Abstract
Objective
To assess the effectiveness of octreotide in preventing postoperative pancreatic fistula. Pancreatic fistula is one of the most common complications after elective pancreatic surgery. Several clinical trials have evaluated the use of octreotide to prevent the development of pancreatic fistula after pancreatic surgery with conflicting recommendations.
Methods
We undertook a meta-analysis of 7 identified randomized controlled trials, reporting comparisons between octreotide and a control. The primary outcome was the incidence of postoperative pancreatic fistula, and the secondary outcome was the postoperative mortality.
Results
Seven studies, involving 1359 patients, met the inclusion criteria for this review. In these studies, sample sizes ranged from 75 to 252 patients. In total, 679 patients were given octreotide and 680 patients formed the control group. Perioperative octreotide is associated with a significant reduction in the incidence of pancreatic fistula after elective pancreatic surgery, with a relative risk of 0.59 (95% confidence interval 0.41–0.85, p = 0.004). However, this risk reduction was not associated with a significant difference in postoperative mortality (p > 0.05).
Conclusions
The review revealed that perioperative octreotide is associated with a significant reduction in the incidence of pancreatic fistula after elective pancreatic surgery. However, this risk reduction was not associated with a significant difference in postoperative mortality; further studies are warranted to confirm the results of this metaanalysis and to define which patient subgroups might benefit the most from prophylactic octreotide administration.
Abstract
Objectif
Cette méta-analyse visait à évaluer l'efficacité de l'octréotide dans la prévention de la fistule postopératoire du pancréas, une des complications les plus courantes d'une chirurgie élective au pancréas. Plusieurs études cliniques ont évalué l'utilisation de l'octréotide pour prévenir l'apparition d'une fistule du pancréas après une intervention chirurgicale au pancréas. Ces études ont produit des recommandations contradictoires.
Méthodes
Nous avons effectué une méta-analyse de sept études contrôlées randomisées identifiées présentant des comparaisons entre l'octréotide et un témoin. L'incidence de la fistule postopératoire du pancréas a constitué le résultat principal et la mortalité postopératoire, le résultat secondaire.
Résultats
Sept études portant sur 1359 patients satisfaisaient aux critères d'inclusion dans cette méta-analyse. La taille des échantillons sur lesquels ont porté ces études a varié de 75 à 252 patients. Au total, 679 patients ont reçu de l'octréotide et 680 ont constitué le groupe témoin. On établit un lien entre l'octréotide administré au cours de la période périopératoire et une réduction importante de l'incidence de la fistule du pancréas après une intervention chirurgicale élective au pancréas, le RR étant de 0,59 (intervalle de confiance à 95 %, 0,41–0,85, p = 0,004). On n'a toutefois pas établi de lien entre cette réduction du risque et une différence importante au niveau de la mortalité postopératoire (p > 0,05).
Conclusions
L'étude a révélé que l'administration d'octréotide au cours de la période périopératoire est associée à une réduction importante de l'incidence de la fistule du pancréas après une intervention chirurgicale élective au pancréas. Cette réduction du risque n'est toutefois pas associée à une différence importante au niveau de la mortalité postopératoire. D'autres études sont justifiées pour confirmer les résultats de cette méta-analyse et définir les sous-groupes de patients qui pourraient bénéficier le plus de l'administration d'octréotide prophylactique.
More than 31 000 people develop pancreatic adenocarcinoma each year in the United States, and almost all are expected to die from the disease.1 Pancreatic resection remains the only potentially curative treatment for carcinoma of the pancreas and periampullary region.2–8 Resection of the pancreatic head or tail and surgical drainage can also be used for the treatment of chronic pancreatitis to treat disabling pain and focal lesions.9–11 Despite advances in surgical techniques and progress in intensive care unit monitoring, the morbidity rate after pancreatic resection remains as high as 30% to 50%.12
Pancreatic fistula and pancreatic stump-related complications are the main challenge after pancreatic surgery.8,13,14 In patients undergoing pancreaticoduodenectomy, the incidence of pancreatic fistula can range from 5% to 35% in most series.15–19 Because pancreatic fistula has been identified as such a common problem after pancreaticoduodenectomy, several surgical strategies have been described to manage the pancreatic anastomosis or transected gland.20–29
Inhibition of pancreatic exocrine secretion has been proposed to reduce the rate of pancreatic fistula after pancreatic resection.30 Octreotide is a synthetic octapeptide analog of endogenous somatostatin with more potency and longer half-life.31,32 Octreotide exerts its effect by inhibiting serotonin release and the secretion of gastrin, vasoactive intestinal peptide, insulin, glucagon, secretin, motilin and pancreatic polypeptide, thus inhibiting pancreatic exocrine secretion.31,32
Several clinical trials and reviews have evaluated the use of octreotide to prevent the development of pancreatic fistula after pancreatic surgery.33–50 These reports have advocated conflicting recommendations. The objective of this study was to assess the effectiveness of perioperative octreotide in preventing pancreatic fistula after pancreatic resection.
This systematic summary of the results of published randomized controlled clinical trials (RCTs) provides the most complete evidence currently available about the efficacy and safety of perioperative octreotide administration.
Materials and methods
Inclusion and exclusion criteria
We included all published RCTs that included adults (aged over 18 yr) who underwent elective pancreatic resection (defined as surgery for pancreatic cancer, periampullary cancer and chronic pancreatitis). The intervention was octreotide or somatostatin, of a specified dose and duration, given as a prophylactic measure in the intervention group and placebo or no intervention in the control group. The primary outcome was the incidence of pancreatic fistula after pancreatic surgery (defined as postoperative drain output of fluid with amylase content more than 3 times the maximum normal serum value and exceeding 10 mL/24 h for more than 3 d). The secondary end point was postoperative death.
We excluded studies that met any 1 of the following criteria: unspecified methods of detection of pancreatic fistula or unspecified period of follow-up, surgery for acute pancreatitis and pancreatic trauma.
Literature search and data extraction
Studies were identified by searching MEDLINE, EMBASE and the Cochrane Controlled Trial Register (CCTR) on the Cochrane Library, from the earliest achievable date of each database to September 2004; we also manually searched reference lists of retrieved trials. We used the following terms and keywords: “octreotide” or “octreotide acetate” or “somatostatin analog” and “fistula” or “pancreatic fistula.” We limited studies to those identified simultaneously by the highly sensitive search strategy for identifying clinical controlled trials in MEDLINE.51
The studies retrieved by the search strategy were reviewed independently by 2 reviewers, and relevant studies were selected according to the definitions in the inclusion and exclusion criteria. Disagreements were resolved by consensus. A bibliographic software (Reference Manager V.10) was used to download all references and ensured references were not duplicated.
Data were independently extracted by the same 2 reviewers. Collected data included general demographic characteristics (e.g., mean age, sex distribution), dose of octreotide, duration of the intervention and incidence of pancreatic fistula. Where studies evaluated multiple treatment arms, comparisons were made, where possible, between octreotide and placebo. We also acquired data for quality assessment. Reasons for exclusion were documented for all excluded studies. The 2 reviewers independently extracted data on to predesigned data abstraction forms. After collection, data were checked and cross-checked. Discrepancies were resolved by consensus with the senior author.
Two reviewers independently assessed the methodological quality of all included studies, using a van Tulder quality assessment scoring system.52
Statistical methods
One reviewer entered all data into the meta-analysis software package (RevMan 4.2); the second reviewer cross-checked the printout against his own data abstraction forms. All statistical analyses were performed with the RevMan 4.2 package.
First we considered clinical homogeneity of the included studies, by assessing the study population, intervention, comparison group and outcome. Statistical homogeneity was assessed by the RevMan software. Where significant heterogeneity was present, we examined the studies for the reasons of heterogeneity. Data were considered to be heterogeneous if the chi-squared value generated by RevMan heterogeneity test was associated with a p value less than 0.1. Where significant heterogeneity was present, attempts were made to explain the differences on the basis of the patient's clinical characteristics and interventions of the included studies. We used the random-effects method to address heterogeneity, where appropriate.53,54 Where heterogeneity prohibited pooling, data were presented as a qualitative overview. For the outcome of interest (incidence of pancreatic fistula), we used a relative risk (RR) with a 95% confidence interval (CI). We used a fixed-effects model for pooling where the trials were homogenous (see above). Where heterogeneity was evident, we used random effects models for pooling.53,54 We performed a subgroup analysis according to the number of centres that participated in each trial (i.e., single centre v. multicentre).
Results
We identified 14 references from the search strategy and excluded 7 studies after examining the entire manuscript. The reasons for exclusion were as follows: the outcome of interest was not measured (1 study),40 it was a different target population (1 study),33 octreotide was used for the treatment (not as a prophylactic measure) of pancreatic fistula (1 study)44 and other reasons (4 studies).34,36,38,46
Seven studies, involving 1359 patients, met the inclusion criteria for this review. In these studies, sample sizes ranged from 75 to 252 patients. In total, 679 patients were given octreotide and 680 patients formed the control group. Six of the 7 studies were randomized placebo-controlled trials; the remaining study was an RCT of octreotide versus no treatment. Five studies were multicentre: Germany (2), Italy (2) and France (1). The remaining studies were from a single centre — the United States (2). The mean age of participants ranged from 47 to 65 years and the included patients in all studies were scheduled for elective pancreatic surgery. Table 1 summarizes the characteristics of the 7 studies.
Table 1
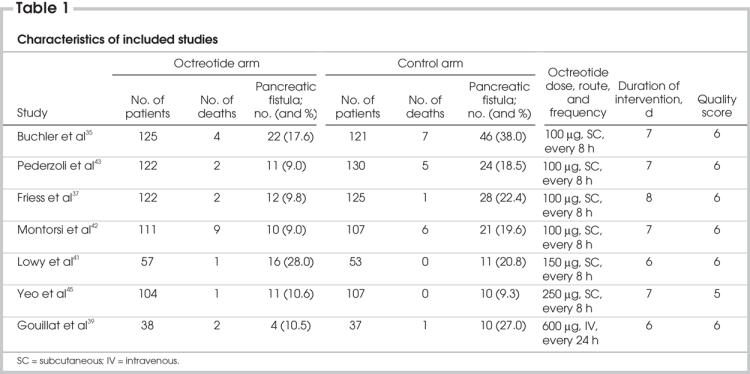
The daily dose of octreotide ranged from 100 μg to 300 μg administered subcutaneously, and the duration of intervention ranged from 6 to 8 days (Table 1).
Of 679 patients in the pooled intervention (octreotide) group, 86 had a pancreatic fistula postoperatively, compared with 150 of 680 patients in the pooled control group. There was statistically significant heterogeneity (I2 = 48.7%, p = 0.07). The pooled analysis under the random-effects model showed that octreotide was associated with a significant risk reduction of postoperative pancreatic fistula (RR 0.59, 95% CI 0.41–0.85; p = 0.004). The number needed to treat (NNT) to prevent 1 patient from having pancreatic fistula was 10 (Fig. 1). A funnel plot to assess for publication bias is shown in Figure 2. The plot approximately resembles a symmetric inverted funnel (the 95% CI); all of the studies included in our final meta-analysis lie within the inverted funnel.
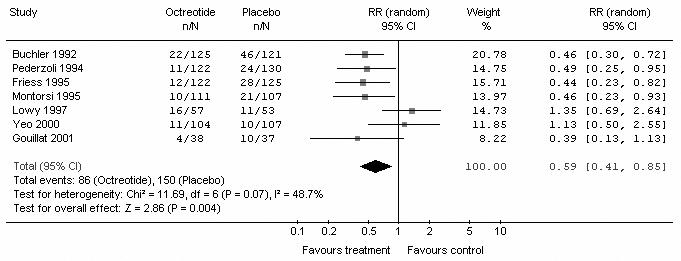
FIG. 1. Meta-analysis of included studies to compare the incidence of pancreatic fistula in octreotide and control groups. RR = relative risk; CI = confidence interval.
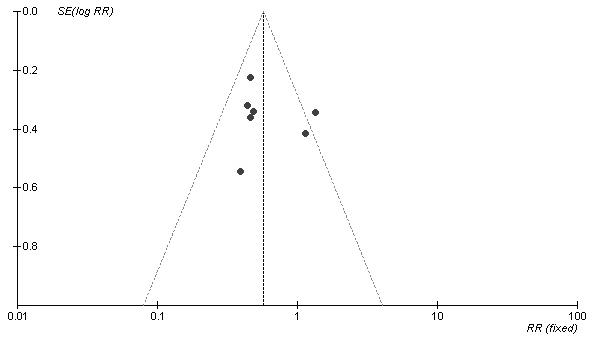
FIG. 2. Funnel plot. SE[log RR] = standard error of log relative risk.
Subgroup analysis was performed according to the number of centres that participated in each trial. Five trials were from multicentres, and the remaining 2 were from single centres. In the multicentre subgroup, of 518 patients in the pooled intervention (octreotide) arm 59 had pancreatic fistula postoperatively, compared with 129 of 520 patients in the pooled control arm. There was no heterogeneity (I2 = 0%, p = 1.00). The pooled analysis under the fixed-effects model showed that octreotide was associated with a significant risk reduction of postoperative pancreatic fistula (Fig. 3) (RR 0.46, 95% CI 0.34–0.60; p < 0.001). In the single-centre subgroup, there were 161 patients in the pooled intervention (octreotide) arm, 27 of whom had a pancreatic fistula postoperatively, compared with 21 of 160 patients in the pooled control arm. There was no heterogeneity (I2 = 0%, p = 0.74). The pooled analysis under the fixed-effect model showed that octreotide was not associated with a significant risk reduction of postoperative pancreatic fistula (Fig. 4) (RR 1.25, 95% CI 0.74–2.10; p = 0.4).
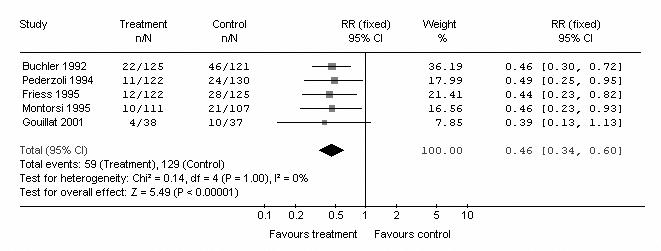
FIG. 3. Subgroup meta-analysis of multicentre studies to compare the incidence of pancreatic fistula in octreotide and control arms. RR = relative risk; CI = confidence interval.
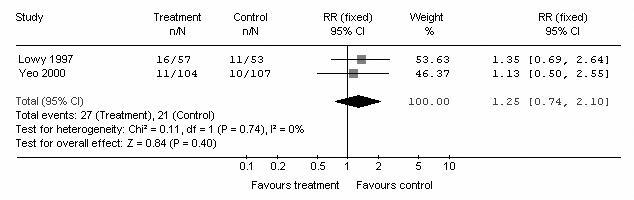
FIG. 4. Subgroup meta-analysis of single-centre studies to compare the incidence of pancreatic fistula in octreotide and control arms. RR = relative risk; CI = confidence interval.
The secondary end point (death after pancreatic surgery) was analyzed according to the length of follow-up. Three studies reported the 90-day mortality.35,37,43 The pooled analysis showed that octreotide was not associated with a significant risk reduction of a postoperative 90-day death (RR 0.62, 95% CI 0.26–1.48; p = 0.28), 1 study reported a 60-day mortality,42 with no significant risk reduction (RR 1.45, 95% CI 0.53– 3.92; p = 0.47), 2 studies reported a 30-day mortality,39,41 with no significant risk reduction (RR 2.23, 95% CI 0.34–14.77; p = 0.4); and 1 study reported an inhospital mortality,45 with no significant risk reduction (Fig. 5) (RR 3.09, 95% CI 0.13– 74.9; p = 0.49).
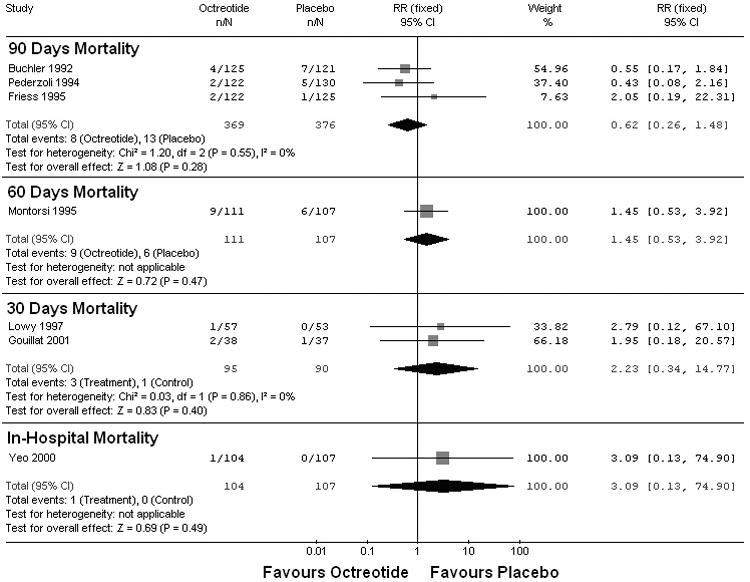
FIG. 5. Meta-analysis of included studies to compare postoperative mortality stratified by length of follow-up. RR = relative risk; CI = confidence interval.
Discussion
The concept of inhibiting exocrine pancreatic secretion to prevent postoperative complications after pancreatic surgery originated in 1979, with Klempa and colleagues;30 they reported lower complication rates after a perioperative continuous infusion of somatostatin (250 mg/h) in patients who underwent a Whipple procedure. The synthetic somatostatin analogue, octreotide (SMS 201–995) is more favourable for clinical use because it possesses a longer half-life.32 Many surgeons now routinely administer perioperative octreotide to patients undergoing elective pancreatic resection.
The focus of this meta-analysis was to address the following controversial question: Is perioperative octreotide effective in reducing the incidence of pancreatic fistula and death after elective pancreatic resection?
Pancreatic fistula remains a challenging problem after pancreatic surgery. It is the most frequent complication after pancreatic resection, occurring in 5% to 35%15–19 of patients. Activated exocrine pancreatic secretion is thought to be a main etiological factor. It has been reported that the mortality after pancreatic surgery is between 3% and 10%.4,9,10,14
Our literature review identified 7 studies that were eligible for this meta-analysis: 4 European multicentre randomized, controlled double-blinded trails, 2 American RCTs and 1 French study.35,37,39,41–43,45
The 4 European studies directly opposed the results of the 2 American studies. Each of the 4 European trials reported a lower incidence of pancreatic fistula in the octreotide group. These trials were performed in many centres by many different surgeons, which might explain the high rate of pancreatic fistula in each of their placebo groups (19%–37%). This contrasts with the American studies, which were done in specialized centres with high-volume experienced surgeons. The rate of pancreatic fistula in the placebo group ranged from 6% to 9%. This low incidence cannot be explained by octreotide administration alone. The surgeon's experience, the type of anastomosis and the quality of the tissue are important determinants.
The definition of a pancreatic fistula is very important. In this meta-analysis, we defined pancreatic fistula as a postoperative drain output of fluid, with an amylase content of more than 3 times the serum level, exceeding 10 mL per 24 hours for more than 3 days; this biochemical leak is more liberal and is adopted by European studies. Yeo and colleagues45 adopted a more conservative definition (> 50 mL after postoperative day 10). They considered biochemical leak to be unimportant, because the most leaks resolve without squelae. Lowy and colleagues41 separated both leaks. The clinical leak rate was 12% in the octreotide group, versus 6% in the placebo group, whereas the total leak rate (clinical and biochemical) was 28% in the octreotide group versus 21% in the control group. The discrepancy in the rate of pancreatic fistula between the European and American trials may partially a result of the inclusion of more biochemical leaks in the early trials.
In the European studies, patients were stratified into 2 groups: high-risk patients (soft pancreas, nonpancreatic and periampullary tumours) and low-risk patients (fibrotic pancreas, chronic pancreatitis). The low-risk group of patients had more favourable pancreatic tissue with which to create an anastomosis, due to the fibrosis of the gland, a larger pancreatic duct and, perhaps, a reduced overall pancreatic exocrine function.
Lowy and colleagues41 reported a single-centre trial at the University of Texas MD Anderson Cancer Center in Houston, Texas. All 120 patients underwent pancreaticoduodenectomy, with use of a standard operative technique. The rate of pancreatic fistula was 6% in the control group and 12% in the octreotide group. This study has received some criticism, because 46 of 110 patients received preoperative chemoradiation, and 64 of 110 patients received intraoperative radiation; this could have resulted in a lower complication rate.41 Yeo and colleagues45 at Johns Hopkins Hospital, Baltimore, studied 211 patients undergoing pancreaticoduodenectomy. The rate of pancreatic fistula was 9% in the control group and 11% in the octreotide group. They concluded that prophylactic octreotide has no benefit and should be eliminated at a considerable cost savings.
Octreotide is a well-tolerated drug with few side effects. The main side effect is pain at the injection site. Buchler and others35,36 reported 31 of 125 patients with this problem; however, it did not require discontinuation of the treatment. Other side effects include hot flashes, rash, fever, nausea, emesis and asymptomatic biliary sludge. No major systemic side effects, such as glucose imbalance, were encountered in any of these studies.35,37,41–43,45
Factors influencing the risk of pancreatic fistula development after pancreatic surgery include the type of surgery (Whipple v. distal pancreatectomy), consistency of the gland and, most importantly, the surgeon's experience. Lerut and colleagues55 described the influence of age, preoperative renal insufficiency and emergency surgery, whereas Yeo and colleagues45 described a strong association between the texture of the gland and fistula formation, with a higher fistula rate in soft-texture glands.
The type of surgery could influence the rate of pancreatic fistula development. Montorsi and colleagues42 found no statistical difference in the rate of pancreatic fistula in patients who underwent pancreaticoduodenectomy; this was supported by the findings of Yeo and colleagues45 and Lowy and colleagues.41 On the contrary, pancreatic fistula was significantly lower in the octreotide group than in the placebo group for patients who underwent distal pancreatectomy and local pancreatic resection, when combined together.
The pooled analysis of these 1359 patients — 679 in the octreotide group and 680 in the control group — showed that prophylactic octreotide is associated with a significant reduction of postoperative pancreatic fistula. Further research is warranted to define subgroups of patients who may benefit most from octreotide administration.
Conclusions
This review revealed that use of perioperative octreotide is associated with a significant reduction in the incidence of pancreatic fistula after elective pancreatic surgery. However, this risk reduction was not associated with a significant difference in postoperative mortality; further studies are warranted to confirm the result of this meta-analysis and to define which patients' subgroups may benefit the most from prophylactic octreotide administration.
Competing interests: None declared.
Accepted for publication Mar. 30, 2006
Correspondence to: Dr. Abdullah A. Alghamdi, Department of Surgery, St. Joseph's Health Centre, University of Toronto, 30 The Queensway, Toronto ON M6R 1B5; fax: 416 530-6844; Abdullah.Alghamdi@utoronto.ca
References
- 1.Jemal A, Tiwari RC, Murray T, et al. Cancer statistics, 2004. CA Cancer J Clin 2004;54:8-29. [DOI] [PubMed]
- 2.Grace PA, Pitt HA, Longmire WP. Pylorus preserving pancreatoduodenectomy: an overview. Br J Surg 1990;77:968-74. [DOI] [PubMed]
- 3.Neoptolemos JP, Talbot IC, Carr-Locke DL, et al. Treatment and outcome in 52 consecutive cases of ampullary carcinoma. Br J Surg 1987;74:957-61. [DOI] [PubMed]
- 4.Peters JH, Carey LC. Historical review of pancreaticoduodenectomy. Am J Surg 1991;161:219-25. [DOI] [PubMed]
- 5.Singh SM, Longmire WP Jr, Reber HA. Surgical palliation for pancreatic cancer. Ann Surg 1990;212:132-9. [DOI] [PMC free article] [PubMed]
- 6.Spencer MP, Sarr MG, Nagorney DM. Radical pancreatectomy for pancreatic cancer in the elderly. Is it safe and justified? Ann Surg 1990;212:140-3. [DOI] [PMC free article] [PubMed]
- 7.van Heerden JA, McIlrath DC, Ilstrup DM, et al. Total pancreatectomy for ductal adenocarcinoma of the pancreas: an update. World J Surg 1988;12:658-62. [DOI] [PubMed]
- 8.Warshaw AL, Swanson RS. Pancreatic cancer in 1988. Possibilities and probabilities. Ann Surg 1988;208:541-53. [DOI] [PMC free article] [PubMed]
- 9.Moossa AR. Surgical treatment of chronic pancreatitis: an overview. Br J Surg 1987;74:661-7. [DOI] [PubMed]
- 10.Rossi RL, Rothschild J, Braasch JW, et al. ReMine SG. Pancreatoduodenectomy in the management of chronic pancreatitis. Arch Surg 1987;122:416-20. [DOI] [PubMed]
- 11.Prinz RA, Greenlee HB. Pancreatic duct drainage in 100 patients with chronic pancreatitis. Ann Surg 1981;194:313-20. [DOI] [PMC free article] [PubMed]
- 12.Yeo CJ, Cameron JL, Sohn TA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg 1997;226:248-57. [DOI] [PMC free article] [PubMed]
- 13.McGuire GE, Pitt HA, Lillemoe KD, et al. Reoperative surgery for periampullary adenocarcinoma. Arch Surg 1991;126:1205-10. [DOI] [PubMed]
- 14.Schirmer WJ, Rossi RL, Braasch JW. Common difficulties and complications in pancreatic surgery. Surg Clin North Am 1991;71:1391-417. [DOI] [PubMed]
- 15.Crist DW, Sitzmann JV, Cameron JL. Improved hospital morbidity, mortality, and survival after the Whipple procedure. Ann Surg 1987;206:358-65. [DOI] [PMC free article] [PubMed]
- 16.Grace PA, Pitt HA, Tompkins RK, et al. Decreased morbidity and mortality after pancreatoduodenectomy. Am J Surg 1986; 151:141-9. [DOI] [PubMed]
- 17.Miedema BW, Sarr MG, van Heerden JA, et al. Complications following pancreaticoduodenectomy. Current management. Arch Surg 1992;127:945-9. [DOI] [PubMed]
- 18.Trede M, Schwall G. The complications of pancreatectomy. Ann Surg 1988;207:39-47. [DOI] [PMC free article] [PubMed]
- 19.Trede M. The surgical treatment of pancreatic carcinoma. Surgery 1985;97:28-35. [PubMed]
- 20.Madiba TE, Thomson SR. Restoration of continuity following pancreaticoduodenectomy. Br J Surg 1995;82:158-65. [DOI] [PubMed]
- 21.Yeo CJ, Cameron JL, Maher MM, et al. A prospective randomized trial of pancreaticogastrostomy versus pancreaticojejunostomy after pancreaticoduodenectomy. Ann Surg 1995;222:580-8. [DOI] [PMC free article] [PubMed]
- 22.Mason GR, Freeark RJ. Current experience with pancreatogastrostomy. Am J Surg 1995;169:217-9. [DOI] [PubMed]
- 23.Hiraoka T, Kanemitsu K, Tsuji T, et al. A method for safe pancreaticojejunostomy. Am J Surg 1993;165:270-2. [DOI] [PubMed]
- 24.Matsumoto Y, Fujii H, Miura K, et al. Successful pancreatojejunal anastomosis for pancreatoduodenectomy. Surg Gynecol Obstet 1992;175:555-62. [PubMed]
- 25.Gall FP, Gebhardt C, Meister R, et al. Severe chronic cephalic pancreatitis: use of partial duodenopancreatectomy with occlusion of the pancreatic duct in 289 patients. World J Surg 1989;13:809-16. [DOI] [PubMed]
- 26.Kingsnorth AN. Duct to mucosa isolated Roux loop pancreaticojejunostomy as an improved anastomosis after resection of the pancreas. Surg Gynecol Obstet 1989;169:451-3. [PubMed]
- 27.Di Carlo V, Chiesa R, Pontiroli AE, et al. Pancreatoduodenectomy with occlusion of the residual stump by Neoprene injection. World J Surg 1989;13:105-10. [DOI] [PubMed]
- 28.Kapur BM. Pancreaticogastrostomy in pancreaticoduodenal resection for ampullary carcinoma: experience in thirty-one cases. Surgery 1986;100:489-93. [PubMed]
- 29.Goldsmith HS, Ghosh BC, Huvos AG. Ligation versus implantation of the pancreatic duct after pancreaticoduodenectomy. Surg Gynecol Obstet 1971;132:87-92. [PubMed]
- 30.Klempa I, Schwedes U, Usadel KH. Prevention of postoperative pancreatic complications following duodenopancreatectomy using somatostatin. Chirurg 1979;50:427-31. [PubMed]
- 31.Kohler E, Beglinger C, Dettwiler S, et al. Effect of a new somatostatin analogue on pancreatic function in healthy volunteers. Pancreas 1986;1:154-9. [DOI] [PubMed]
- 32.Bauer W, Briner U, Doepfner W, et al. Pless. SMS 201-995: a very potent and selective octapeptide analogue of somatostatin with prolonged action. Life Sci 1982;31:1133-40. [DOI] [PubMed]
- 33.Amirata E, Livingston DH, Elcavage J. Octreotide acetate decreases pancreatic complications after pancreatic trauma. Am J Surg 1994;168:345-7. [DOI] [PubMed]
- 34.Bassi C, Falconi M, Lombardi D, et al. Prophylaxis of complications after pancreatic surgery: results of a multicenter trial in Italy. Digestion 1994;55:41-7. [DOI] [PubMed]
- 35.Buchler M, Friess H, Klempa I, et al. Role of octreotide in the prevention of postoperative complications following pancreatic resection. Am J Surg 1992;163:125-30. [DOI] [PubMed]
- 36.Buchler M, Friess H. Prevention of postoperative complications following pancreatic surgery. Digestion 1993;54:41-6. [DOI] [PubMed]
- 37.Friess H, Beger HG, Sulkowski U, et al. Randomized controlled multicentre study of the prevention of complications by octreotide in patients undergoing surgery for chronic pancreatitis. Br J Surg 1995;82:1270-3. [DOI] [PubMed]
- 38.Friess H, Buchler MW. Efficacy of somatostatin and its analogues in pancreatic surgery and pancreatic disorders. Digestion 1996;57:97-102. [DOI] [PubMed]
- 39.Gouillat C, Chipponi J, Baulieux J, et al. Randomized controlled multicentre trial of somatostatin infusion after pancreaticoduodenectomy. Br J Surg 2001;88:1456-62. [DOI] [PubMed]
- 40.Lange JR, Steinberg SM, Doherty GM, et al. A randomized, prospective trial of postoperative somatostatin analogue in patients with neuroendocrine tumors of the pancreas. Surgery 1992;112:1033-7. [PubMed]
- 41.Lowy AM, Lee JE, Pisters PW, et al. Prospective, randomized trial of octreotide to prevent pancreatic fistula after pancreaticoduodenectomy for malignant disease. Ann Surg 1997;226:632-41. [DOI] [PMC free article] [PubMed]
- 42.Montorsi M, Zago M, Mosca F, et al. Efficacy of octreotide in the prevention of pancreatic fistula after elective pancreatic resections: a prospective, controlled, randomized clinical trial. Surgery 1995;117: 26-31. [DOI] [PubMed]
- 43.Pederzoli P, Bassi C, Falconi M, et al. Efficacy of octreotide in the prevention of complications of elective pancreatic surgery. Br J Surg 1994;81:265-9. [DOI] [PubMed]
- 44.Sancho JJ, di Costanzo J, Nubiola P, et al. Randomized double-blind placebo-controlled trial of early octreotide in patients with postoperative enterocutaneous fistula. Br J Surg 1995;82:638-41. [DOI] [PubMed]
- 45.Yeo CJ, Cameron JL, Lillemoe KD, et al. Does prophylactic octreotide decrease the rates of pancreatic fistula and other complications after pancreaticoduodenectomy? Results of a prospective randomized placebo-controlled trial. Ann Surg 2000;232:419-29. [DOI] [PMC free article] [PubMed]
- 46.Fiess H, Klempa I, Hermanek P, et al. Prophylaxis of complications after pancreatic surgery. Digestion 1994;55:35-40. [DOI] [PubMed]
- 47.Gouillat C, Gigot JF. Pancreatic surgical complications, the case for prophylaxis. Gut 2001;49 Suppl 4:iv32-9. [DOI] [PMC free article] [PubMed]
- 48.Li-Ling J, Irving M. Somatostatin and octreotide in the prevention of postoperative pancreatic complications and the treatment of enterocutaneous pancreatic fistulas: a systematic review of randomized controlled trials. Br J Surg 2001;88:190-9. [DOI] [PubMed]
- 49.Poon RT, Lo SH, Fong D, et al. Prevention of pancreatic anastomotic leakage after pancreaticoduodenectomy. Am J Surg 2002;183:42-52. [DOI] [PubMed]
- 50.Stojadinovic A, Brooks A, Hoos A, et al. An evidence-based approach to the surgical management of resectable pancreatic adenocarcinoma. J Am Coll Surg 2003;196:954-64. [DOI] [PubMed]
- 51.Robinson KA, Dickersin K. Development of a highly sensitive search strategy for the retrieval of reports of controlled trials using PubMed. Int J Epidemiol 2002;31:150-3. [DOI] [PubMed]
- 52.van Tulder M, Furlan A, Bombardier C, et al. Updated method guidelines for systematic reviews in the cochrane collaboration back review group. Spine 2003;28:1290-9. [DOI] [PubMed]
- 53.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. [DOI] [PubMed]
- 54.Altman DG, Smith GD, Egger M. Systematic reviews in health care: meta-analysis in context. 2nd ed. London: BMJ Publishing; 2001. p. 285-312.
- 55.Lerut J, Gianello P, Reynaert M, et al. Postoperative pancreatic fistulas: clinical study of a series of 114 consecutive cephalic duodeno-pancreatectomies. Acta Chir Belg 1985;85:205-10. [PubMed]


