Abstract
Intraabdominal adhesions develop after abdominal surgery as part of the normal healing processes that occur after damage to the peritoneum. Over the last 2 decades, much research has gone into understanding the biochemical and cellular processes that lead to adhesion formation. The early balance between fibrin deposition and degradation seems to be the critical factor in adhesion formation. Although adhesions do have some beneficial effects, they also cause significant morbidity, including adhesive small bowel obstruction, infertility and increased difficulty with reoperative surgery. Several strategies have been employed over the years to prevent adhesion formation while not interfering with wound healing. This article summarizes much of our current understanding of adhesion formation and strategies that have been employed to prevent them.
Abstract
Les adhérences intra-abdominales font leur apparition après une chirurgie à l'abdomen dans le cours des mécanismes de guérison normaux suivant un dommage au péritoine. Au cours des deux dernières décennies, on a effectué beaucoup de recherches afin de comprendre les phénomènes biochimiques et cellulaires à l'origine de la formation d'adhérences. L'équilibre précoce entre le dépôt de fibrine et sa dégradation semble jouer un rôle critique dans la formation d'adhérences. Même si les adhérences ont certains effets bénéfiques, elles causent aussi une morbidité importante, y compris l'occlusion de l'intestin grêle, l'infécondité et les difficultés accrues dans le cas d'interventions chirurgicales ultérieures. On a suivi au fil des ans plusieurs stratégies pour prévenir la formation d'adhérences sans nuire à la guérison de la plaie. Cet article résume une grande partie des connaissances actuelles au sujet de la formation d'adhérences, ainsi que les stratégies que l'on a suivies pour les prévenir.
Postoperative adhesions form after trauma to the peritoneal cavity and are a result of the biochemical and cellular response that occurs in an attempt to repair the peritoneum. Although there are beneficial effects to adhesions, they are the leading cause of small intestinal obstruction after abdominal surgery and can be the source of significant morbidity, in some cases leading to mortality. This review aims to provide general surgeons with a broad overview of what is currently known about adhesions, the cellular and molecular events that are involved in their formation, the latest re-search developments in this area and the current available methods of prevention.
Background
Peritoneal adhesions can be defined as abnormal fibrous bands between organs or tissues or both in the abdominal cavity that are normally separated.1–3 Adhesions may be acquired or congenital; however, most are acquired as a result of peritoneal injury, the most common cause of which is abdomino-pelvic surgery.4 Less commonly, adhesions may form as the result of inflammatory conditions, intraperitoneal infection or abdominal trauma.4
It is estimated that 93% to 100% of patients who undergo transperitoneal surgery will develop postoperative adhesions.5 The extent of adhesion formation varies from one patient to another and is most dependent on the type and magnitude of surgery performed, as well as whether any postoperative complications develop.6 Another surgical factor that has been shown to contribute to adhesion formation is intraperitoneal foreign bodies, including mesh, glove powder, suture material and spilled gallstones.7 Fortunately, most patients with adhesions do not experience any overt clinical symptoms. For others, adhesions may lead to any one of a host of problems and can be the cause of significant morbidity and mortality.8
Adhesions and small bowel obstruction (SBO)
Intraabdominal adhesions are the most common cause of SBO in industrialized countries, accounting for approximately 65% to 75% of cases.5 There is a wide range of values reported in the literature for the risk of developing adhesive SBO after transperitoneal surgery, depending on the series of patients, how they were evaluated and the types of surgical procedures performed. In general, procedures in the lower abdomen, pelvis or both and those resulting in damage to a large peritoneal surface area tend to put patients at higher risk for subsequent adhesive obstruction.4 It is estimated that the risk of SBO is 1% to 10% after appendectomy,9,10 6.4% after open cholecystectomy,9 10% to 25% after intestinal surgery11,12 and 17% to 25% after restorative proctocolectomy (IPAA).13–16
The relation between postoperative adhesions and intestinal obstruction is not a new concept. In 1872, Thomas Bryant described a fatal case of intestinal obstruction caused by intra-abdominal adhesions that developed after removal of an ovarian tumour.17 Since Bryant's report, a significant amount of time and money has been invested into research on intraabdominal adhesions, with a primary focus on the development of methods to prevent their formation. Despite substantial work in this area, little progress has been made; to this day, no clinical standard exists for any preventive measure, either surgical or pharmacological, to control the formation of postoperative adhesions.4
Other complications of adhesions
SBO is probably the most severe consequence of intraabdominal adhesions, but it is not the only one, and the adverse effects of adhesions are not limited to the gut.4 For example, in the gynecological literature, it has been found that adhesions are a leading cause of secondary infertility in women (responsible for 15%–20% of cases)18 and, although controversial, there is evidence to suggest that they may be a cause of longer-term abdominal and pelvic pain.19 For patients with chronic renal failure, adhesions may make peritoneal dialysis impossible, and their presence may preclude the use of intraperitoneal chemotherapy in those patients who are candidates.4,6 For general surgeons, the presence of adhesions often makes reoperative surgery difficult and may increase the complication rate of the intended surgical procedure.20 In the current era of advanced laparoscopic surgery, adhesions have taken on an even greater significance, frequently making laparoscopic approaches more difficult and, in some cases, entirely impossible.4 Even with open reoperative surgery, extensive adhesiolysis is often necessary to ensure adequate exposure, not uncommonly resulting in prolonged operating times, increased blood loss and other complications.4,20,21 Inadvertent enterotomy is probably the best recognized complication of adhesiolysis, with an incidence of approximately 20% in reoperative surgery.20 These cases result in a poorer outcome for the patient, with prolonged hospitalization and a higher incidence of intensive care unit admissions.20
Socioeconomic burden of adhesive SBO
The consequences of postoperative adhesion formation have become a significant burden socioeconomically, and the treatment of adhesion-related disease uses a significant portion of health care resources and dollars.8 From a large-scale epidemiological study in Scotland, for example, 5.7% of hospital readmissions over a 10-year period were found to be directly related to adhesions, and 3.8% of these admissions required operative management.8 In 1994, the estimated financial impact for direct patient care owing to adhesion-related disorders in the United States was US$1.3 billion.22 In Sweden, it is estimated that the health care burden owing to adhesive disease reaches $13 million annually.23 As the cost of health care continues to escalate and the number of patients requiring surgical care increases with the aging population, the financial burden of adhesions will continue to expand. Given the far-reaching consequences of postoperative adhesions, it is important that they not be viewed as an inevitable consequence of surgery for which little can be done.24 This knowledge should provide the impetus for further research in this area, to improve our understanding of the pathophysiology of adhesions and to enable the development of methods to alter the biological events that are necessary for their formation.
Understanding the pathophysiology of adhesion formation
Holmdahl and Ivarsson25 have suggested that the inability to discover effective ways to reduce or abolish adhesion formation over the years has been due to a lack of insight into the basic tenets of peritoneal tissue repair. Only in the last 15 to 20 years have researchers started to unravel the complexities of this process, which involves several different cell types, cytokines, coagulation factors and proteases, all acting together to restore tissue integrity.25 Although our understanding is far from complete, studies of adhesion formation thus far have determined what is believed to be the central pathophysiological mechanism leading to ad-hesion development.24,26 This is discussed below. If effective preventative and treatment strategies are to be developed, a more comprehensive understanding of this process at both the cellular and the molecular level, as well as the identification of inflammatory mediators involved, is essential. The key to preventing post-operative adhesions will most likely be based on selective inhibition of one or more of the critical factors required for their formation.
Peritoneal wound healing differs from skin in both the mode of epithelialization and the consequences of fibrin deposition. To understand how the peritoneum responds to injury, some basic knowledge about its structure is required. The peritoneum consists of a single outer layer of mesothelial cells that are loosely anchored to a basement membrane and that detach readily with even the slightest trauma.21,25,27
The submesothelial layer consists of components of the extracellular matrix, along with capillaries and lymphatics.21,23,25 Fluid resorption and diffusion occurs freely across these layers.21 The fluid in the peritoneal cavity contains several different cell types, including leukocytes and macrophages.25 These cells, along with the mesothelium, secrete various cellular mediators that have roles in peritoneal healing, enabling modulation of the inflammatory response over a large surface area.21
The process of postoperative adhesion formation constitutes a complex interaction of biochemical events involved in inflammation, tissue repair, angiogenesis and innervation.28 Peritoneal injury occurs at the site of the actual procedure and in areas remote from the operative field, as a result of tissue and organ retraction during the course of surgery.1 Surgical trauma to the peritoneum can occur by various mechanisms: cutting, abrasion, ischemia, desiccation and coagulation.4 The latter 2 types of injury are unique in that they are directly toxic to the mesothelial cells that line the peritoneal cavity and to the underlying connective tissue.4 Ischemic injury is typically the result of tissue and organ retraction. Regardless of the mechanism, however, the response of the peritoneum to surgical trauma is the same25 (Fig. 1). Immediately after injury, there is bleeding and an increase in vascular permeability with fluid leakage from injured surfaces.21,25,28 Simultaneously, a posttraumatic inflammatory response occurs, with infiltration of inflammatory cells, release of pro-inflammatory cytokines and activation of the complement and coagulation cascades.25,27
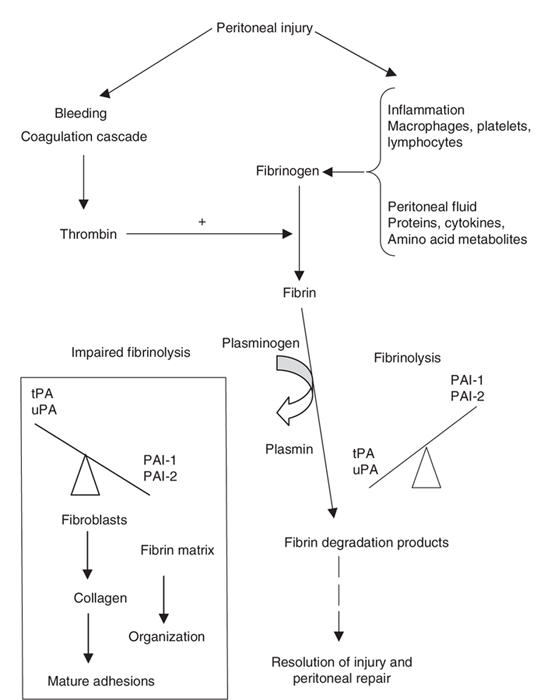
FIG. 1. Biological events involved in peritoneal tissue repair and adhesion formation.
PAI-1 = plasminogen activator inhibitors group 1; tPA = tissue plasminogen activator; uPA= urokinase-like plasminogen activator.
The fluid exudate released from injured peritoneal surfaces is rich in plasma proteins — especially fibrinogen.4,27 Activation of the coagulation cascade results in the formation of thrombin, which is necessary for the conversion of fibrinogen to fibrin.27 Fibrin functions to restore injured tissues and, once generated, is deposited along peritoneal surfaces. Fibrin is a tacky substance and causes adjacent organs or injured serosal surfaces to coalesce.24 Under normal circumstances, the formation of a fibrin matrix during wound healing is only temporary, and degradation of these filmy fibrinous adhesions by locally released proteases of the fibrinolytic system occurs within 72 hours of injury.2 Thus the process of fibrinolysis is not confined to the degradation of intravascular thrombi; it also has a key role in tissue remodelling and repair.25 Fibrinolysis allows mesothelial cells to proliferate and the peritoneal defect to be restored within 4 to 5 days, preventing the permanent attachment of adjacent surfaces.2,29 Adequate blood supply is critical for fibrinolysis, and since peritoneal injury results in ischemia, it also interferes with fibrinolysis.6 If fibrinolysis does not occur within 5 to 7 days of peritoneal injury, or if local fibrinolytic activity is reduced, the fibrin matrix persists.25 If this occurs, the temporary fibrin matrix gradually becomes more organized as collagen-secreting fibroblasts and other reparative cells infiltrate the matrix.4,24 The organization of fibrin bands over time and their transformation into mature fibrous adhesions is what enables them to persist.2 These “mature” adhesions are not simply composed of connective tissue; studies have demonstrated that, over time, they become highly organized cellular structures that contain arter-ioles, venules, capillaries and nerve fibres in addition to collagen.30
As described above, the fibrinolytic system has a key role in peritoneal wound healing, and disruption of this system results in adhesion formation. In addition to activators of fibrinolysis, there are also inhibitors that exist to maintain balance in the system (that is, to prevent excessive fibrin deposition and degradation). There are 2 major activators in the fibrinolytic system: tissue plasminogen activator (tPA) and urokinase-like plasminogen activator (uPA), both of which are capable of activating plasminogen to plasmin.2 Plasmin is a broad-range protease capable of degrading various molecules in the extracellular matrix (ECM), including fibrin.21,25 Of the 2 plasminogen activators, tPA is the most important in peritoneal wound healing because it has a specific affinity for fibrin that uPA lacks; it is responsible for 95% of the plasmin generated in the response to peritoneal injury.31 There is also a group of glycoproteins that act as inhibitors of fibrinolysis and are collectively referred to as plasminogen activator inhibitors (PAI). Two groups of PAIs exist: PAI-1 and PAI-2. However, PAI-1 is recognized as the dominant inhibitor of fibrinolysis in plasma.27,31,32 PAI-1 specifically prevents the formation of plasmin by directly binding to and inhibiting the activities of tPA and uPA, thereby preventing the degradation of fibrin.21
If fibrinolysis is a normal part of peritoneal healing, one may ask, “what allows fibrin to become organized and fibrous adhesions to persist?” In 1983, Moore and colleagues33 demonstrated that the peritoneum has powerful coagulation and fibrinolytic capacity. As discussed above, under normal conditions (i.e., in an undisturbed abdominal cavity), fibrinolytic capacity exceeds coagulation.33 Additional studies have shown that, in conditions where there is peritoneal injury, relative ischemia or both (such as when a patient has peritonitis or is undergoing surgery), peritoneal fibrinolytic capacity is depressed,31 and the relation between fibrinolysis and coagulation is reversed. Further, the reduction in peritoneal fibrinolysis after an operation seems to be inversely correlated to the degree of adhesion formation.34 Given these findings, it is believed that the decline in peritoneal fibrinolytic capacity after surgery is the common central pathway leading to adhesion formation.26,31
Both animal and human studies have shown that 2 major changes mediate the decline in fibrinolysis: a decrease in local tPA activity31 and an increase in PAI-1 locally and system-ically.35 The reason for decreased activity of tPA appears to be 2-fold: a reduction in the absolute amount of tPA released by the injured peritoneum and the result of quenching any remaining tPA activity by PAI-1.25,32 The importance of tPA and PAI-1 in adhesion formation is further supported by studies in which it was discovered that patients with the most severe adhesions overexpress PAI-1 and have depressed tPA activity.31,32 Further, after surgery, tPA knockout mice seem to be more susceptible to adhesion formation, compared with uPA-deficient or wild-type mice.2 Although the specific molecular and biochemical events mediating the change in fibrinolytic activity have yet to be fully elucidated, it appears that cytokines, growth factors and angiogenesis factors, all of which are released by activated macrophages and other inflammatory cells in response to peritoneal injury, may have important roles in regulating this change.
Elucidating the role of inflammatory mediators in adhesion formation has become the main current focus of research in this area. It is known that specific cytokines and growth factors are responsible for upregulating the expression of genes whose products may help to initiate adhesion formation, likely by coordinating the events responsible for the decline in fibrinolysis.21,25 Examples include genes for the neurokinin-1 (NK-1) receptor, transforming growth factor beta (TGF-β), substance P (SP), intracellular adhesion molecule (ICAM-1) and vascular cell adhesion molecule (VCAM-1). An increase in the levels of mRNA transcribed from each of these genes has been found in the peritoneal tissue of rats early after surgical trauma.28
TGF-β is the most thoroughly studied cytokine in adhesion formation.25 TGF-β is a potent cytokine and growth factor that initiates, modulates and terminates tissue repair, and both TGF-β and its receptor are elevated in peritoneal tissue and fluid after transperitoneal surgery.36 In vitro studies suggest that TGF-β contributes to a decrease in peritoneal fibrinolytic capacity and may have a role in preventing the early dissolution of fibrinous adhesions.37 In vivo evidence for a role of TGF-β in promoting adhesion formation comes from studies using an animal model of surgically induced adhesions, in which animals were given either intraperitoneal recombinant TGF-β or placebo at the time of laparotomy. The animals that received TGF-β had significantly more adhesions than the control group when reexamined several days later.38 Similarly, in a separate study, animals treated with a TGF-β neutralizing antibody had reduced adhesion formation after surgery, compared with controls.39 The exact mechanism through which TGF-β mediates this response is not known; however, early studies suggest that it may involve the local regulation of PAI-1.40
Several proinflammatory interleukins have been studied for their potential role in adhesion formation. Although the role of many of these interleukins has yet to be defined, the role of interleukin-1 (IL-1) in the pathophysiology of adhesion formation is becoming clear. Studies have suggested that, in addition to promoting inflammation and primary coagulation, IL-1 also contributes to the overall decrease in local fibrinolytic capacity that is necessary for adhesions to form. The increased level of IL-1β that has been measured in peritoneal fluid postoperatively supports a local action for this substance in the peritoneal cavity.41 In vivo, IL-1β has been found to stimulate the release of PAI-1 in human mesothelial cells,42 suggesting that it may play a part in inhibiting local fibrin degradation. Further support for its role in promoting adhesion formation and initiating tissue repair comes from a study in which rats treated with an anti–IL-1 preparation developed significantly less surgically induced adhesions than did the controls.43
Recently, substance P (SP) has received attention with respect to its role in adhesion formation. SP is a neuropeptide that belongs to the tachykinin family of peptides, to which the NK receptors also belong. SP can be found in a variety of locations, including peritoneal fluid, and it has many biological effects — most of which involve mediation of the inflammatory reaction.28 Through high-affinity binding to the NK-1 receptor, SP has been shown to affect the expression of intracellular adhesion molecules (such as ICAM-1 and VCAM-1) and TGF-β in several cell types, all of which have also been shown to have a role in adhesion formation.28 Further support for a role of SP in adhesion formation comes from studies demonstrating the presence of SP-containing sensory neurons in peritoneal adhesions19,28 and animal studies with neural endopeptidase knockout mice.44 Neural endopeptidase is a cell surface enzyme that degrades SP, and mice lacking this enzyme develop intraabdominal adhesions more readily than their wild-type counterparts. Given these findings, it is likely that SP plays a central role in coordinating the pathogenesis of adhesion formation, and further investigations are warranted.
With respect to a role for the NK-1 receptor (NK-1R) in adhesion formation, initial experiments by Reed and colleagues28 demonstrated that there is a significant increase in mRNA levels for both NK-1R and SP in peritoneal adhesion tissue by day 3 after surgery. Additional experiments showed that administration of a NK-1R antagonist (NK-1RA) to rats after surgery significantly reduced adhesion formation by 45%, compared with controls.45 NK-1RA blocks the binding of SP to NK-1, further supporting a role for both SP and NK-1 in adhesion formation. Evidence that SP and NK-1 specifically affect fibrinolysis comes from the same study, in which peritoneal samples were collected from nonoperated controls and from both experimental groups of animals (those who received the NK-1RA or placebo) 24 hours postsurgery. These investigators found that NK-1RA administration led to a significant increase in the expression of mRNA for tPA in both peritoneal fluid and tissue, compared with the operated and nonoperated controls. With the use of zymography, investigators found that the fibrinolytic activity was also increased in the corresponding tissue samples.45
Preventative strategies
The goal of adhesion prevention is to abolish or reduce the incidence, severity, extent and consequences of adhesions while retaining normal healing and preventing infection.4 Over the years, several strategies to prevent postoperative adhesion formation have been proposed, based on what has been learned about the underlying pathophysiology. Unfortunately, although numerous different strategies have been evaluated, few have been successful, and some have even been deleterious. To this day, there are no means of completely preventing postoperative adhesion formation. The only method available to treat adhesions that have already formed is surgical adhesiolysis. Lysis of adhesions is typically only performed in patients who develop complications from adhesions, such as SBO, pain or infertility, since most of the adhesions that are surgically removed will simply reform.5,24
Strict adherence to meticulous surgical technique has been advocated for many years by surgeons and surgical texts as a means to reduce adhesion formation after transperitoneal surgery.4 Although such efforts rarely prevent adhesions in most patients, the principle of good surgical technique to decrease peritoneal injury should not be discounted, because such practices can also influence the risk of developing com-plications associated with surgical procedures.6 The measures that have been described and advocated for decreasing adhesion formation include minimizing peritoneal foreign body exposure (e.g., using suture material only as necessary, eliminating glove powder by washing gloved hands before surgery), careful tissue handling, using cautery and retractors sparingly, ensuring meticulous hemostasis while avoiding dessication and ischemia, administering prophylaxis against infection and avoiding the use of overheated irrigation fluids.4,6
Given that strict adherence to careful surgery does not eliminate or prevent adhesion formation, there are some surgical adjuvants that have been developed and evaluated for the purpose of decreasing postsurgical adhesion formation. An in-depth, comprehensive discussion of each agent is beyond the scope of this review; therefore, a general overview of these agents will be provided. There are 6 main mechanisms that interfere with adhesion formation: those that decrease peritoneal damage, those that decrease the initial inflammatory response, those that prevent fibrin formation, those that increase fibrinolysis, those that prevent collagen deposition and those that act as barriers to adhesion formation (Table 1).
Table 1
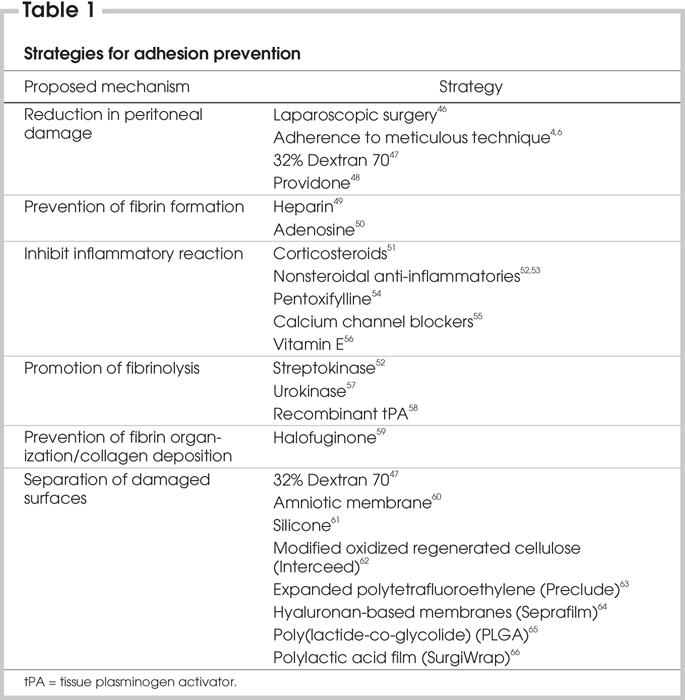
The agents that act most directly to reduce adhesions do so by decreasing the deposition of fibrin, which is absolutely necessary for adhesion formation to occur. These agents include nonsteroidal antiinflammatory drugs (NSAIDs), which interfere with prostaglandin synthesis and decrease the initial inflammatory response, and anticoagulants such as heparin. The results from studies using NSAIDs have been conflicting in terms of their effectiveness in reducing adhesions,52,67 and their use is controversial due to the risk of bleeding. Immunomodulators, such as corticosteroids, have also been tested for their ability to prevent adhesions,51 but their effectiveness has been found to be equivocal68 or even deleterious in some studies.69
Once fibrin is formed, another method of adhesion prevention is to eliminate fibrin, usually by enzymatic degradation.6 Examples of agents that degrade fibrin are streptokinase and the synthetic tissue plasminogen activators. Unfortunately, although successful in reducing adhesion formation in animal models, the use of recombinant tissue plasminogen activator (rtPA) is limited not only by the significant cost and intra-peritoneal administration that is required, but also by the risk of hemorrhage that exists.58,70
There are other miscellaneous agents that have been tried with limited success. One agent that deserves to be mentioned is halofuginone, an inhibitor of type I collagen synthesis. Halofuginone acts to prevent the formation of permanent fibrous adhesions by decreasing collagen deposition in the fibrin matrix. Although effective in reducing adhesion formation in animal models, it has yet to be evaluated in humans.59,71 Concerns have been raised about the safety of halofuginone, specifically, the effects it may have on the biosynthesis of other critical matrix proteins and, therefore, the potential for impairing normal wound healing.6,59
The most promising group of agents to be evaluated for their effectiveness in decreasing surgically induced adhesions are known as barriers. Barriers exist in the form of a membrane or gel, and they act to separate damaged or injured peritoneal surfaces that may be at risk for adhesions. These agents exert their effects locally, at the specific site where they have been applied, and have no effect on remote areas in the peritoneal cavity. An ideal barrier does not yet exist; however, in creating one, the following characteristics should be kept in mind: antiadhesive, biocompatible, resorbable, adherent to the traumatized surface, effective on an oozing surface, applicable through the laparoscope and inexpensive.72
The first barrier to demonstrate efficacy in humans is composed of modified oxidized regenerated cellulose and is known as Interceed (Johnson & Johnson, New Brunswick, NJ).62 Although studies have found it to be successful in reducing adhesion formation in gynecological procedures, its use in general surgical procedures is not known. Further, it has been suggested that the efficacy of Interceed is significantly reduced in the presence of blood. In fact, it has been observed that adhesion formation can actually increase if the Interceed barrier is placed in areas where blood accumulation cannot be prevented (e.g., the pelvis), making it less acceptable to use.62
The Preclude Peritoneal Membrane (W.L. Gore & Associates, The Netherlands) is another barrier that has been evaluated and found to be successful in decreasing postoperative adhesions. It consists of expanded polytetrafluoroethylene (PTFE), which is also used to make Gore-Tex. Animal studies evaluating PTFE as an antiadhesion barrier have found it to be effective in preventing pelvic adhesions only if sufficient in size to cover the entire peritoneal defect, with at least a 1-cm overlap onto normal peritoneum.73 Unfortunately, PTFE is not bioabsorbable and requires suturing to keep it in place, making it undesirable for use as a barrier to prevent adhesions, especially in cases where future reoperative surgery is likely.65 In addition, the cost of PTFE and the large size required for it to be effective, makes routine use of it after abdomino-pelvic surgery difficult to justify.
The most extensively studied barrier, and the most efficacious to date, is a hyaluronan-based agent that is available both as a viscous solution and as a membrane. Hyaluronan is a naturally occurring polysaccharide that is present in virtually all tissues and bodily fluids of vertebrate animals and plays several roles in cellular biology.64 Studies have suggested that hyaluronan-based agents have the potential to act by different mechanisms to decrease adhesion formation. For example, sodium hyaluronate seems to improve peritoneal healing by facilitating cell detachment and migration and by increasing the proliferation rate of mesothelial cells, thereby helping to restore denuded areas of the mesothelial lining.74 Other studies have suggested that hyaluronan might also increase the fibrinolytic response of mesothelial cells, although this has not yet been demonstrated in vivo.74
The bioresorbable membrane that consists of hyaluronan and carbo-xymethylcellulose is most commonly known as Seprafilm (Genzyme Corporation, Cambridge, Mass.). This membrane was introduced in 1996 for use as a barrier to decrease postsurgical adhesions. The same components of Seprafilm also exist as a solution known as Sepracoat (Genzyme Corporation, Cambridge, Mass.). This viscous, gel-like solution was developed for use as a coating during surgery to protect tissues against operative trauma; it was hoped that Sepracoat would act postoperatively as a medium to keep the intestines separated until the mesothelial lining was restored.27 Unfortunately, Sepracoat is short-lived in the peritoneal cavity and has only moderate efficacy against the formation of de novo adhesions, limiting its widespread use.75 Conversely, Seprafilm has been evaluated in human studies, all of which demonstrated a significant reduction in the formation of adhesions with use of this membrane.3,64,76 Unlike its counterpart Sepracoat, which is applied during surgery, Seprafilm sheets are placed at potential sites of adhesion formation at the end of the procedure, just before closure. The Seprafilm membrane hydrates to form a gel-like barrier within the next 24 to 48 hours. It slowly resorbs within 7 days of placement and is fully excreted by 28 days. The hyaluronan in Seprafilm is degraded in the same manner as the endogenous form.27
Both animal and human studies have found a significant decrease in adhesion formation with the use of Seprafilm. Becker and colleagues64 evaluated the use of Seprafilm after colectomy and IPAA with diverting loop ileostomy in patients with ulcerative colitis or familial adenomatous polyposis. They found that the use of Seprafilm halved the incidence of adhesions and significantly reduced the extent and severity of adhesions to the anterior abdominal wall when patients were reexplored laparoscopically at the time of ileostomy closure 8 to 12 weeks later. Fifty-one percent of patients in the treatment group and 6% of the control group were free of such adhesions at second look laparoscopy.
In a large multicentre trial, Beck and colleagues76 evaluated the use of Seprafilm in patients undergoing various types of abdomino-pelvic procedures. Just before closure of the abdomen, each of the 1791 participating patients was randomized to receive Seprafilm or no treatment. The main objective of this study was to prospectively evaluate the long-term effectiveness of Seprafilm for the reduction of adhesion-related postoperative bowel obstruction after abdomino-pelvic surgery; the results are pending. A secondary objective was to evaluate the safety of Seprafilm by looking at the incidence of postoperative abscess formation and pulmonary embolism. Although there were no significant differences found between the treatment and control groups, a subgroup analysis demonstrated that when Seprafilm was wrapped around a fresh anastomosis, there was a significant increase in the number of anastomotic leak–related events (e.g., peritonitis, fistula or abscess formation or both, anastomotic leak and sepsis). Given these findings, they concluded that, although the use of Seprafilm in the peritoneal cavity seems to be safe, it should not be used in areas that are in close proximity to fresh intestinal anastomoses.
The other human studies that evaluated the effectiveness of Sepra-film for the reduction in postoperative adhesion formation are limited in that they failed to evaluate the clinically relevant outcomes. That is, it remains to be seen whether there is a reduction in long-term morbidity, particularly in the incidence of SBO, as a result of using Seprafilm. The trial by Beck and colleagues76 was designed specifically to address this question; the results, however, are still pending.
More recently, 2 novel absor-bable antiadhesion barriers have been assessed in animal models; a nanostructured barrier made by electrospinning copolymers of polylactidecoglycolide (PLGA) and mixing it with cefoxitin65 and a polylactic acid film (SurgiWrap).66 The efficacy of these barriers in reducing adhesion formation was evaluated in a rat model of surgically induced adhesions. Both studies showed significantly decreased rates of adhesion formation in the treated animals. Further studies are needed to evaluate these novel compounds in terms of their safety profile and their efficacy in the reduction of peritoneal adhesions in humans.
A final strategy to decrease adhesion formation is to cause less operative trauma to the peritoneum. Laparoscopic surgery has the theoretical advantage of inducing fewer adhesions than open surgery, because there is typically less peritoneal damage incurred with the former technique. The purported advantages of laparoscopic surgery are supported by studies that have recently emerged, comparing rates of adhesion formation after laparoscopic surgery to conventional open surgery. Fifteen studies published from 1987 to 2001 were identified and recently reviewed by Gutt and colleagues.46 Unfortunately, they were unable to carry out a metaanalysis, due to the significant diversity of the studies in terms of their designs, the end points evaluated and the adhesion scoring systems that were used. Most of the studies were experimental and used animal models to look at rates of adhesion formation. Only 3 of the studies identified by the reviewers were clinical. The reviewers found that all of the clinical studies and most of the experimental studies showed a reduction in the formation of adhesions after laparoscopic surgery, compared with open surgery. These findings are promising, especially given the recent advances that have been made in laparoscopic techniques and the increasing number of procedures that can now be performed this way. Further investigations (particularly human trials) are warranted before such conclusions can be made unequivocally. The existing studies are not without limitations, the most significant of which was the incomplete assessment of adhesion formation.
Summary
The formation of peritoneal adhesions continues to plague patients, surgeons and society. Although research in this area is ongoing, there is currently no method that is 100% effective in adhesion prevention, nor is there any way to permanently remove them once they have formed. As our understanding of the specific mechanisms involved in peritoneal repair evolves, it seems likely that specific targets for adhesion prevention will be identified and evaluated. The bioresorbable membrane Sepra-film is currently the most effective adjuvant to decrease adhesion formation, and this barrier may be considered for use in patients in whom the formation of adhesions postoperatively is particularly undesirable. The long-term outcomes with this agent remain unknown. Newer products are being developed that seem promising, but their efficacy has yet to be proven in clinical trials. Until then, surgeons should continue to be meticulous in their operative technique and should seek to minimize injury to the peritoneal surface.
Competing interests: None declared.
Accepted for publication June 17, 2005
Correspondence to: Dr. Anthony R. MacLean, Department of Surgery, Foothills Medical Centre, 1403–29th St. N.W., Calgary AB T2N 2T9; Tony.MacLean@calgaryhealthregion.ca
References
- 1.Vrijland WW, Jeekel J, van Geldorp HJ, et al. Abdominal adhesions: intestinal obstruction, pain, and infertility. Surg Endosc 2003;17:1017-22. [DOI] [PubMed]
- 2.Sulaiman H, Dawson L, Laurent GJ, et al. Role of plasminogen activators in peritoneal adhesion formation. Biochem Soc Trans 2002;30:126-31. [DOI] [PubMed]
- 3.Vrijland WW, Tseng LN, Eijkman HJ, et al. Fewer intraperitoneal adhesions with use of hyaluronic acid-carboxymethylcellulose membrane: a randomized clinical trial. Ann Surg 2002;235:193-9. [DOI] [PMC free article] [PubMed]
- 4.Dijkstra FR, Nieuwenhuijzen M, Reijnen MM, et al. Recent clinical developments in pathophysiology, epidemiology, diagnosis and treatment of intra-abdominal adhesions. Scand J Gastroenterol Suppl 2000;232:52-9. [PubMed]
- 5.Menzies D, Ellis H. Intestinal obstruction from adhesions–how big is the problem? Ann R Coll Surg Engl 1990;72:60-3. [PMC free article] [PubMed]
- 6.Monk BJ, Berman ML, Montz FJ. Adhesions after extensive gynecologic surgery: clinical significance, etiology, and prevention. Am J Obstet Gynecol 1994;170:1396-403. [DOI] [PubMed]
- 7.Johnston S, O'Malley K, McEntee G, et al. The need to retrieve the dropped stone during laparoscopic cholecystectomy. Am J Surg 1994;167:608-10. [DOI] [PubMed]
- 8.Ellis H, Moran BJ, Thompson JN, et al. Adhesion-related hospital readmissions after abdominal and pelvic surgery: a retrospective cohort study. Lancet 1999;353:1476-80. [DOI] [PubMed]
- 9.Zbar RI, Crede WB, McKhann CF, et al. The postoperative incidence of small bowel obstruction following standard, open appendectomy and cholecystectomy: a six-year retrospective cohort study at Yale-New Haven Hospital. Conn Med 1993;57:123-7. [PubMed]
- 10.Ahlberg G, Bergdahl S, Rutqvist J, et al. Mechanical small-bowel obstruction after conventional appendectomy in children. Eur J Pediatr Surg 1997;7:13-5. [DOI] [PubMed]
- 11.Beck DE, Opelka FG, Bailey HR, et al. Incidence of small-bowel obstruction and adhesiolysis after open colorectal and general surgery. Dis Colon Rectum 1999;42: 241-8. [DOI] [PubMed]
- 12.Nieuwenhuijzen M, Reijnen MM, Kuijpers JH, et al. Small bowel obstruction after total or subtotal colectomy: a 10-year retrospective review. Br J Surg 1998;85:1242-5. [DOI] [PubMed]
- 13.MacLean AR, Cohen Z, MacRae HM, et al. Risk of small bowel obstruction after the ileal pouch-anal anastomosis. Ann Surg 2002;235:200-6. [DOI] [PMC free article] [PubMed]
- 14.Fazio VW, Ziv Y, Church JM, et al. Ileal pouch-anal anastomoses complications and function in 1005 patients. Ann Surg 1995;222:120-7. [DOI] [PMC free article] [PubMed]
- 15.Marcello PW, Roberts PL, Schoetz DJ Jr, et al. Obstruction after ileal pouch-anal anastomosis: a preventable complication? Dis Colon Rectum 1993;36:1105-11. [DOI] [PubMed]
- 16.Francois Y, Dozois RR, Kelly KA, et al. Small intestinal obstruction complicating ileal pouch-anal anastomosis. Ann Surg 1989;209:46-50. [DOI] [PMC free article] [PubMed]
- 17. Bryant T. Clinical lectures on intestinal obstruction. Med Times Gaz 1872;1:363-5.
- 18.Stovall TG, Elder RF, Ling FW. Predictors of pelvic adhesions. J Reprod Med 1989;34:345-8. [PubMed]
- 19.Sulaiman H, Gabella G, Davis MC, et al. Presence and distribution of sensory nerve fibers in human peritoneal adhesions. Ann Surg 2001;234:256-61. [DOI] [PMC free article] [PubMed]
- 20.Van Der Krabben AA, Dijkstra FR, Nieuwenhuijzen M, et al. Morbidity and mortality of inadvertent enterotomy during adhesiotomy. Br J Surg 2000;87:467-71. [DOI] [PubMed]
- 21.Cheong YC, Laird SM, Li TC, et al. Peritoneal healing and adhesion formation/ reformation. Hum Reprod Update 2001;7:556-66. [DOI] [PubMed]
- 22.Ray NF, Denton WG, Thamer M, et al. Abdominal adhesiolysis: inpatient care and expenditures in the United States in 1994. J Am Coll Surg 1998;186:1-9. [DOI] [PubMed]
- 23.Ivarsson ML, Holmdahl L, Franzen G, et al. Cost of bowel obstruction resulting from adhesions. Eur J Surg 1997;163:679-84. [PubMed]
- 24.Holmdahl L. Making and covering of surgical footprints. Lancet 1999;353:1456-7. [DOI] [PubMed]
- 25.Holmdahl L, Ivarsson ML. The role of cytokines, coagulation, and fibrinolysis in peritoneal tissue repair. Eur J Surg 1999;165:1012-9. [DOI] [PubMed]
- 26.Buckman RF, Woods M, Sargent L, et al. A unifying pathogenetic mechanism in the etiology of intraperitoneal adhesions. J Surg Res 1976;20:1-5. [DOI] [PubMed]
- 27.Reijnen MM, Bleichrodt RP, Van Goor H. Pathophysiology of intra-abdominal adhesion and abscess formation, and the effect of hyaluronan. Br J Surg 2003;90:533-41. [DOI] [PubMed]
- 28.Reed KL, Fruin AB, Bishop-Bartolomei KK, et al. Neurokinin-1 receptor and substance P messenger RNA levels increase during intraabdominal adhesion formation. J Surg Res 2002;108:165-72. [DOI] [PubMed]
- 29.Rout UK, Diamond MP. Role of plasminogen activators during healing after uterine serosal lesioning in the rat. Fertil Steril 2003;79:138-45. [DOI] [PubMed]
- 30.Herrick SE, Mutsaers SE, Ozua P, et al. Human peritoneal adhesions are highly cellular, innervated, and vascularized. J Pathol 2000;192:67-72. [DOI] [PubMed]
- 31.Holmdahl L, Eriksson E, al Jabreen M, et al. Fibrinolysis in human peritoneum during operation. Surgery 1996;119:701-5. [DOI] [PubMed]
- 32.Ivarsson ML, Bergstrom M, Eriksson E, et al. Tissue markers as predictors of postoperative adhesions. Br J Surg 1998;85:1549-54. [DOI] [PubMed]
- 33.Moore KL, Bang NU, Broadie TA, et al. Peritoneal fibrinolysis: evidence for the efficiency of the tissue-type plasminogen activator. J Lab Clin Med 1983;101:921-9. [PubMed]
- 34.Raftery AT. Effect of peritoneal trauma on peritoneal fibrinolytic activity and intraperitoneal adhesion formation. An experimental study in the rat. Eur Surg Res 1981;13:397-401. [DOI] [PubMed]
- 35.Vipond MN, Whawell SA, Thompson JN, et al. Peritoneal fibrinolytic activity and intra-abdominal adhesions. Lancet 1990;335:1120-2. [DOI] [PubMed]
- 36.Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med 1994;331:1286-92. [DOI] [PubMed]
- 37.Tietze L, Elbrecht A, Schauerte C, et al. Modulation of pro-and antifibrinolytic properties of human peritoneal mesothelial cells by transforming growth factor beta1 (TGF-beta1), tumor necrosis factor alpha (TNF-alpha) and interleukin 1beta (IL-1beta). Thromb Haemost 1998;79:362-70. [PubMed]
- 38.Williams RS, Rossi AM, Chegini N, et al. Effect of transforming growth factor beta on postoperative adhesion formation and intact peritoneum. J Surg Res 1992;52: 65-70. [DOI] [PubMed]
- 39.Lucas PA, Warejcka DJ, Young HE, et al. Formation of abdominal adhesions is inhibited by antibodies to transforming growth factor-beta1. J Surg Res 1996;65:135-8. [DOI] [PubMed]
- 40.Holmdahl L, Kotseos K, Bergstrom M, et al. Overproduction of transforming growth factor-beta1 (TGF-beta1) is associated with adhesion formation and peritoneal fibrinolytic impairment. Surgery 2001;129:626-32. [DOI] [PubMed]
- 41.Tsukada K, Katoh H, Shiojima M, et al. Concentrations of cytokines in peritoneal fluid after abdominal surgery. Eur J Surg 1993;159:475-9. [PubMed]
- 42.Whawell SA, Thompson JN. Cytokine-induced release of plasminogen activator inhibitor-1 by human mesothelial cells. Eur J Surg 1995;161:315-8. [PubMed]
- 43.Kaidi AA, Gurchumelidze T, Nazzal M, et al. Tumor necrosis factor-alpha: a marker for peritoneal adhesion formation. J Surg Res 1995;58:516-8. [DOI] [PubMed]
- 44.Sturiale S, Barbara G, Qiu B, et al. Neutral endopeptidase (EC 3.4.24.11) terminates colitis by degrading substance P. Proc Natl Acad Sci U S A 1999;96:11653-8. [DOI] [PMC free article] [PubMed]
- 45.Reed KL, Fruin AB, Gower AC, et al. A neurokinin 1 receptor antagonist decreases postoperative peritoneal adhesion formation and increases peritoneal fibrinolytic activity. Proc Natl Acad Sci U S A 2004;101:9115-20. [DOI] [PMC free article] [PubMed]
- 46.Gutt CN, Oniu T, Schemmer P, et al. Fewer adhesions induced by laparoscopic surgery? Surg Endosc 2004;18:898-906. [DOI] [PubMed]
- 47.Reduction of postoperative pelvic adhesions with intraperitoneal 32% dextran 70: a prospective, randomized clinical trial. Fertil Steril 1983;40:612-9. [DOI] [PubMed]
- 48.Goldberg EP, Sheets JW, Habal MB. Peritoneal adhesions: prevention with the use of hydrophilic polymer coatings. Arch Surg 1980;115:776-80. [DOI] [PubMed]
- 49.Fukasawa M, Girgis W, diZerega GS. Inhibition of postsurgical adhesions in a standardized rabbit model: II. Intraperitoneal treatment with heparin. Int J Fertil 1991;36:296-301. [PubMed]
- 50.Jackson EK. Intraperitoneal administration of adenosine inhibits formation of abdominal adhesions. Dis Colon Rectum 2004;47:1390-6. [DOI] [PubMed]
- 51.Replogle RL, Johnson R, Gross RE. Prevention of postoperative intestinal adhesions with combined promethazine and dexamethasone therapy: experimental and clinical studies. Ann Surg 1966;163:580-8. [DOI] [PMC free article] [PubMed]
- 52.Montz FJ, Monk BJ, Lacy SM, et al. Ketorolac tromethamine, a nonsteroidal anti-inflammatory drug: ability to inhibit post-radical pelvic surgery adhesions in a porcine model. Gynecol Oncol 1993;48:76-9. [DOI] [PubMed]
- 53.Siegler AM, Kontopoulos V, Wang CF. Prevention of postoperative adhesions in rabbits with ibuprofen, a nonsteroidal anti-inflammatory agent. Fertil Steril 1980;34:46-9. [PubMed]
- 54.Steinleitner A, Lambert H, Kazensky C, et al. Pentoxifylline, a methylxanthine derivative, prevents postsurgical adhesion reformation in rabbits. Obstet Gynecol 1990;75:926-8. [PubMed]
- 55.Steinleitner A, Kazensky C, Lambert H. Calcium channel blockade prevents postsurgical reformation of adnexal adhesions in rabbits. Obstet Gynecol 1989;74:796-8. [PubMed]
- 56.de la PF. Ynfante I, Bejarano D, et al. Prevention of peritoneal adhesions by intraperitoneal administration of vitamin E: an experimental study in rats. Dis Colon Rectum 2004;47:2157-61. [DOI] [PubMed]
- 57.Hellebrekers BW, Trimbos-Kemper TC, Trimbos JB, et al. Use of fibrinolytic agents in the prevention of postoperative adhesion formation. Fertil Steril 2000;74:203-12. [DOI] [PubMed]
- 58.Montz FJ, Fowler JM, Wolff AJ, et al. The ability of recombinant tissue plasminogen activator to inhibit post-radical pelvic surgery adhesions in the dog model. Am J Obstet Gynecol 1991;165:1539-42. [DOI] [PubMed]
- 59.Nagler A, Genina O, Lavelin I, et al. Halofuginone, an inhibitor of collagen type I synthesis, prevents postoperative adhesion formation in the rat uterine horn model. Am J Obstet Gynecol 1999;180: 558-63. [DOI] [PubMed]
- 60.Young RL, Cota J, Zund G, et al. The use of an amniotic membrane graft to prevent postoperative adhesions. Fertil Steril 1991;55:624-8. [DOI] [PubMed]
- 61.Yemini M, Shoham Z, Katz Z, et al. Effectiveness of silicone sheeting in preventing the formation of pelvic adhesions. Int J Fertil 1989;34:71-3. [PubMed]
- 62.Prevention of postsurgical adhesions by Interceed (TC7), an absorbable adhesion barrier: a prospective randomized multicenter clinical study. Interceed (TC7) Adhesion Barrier Study Group. Fertil Steril 1989;51:933-8. [PubMed]
- 63.Bellon JM, Bujan J, Contreras LA, et al. Use of nonporous polytetrafluoroethylene prosthesis in combination with polypropylene prosthetic abdominal wall implants in prevention of peritoneal adhesions. J Biomed Mater Res 1997;38:197-202. [DOI] [PubMed]
- 64.Becker JM, Dayton MT, Fazio VW, et al. Prevention of postoperative abdominal adhesions by a sodium hyaluronate-based bioresorbable membrane: a prospective, randomized, double-blind multicenter study. J Am Coll Surg 1996;183:297-306. [PubMed]
- 65.Zong X, Li S, Chen E, et al. Prevention of postsurgery-induced abdominal adhesions by electrospun bioabsorbable nanofibrous poly(lactide-co-glycolide)-based membranes. Ann Surg 2004;240:910-5. [DOI] [PMC free article] [PubMed]
- 66.Avital S, Bollinger TJ, Wilkinson JD, et al. Preventing intra-abdominal adhesions with polylactic acid film: an animal study. Dis Colon Rectum 2005;48:153-7. [DOI] [PubMed]
- 67.Holtz G. Failure of a nonsteroidal anti-inflammatory agent (ibuprofen) to inhibit peritoneal adhesion reformation after lysis. Fertil Steril 1982;37:582-3. [DOI] [PubMed]
- 68.Risberg B. Adhesions: preventive strategies. Eur J Surg Suppl 1997;577:32-9. [PubMed]
- 69.Grosfeld JL, Berman IR, Schiller M, et al. Excessive morbidity resulting from the prevention of intestinal adhesions with steroids and antihistamines. J Pediatr Surg 1973;8:221-6. [DOI] [PubMed]
- 70.James DC, Ellis H, Hugh TB. The effect of streptokinase on experimental intraperitoneal adhesion formation. J Pathol Bacteriol 1965;90:279-87. [DOI] [PubMed]
- 71.Nagler A, Rivkind AI, Raphael J, et al. Halofuginone–an inhibitor of collagen type I synthesis–prevents postoperative formation of abdominal adhesions. Ann Surg 1998;227:575-82. [DOI] [PMC free article] [PubMed]
- 72.Hellebrekers BW, Trimbos-Kemper GC, van Blitterswijk CA, et al. Effects of five different barrier materials on postsurgical adhesion formation in the rat. Hum Reprod 2000;15:1358-63. [DOI] [PubMed]
- 73.Montz FJ, Monk BJ, Lacy SM. The Gore-Tex surgical membrane: effectiveness as a barrier to inhibit postradical pelvic surgery adhesions in a porcine model. Gynecol Oncol 1992;45:290-3. [DOI] [PubMed]
- 74.Reijnen MM, Falk P, Van Goor H, et al. The antiadhesive agent sodium hyaluronate increases the proliferation rate of human peritoneal mesothelial cells. Fertil Steril 2000;74:146-51. [DOI] [PubMed]
- 75.Diamond MP. Reduction of de novo postsurgical adhesions by intraoperative precoating with Sepracoat (HAL-C) solution: a prospective, randomized, blinded, placebo-controlled multicenter study. The Sepracoat Adhesion Study Group. Fertil Steril 1998;69:1067-74. [DOI] [PubMed]
- 76.Beck DE, Cohen Z, Fleshman JW, et al. A prospective, randomized, multicenter, controlled study of the safety of Seprafilm adhesion barrier in abdominopelvic surgery of the intestine. Dis Colon Rectum 2003;46:1310-9. [DOI] [PubMed]


