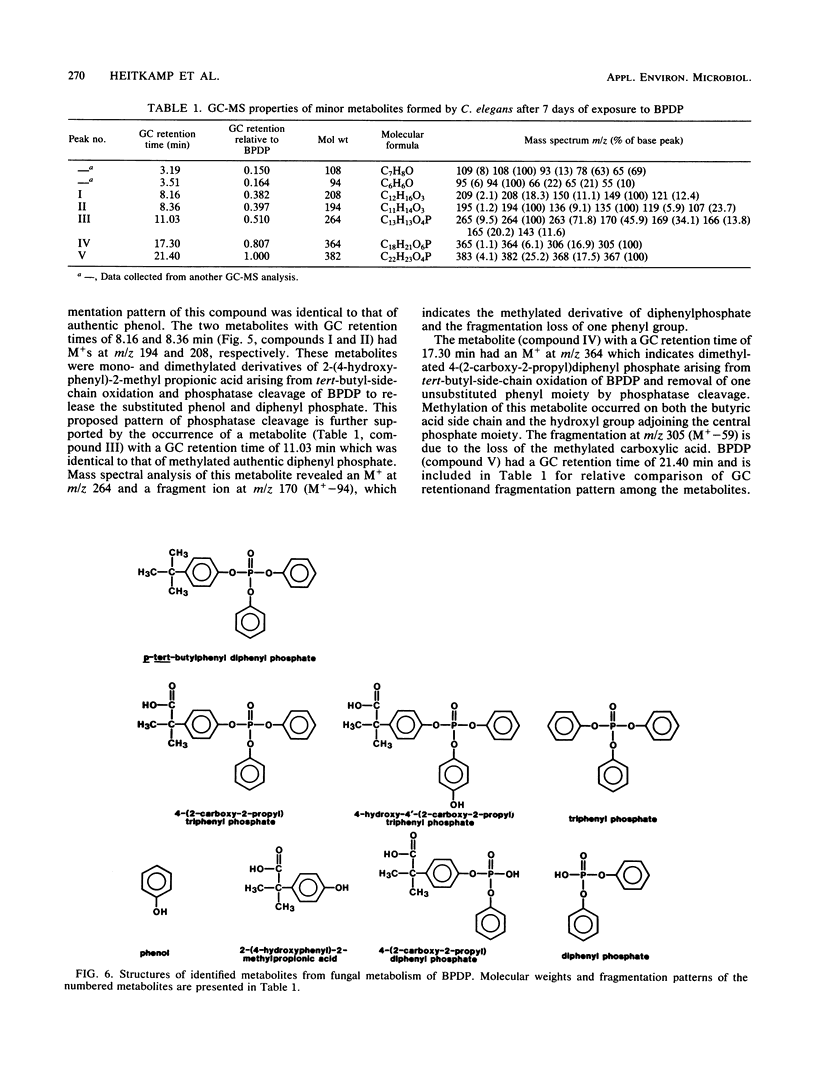
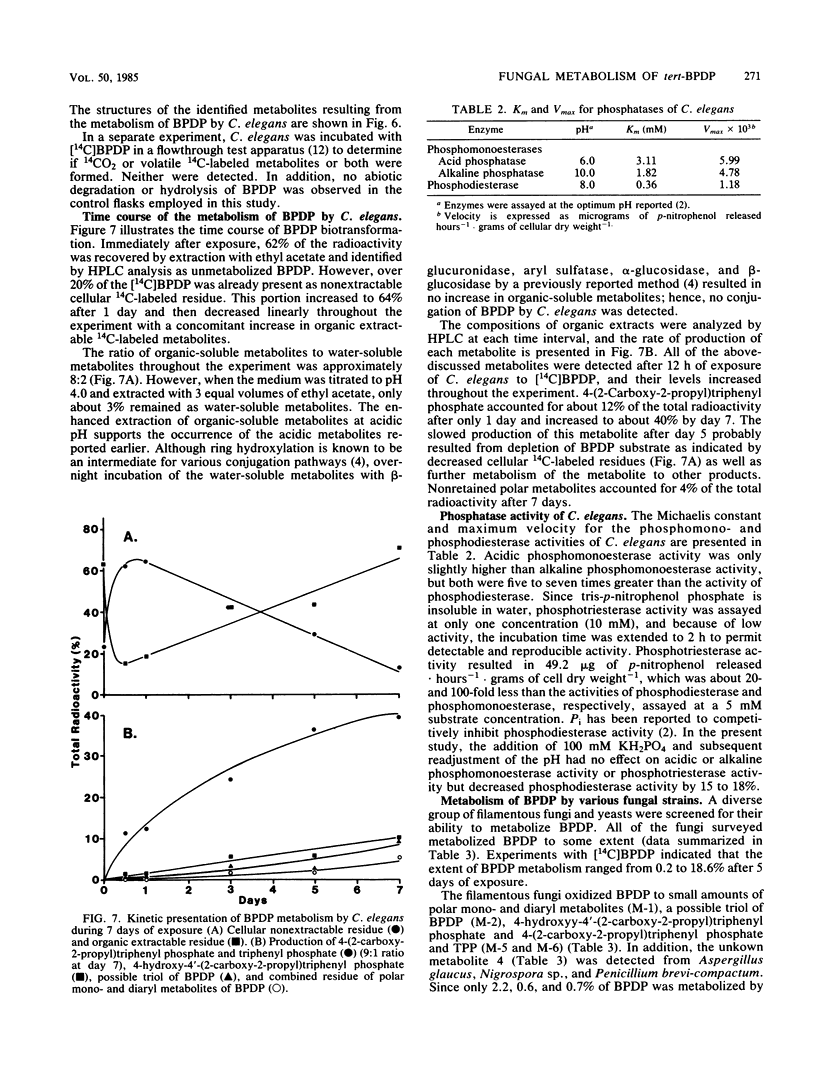
The fungal metabolism of tert-butylphenyl diphenyl phosphate (BPDP) was studied. Cunninghamella elegans was incubated with BPDP for 7 days, and the metabolites formed were separated by thin-layer, gas-liquid, or high-pressure liquid chromatography and identified by 1H nuclear magnetic resonance and mass spectral techniques. C. elegans metabolized BPDP predominantly at the tert-butyl moiety to form the carboxylic acid 4-(2-carboxy-2-propyl)triphenyl phosphate. In addition, 4-hydroxy-4'-(2-carboxy-2-propyl)triphenyl phosphate, triphenyl phosphate, diphenyl phosphate, 4-(2-carboxy-2-propyl)diphenyl phosphate, 2-(4-hydroxyphenyl)-2-methyl propionic acid, and phenol were detected. Similar metabolites were found in the 28 fungal cultures which were examined for their ability to metabolize BPDP. Experiments with [14C]BPDP indicated that C. elegans metabolized 70% of the BPDP after 7 days and that the ratio of organic-soluble metabolites to water-soluble metabolites was 8:2. The results indicate that fungi preferentially oxidize BPDP at the alkyl side chain and at the aromatic rings to form hydroxylated derivatives. The trace levels of mono- and diaryl metabolites and the low level of phosphotriesterase activity measured in C. elegans indicate that phosphatase cleavage is a minor pathway for fungal metabolism of BPDP.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Broadhurst M. G. Use and replaceability of polychlorinated biphenyls. Environ Health Perspect. 1972 Oct;2:81–102. doi: 10.1289/ehp.720281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerniglia C. E., Freeman J. P., Mitchum R. K. Glucuronide and sulfate conjugation in the fungal metabolism of aromatic hydrocarbons. Appl Environ Microbiol. 1982 May;43(5):1070–1075. doi: 10.1128/aem.43.5.1070-1075.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerniglia C. E., Lambert K. J., Miller D. W., Freeman J. P. Transformation of 1- and 2-methylnaphthalene by Cunninghamella elegans. Appl Environ Microbiol. 1984 Jan;47(1):111–118. doi: 10.1128/aem.47.1.111-118.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerniglia C. E. Microbial metabolism of polycyclic aromatic hydrocarbons. Adv Appl Microbiol. 1984;30:31–71. doi: 10.1016/s0065-2164(08)70052-2. [DOI] [PubMed] [Google Scholar]
- Daft J. L. Identification of aryl/alkyl phosphate residues in foods. Bull Environ Contam Toxicol. 1982 Aug;29(2):221–227. doi: 10.1007/BF01606154. [DOI] [PubMed] [Google Scholar]
- Dollahite J. W., Pierce K. R. Neurologic disturbances due to triaryl phosphate toxicity. Am J Vet Res. 1969 Aug;30(8):1461–1464. [PubMed] [Google Scholar]
- Johannsen F. R., Wright P. L., Gordon D. E., Levinskas G. J., Radue R. W., Graham P. R. Evaluation of delayed neurotoxicity and dose-response relationships of phosphate esters in the adult hen. Toxicol Appl Pharmacol. 1977 Aug;41(2):291–304. doi: 10.1016/0041-008x(77)90030-8. [DOI] [PubMed] [Google Scholar]
- Johnson M. K. Organophosphorus esters causing delayed neurotoxic effects: mechanism of action and structure activity studies. Arch Toxicol. 1975 Dec 18;34(4):259–288. doi: 10.1007/BF00353848. [DOI] [PubMed] [Google Scholar]
- Johnson M. K. The delayed neuropathy caused by some organophosphorus esters: mechanism and challenge. CRC Crit Rev Toxicol. 1975 Jun;3(3):289–316. doi: 10.3109/10408447509079861. [DOI] [PubMed] [Google Scholar]
- Jones J. G. Studies on freshwater bacteria: effect of medium composition and method on estimates of bacterial population. J Appl Bacteriol. 1970 Dec;33(4):679–686. doi: 10.1111/j.1365-2672.1970.tb02250.x. [DOI] [PubMed] [Google Scholar]
- Ku Y., Alvarez G. H. Biodegradation of [C]Tri-p-Cresyl Phosphate in a Laboratory Activated-Sludge System. Appl Environ Microbiol. 1982 Mar;43(3):619–622. doi: 10.1128/aem.43.3.619-622.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickard M. A., Whelihan J. A., Westlake D. W. Utilization of triaryl phosphates by a mixed bacterial population. Can J Microbiol. 1975 Feb;21(2):140–145. doi: 10.1139/m75-021. [DOI] [PubMed] [Google Scholar]
- Rushing L. G., Althaus J. R., Thompson H. C., Jr Simultaneous determination of trimellitic anhydride and its trimellitic acid impurity by GC/FID. J Anal Toxicol. 1982 Nov-Dec;6(6):290–293. doi: 10.1093/jat/6.6.290. [DOI] [PubMed] [Google Scholar]
- Turner B. C., Glotfelty D. E. Field air sampling of pesticide vapors with polyurethane foam. Anal Chem. 1977 Jan;49(1):7–10. doi: 10.1021/ac50009a009. [DOI] [PubMed] [Google Scholar]