Abstract
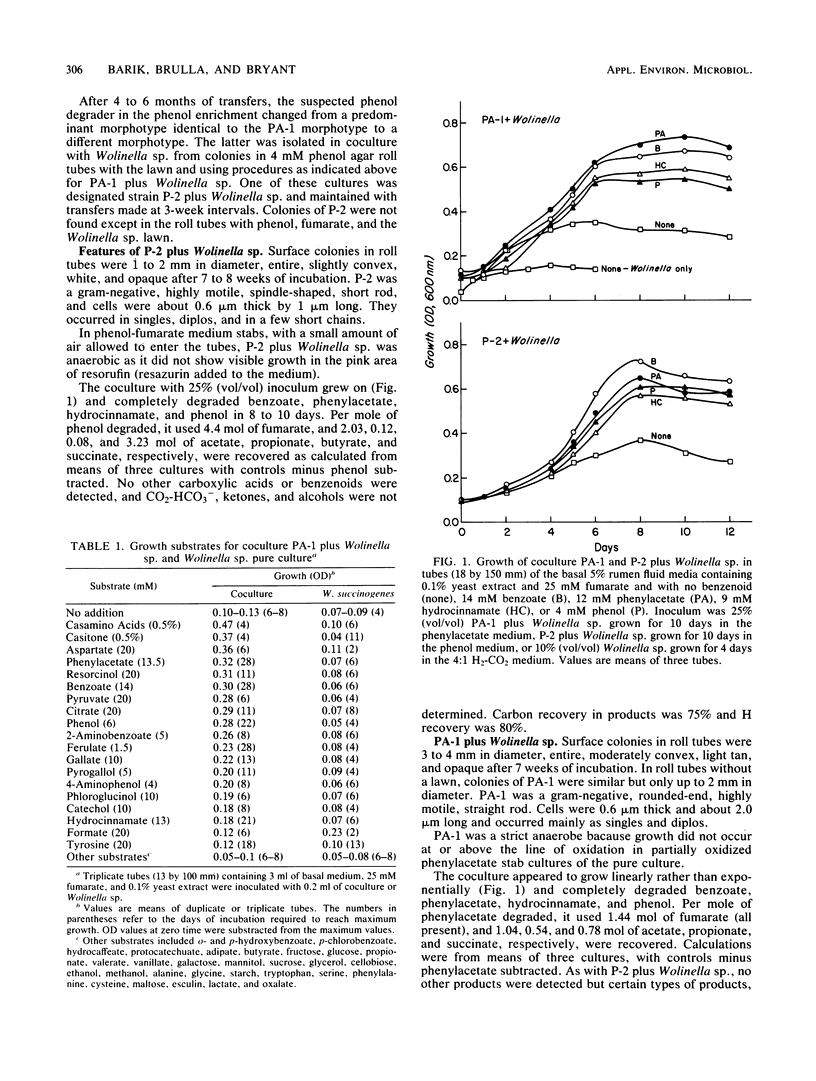
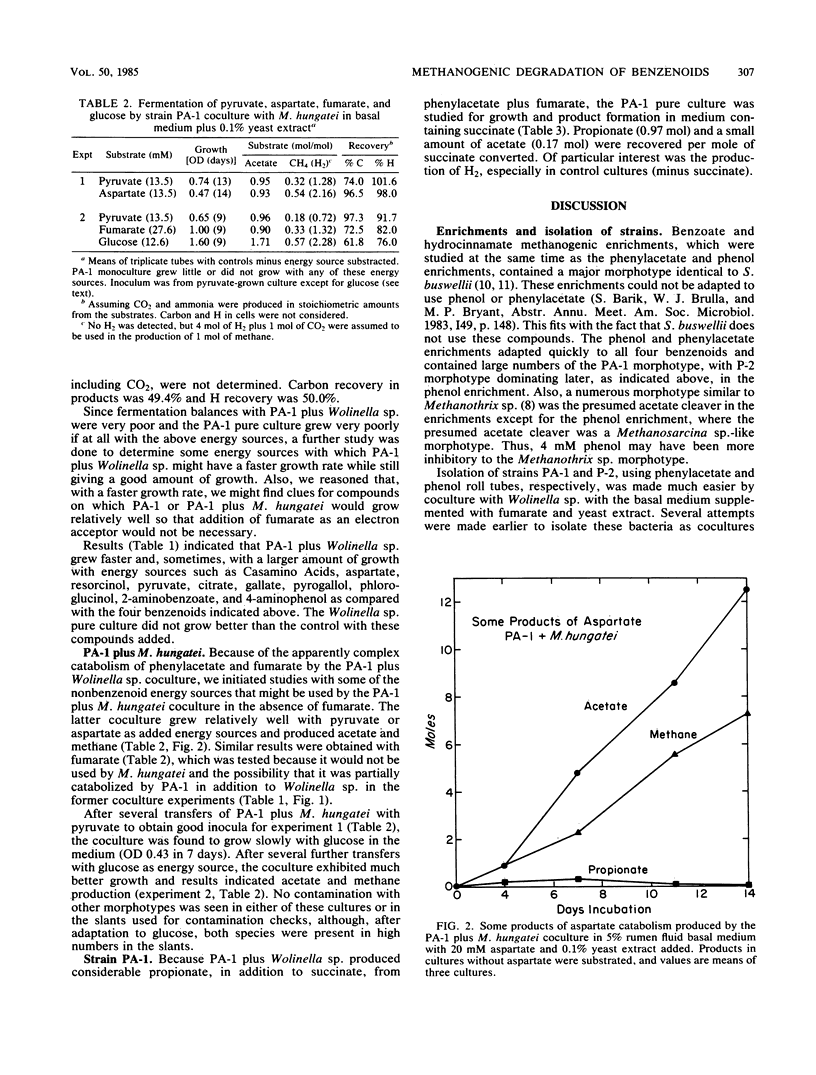
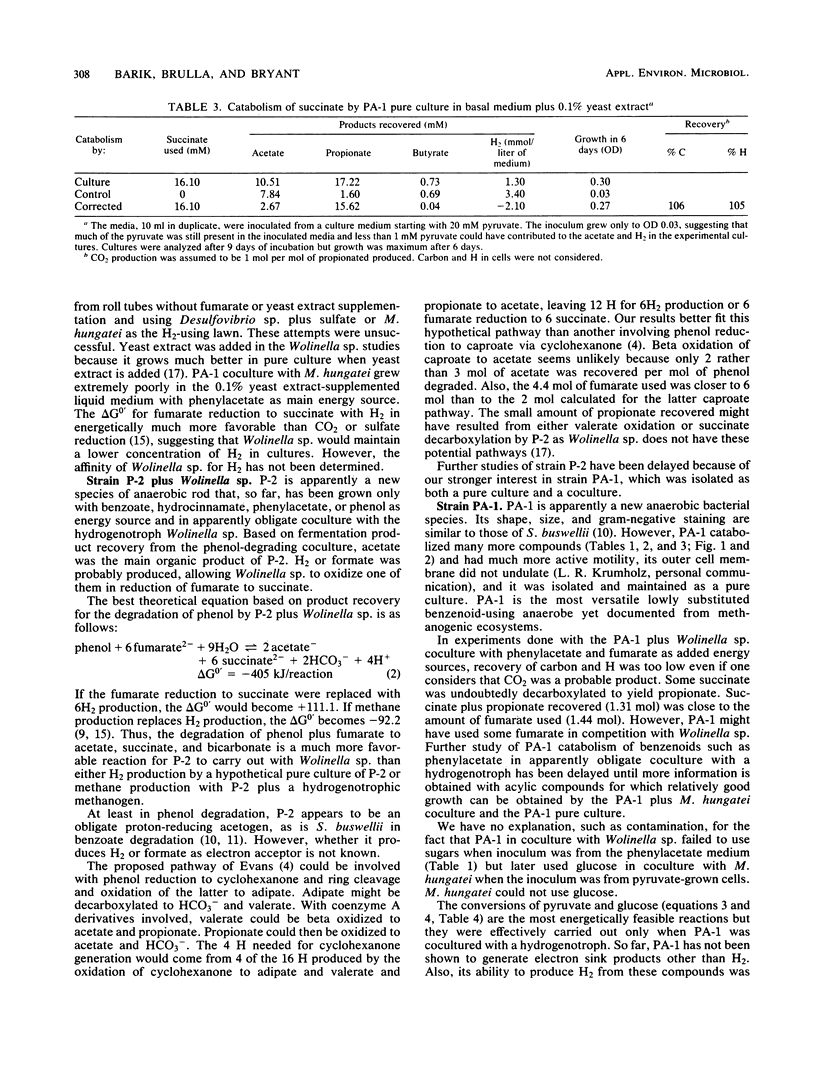
Methanogenic enrichments catabolizing 13 mM phenylacetate or 4 mM phenol were established at 37°C, using a 10% inoculum from a municipal anaerobic digester. By using agar roll tubes of the basal medium plus 0.1% yeast extract-25 mM fumarate, a hydrogenotrophic lawn of Wolinella succinogenes and phenol or phenylacetate, strains P-2 and PA-1, respectively, were isolated in coculture with W. succinogenes. With the lawn deleted, PA-1 was isolated in pure culture. Strain P-2 is apparently a new species of anaerobic, motile, gram-negative, spindle-shaped, small rod that as yet has been grown only in coculture with W. succinogenes. It used phenol, hydrocinnamate, benzoate, and phenylacetate as energy sources. Product recovery by the coculture, per mole of phenol and 4.4 mol of fumarate used, included 2.03, 0.12, 0.08, and 3.23 mol, respectively, of acetate, propionate, butyrate, and succinate. Carbon recovery was 75% and H recovery was 80%, although CO2 and a few other possible products were not determined. That P-2 is an obligate proton-reducing acetogen and possible pathways for its degradation of phenol are discussed. Strain PA-1 is apparently a new species of anaerobic, motile, relatively small, gram-negative rod. It utilized compounds such as phenylacetate, hydrocinnamate, benzoate, phenol, resorcinol, gallate, 4-aminophenol, 2-aminobenzoate, pyruvate, Casamino Acids, and aspartate as energy sources in coculture with W. succinogenes. Per mole of phenylacetate and 1.44 mol of fumarate used, 1.04, 0.53, and 0.78 mol of acetate, propionate, and succinate, respectively, were recovered from the coculture. Only about 50% of the carbon and H were recovered. In coculture with Methanospirillum hungatei, 0.96 mol of acetate and 0.25 mol of methane were recovered per mol of pyruvate used; 0.90 mol of acetate and 0.33 mol of methane, per mol of fumarate used; 0.93 mol of acetate and 0.54 mol of methane, per mol of aspartate used; and 1.71 mol of acetate and 0.57 mol of methane, per mol of glucose used. Carbon and H recoveries, assuming CO2 and ammonia were produced in stoichiometric amounts, were 97 and 98% for pyruvate, 72.5 and 82% for fumarate, 96.5 and 98% for aspartate, and 61.8 and 76% for glucose. No explanation such as contamination could be found for the fact that the coculture PA-1 plus Wolinella sp. did not use glucose; after growth with M. hungatei on pyruvate, however, the latter coculture used glucose. The PA-1 pure culture produced 0.86 mol of propionate per mol of succinate used during growth. PA-1 produced a small amount of H2. Strain PA-1 is the most versatile anaerobic bacterium yet known that catabolizes monobenzenoids in the absence of electron acceptors such as sulfate or nitrate.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balba M. T., Evans W. C. The anaerobic dissimilation of benzoate by Pseudomonas aeruginosa coupled with Desulfovibrio vulgaris, with sulphate as terminal electron acceptor. Biochem Soc Trans. 1980 Oct;8(5):624–625. doi: 10.1042/bst0080624. [DOI] [PubMed] [Google Scholar]
- Bryant M. P. Commentary on the Hungate technique for culture of anaerobic bacteria. Am J Clin Nutr. 1972 Dec;25(12):1324–1328. doi: 10.1093/ajcn/25.12.1324. [DOI] [PubMed] [Google Scholar]
- Evans W. C. Biochemistry of the bacterial catabolism of aromatic compounds in anaerobic environments. Nature. 1977 Nov 3;270(5632):17–22. doi: 10.1038/270017a0. [DOI] [PubMed] [Google Scholar]
- Hilpert W., Schink B., Dimroth P. Life by a new decarboxylation-dependent energy conservation mechanism with Na as coupling ion. EMBO J. 1984 Aug;3(8):1665–1670. doi: 10.1002/j.1460-2075.1984.tb02030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. G., Jones G. A. Isolation and identification of rumen bacteria capable of anaerobic phloroglucinol degradation. Can J Microbiol. 1975 Jun;21(6):794–801. doi: 10.1139/m75-117. [DOI] [PubMed] [Google Scholar]
- WOLIN M. J., WOLIN E. A., JACOBS N. J. Cytochrome-producing anaerobic Vibrio succinogenes, sp. n. J Bacteriol. 1961 Jun;81:911–917. doi: 10.1128/jb.81.6.911-917.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]


