Abstract
Background
Thoracic trauma is commonly treated with tube thoracostomy. The overall complication rate associated with this procedure is up to 30% among all operators. The primary purpose of this study was to define the incidence and risk factors for complications in chest tubes placed exclusively by resident physicians. The secondary objective was to outline the rate of complications occult to postinsertional supine anteroposterior (AP) chest radiographs (CXRs).
Methods
Over a 12-month period at a regional trauma centre, we retrospectively reviewed all severely injured trauma patients (injury severity score ≥ 12) who underwent tube thoracostomy (338/761 patients). Insertional, positional and infective complications were identified. Patients were assessed for complications on the basis of resident operator characteristics, patient demographics, associated injuries and outcomes. Thoracoabdominal CT scans and corresponding CXRs were also used to determine the rate of complications occult to postinsertional supine AP CXR.
Results
Of the patients, 338 (44%,) had CXR and CT imaging. Out of 76 (22%) chest tubes placed by residents in 61 (18%) patients (99% of whom had blunt trauma injuries), there were 17 complications; 6 (35%) were insertional; 9 (53%) were positional and 2 (12%) were infective. Tube placement outside the trauma bay (p = 0.04) and nonsurgical resident operators (p = 0.03) were independently predictive of complications. The rates of complications according to training discipline were as follows: 7% general surgery, 13% internal and family medicine, 25% other surgical disciplines and 40% emergency medicine. Resident seniority, time of day and other factors were not predictive. Six of 11 (55%) positional and intraparenchymal lung tube placements were occult to postinsertional supine AP CXR.
Conclusions
Chest tubes placed by resident physicians are commonly associated with complications that are not identified by postinsertional AP CXR. Thoracic CT is the only way to reliably identify this morbidity. The differential rate of complications according to resident specialty suggests that residents in non–general surgical training programs may benefit from more structured instruction and closer supervision in tube thoracostomy.
Abstract
Contexte
On traite communément les traumatismes au thorax par thoracostomie à drain. Le taux global de complications associées à cette intervention peut atteindre 30 % pour l'ensemble des chirurgiens. Cette étude visait principalement à définir l'incidence et les facteurs de risque de complications dans le cas des drains thoraciques mis en place exclusivement par des médecins résidents. Le deuxième objectif consistait à décrire le taux de complications occultes à une radiographie pulmonaire AP en supination après l'insertion.
Méthodes
Au cours d'une période de 12 mois, nous avons étudié rétrospectivement, dans un centre régional de traumatologie, tous les patients gravement traumatisés (score de gravité du traumatisme ≥ 12) ayant subi une thoracotomie à drain (338/761 patients). On a défini les complications liées à la mise en place, à la position et à l'infection. On a évalué les complications chez les patients en fonction des caractéristiques du chirurgien résident, des caractéristiques démographiques des patients, des traumatismes connexes et des résultats. On a aussi utilisé des tomodensitométries thoracoabdominales et des radiographies pulmonaires correspondantes pour déterminer le taux de complications occultes aux radiographies pulmonaires AP en supination après l'insertion.
Résultats
Parmi les patients, 338 (44 %) ont subi une radiographie pulmonaire et une tomodensitométrie. Des 76 (22 %) drains thoraciques mis en place par des résidents chez 61 (18 %) des patients (dont 99 % avaient été victimes d'un traumatisme contondant), il y a eu 17 complications dont 6 (35 %) étaient liées à l'insertion; 9 (53 %) à la position et 2 (12 %) à une infection. L'insertion du drain en dehors de l'unité de traumatologie (p = 0,04) et des médecins résidents non chirurgiens (p = 0,03) constituaient des prédicteurs indépendants de complications. Les taux de complications selon la discipline de formation étaient les suivants : chirurgie générale (7 %), médecine interne et familiale (13 %), autres disciplines de la chirurgie (25 %) et médecine d'urgence (40 %). L'ancienneté des résidents, l'heure du jour et d'autres facteurs n'étaient pas des prédicteurs. Des 11 drains pulmonaires positionnels et intraparenchymateux, 6 (55 %) étaient occultes à la radiographie pulmonaire AP en supination après l'insertion.
Conclusions
On établit couramment un lien entre des drains thoraciques insérés par des médecins résidents et des complications qui ne sont pas décelées par radiographie pulmonaire AP après l'insertion. La TDM pulmonaire est le seul moyen d'identifier fiablement cette morbidité. Le taux différentiel des complications selon la spécialité du résident indique que les résidents des programmes de formation en chirurgie non générale pourraient bénéficier d'une formation plus structurée et d'une supervision plus rapprochée dans le cas de la thoracostomie à drain.
Thoracic injury accounts for 25% of all trauma deaths.1,2 Despite its severity, less than 10% of blunt chest injuries and 15%–30% of penetrating thoracic trauma require thoracotomy.3 Most patients are cared for with simple interventions such as chest tube thoracostomy.4–6
While the ultimate goal of draining the pleural cavity has remained constant, the actual technique of chest tube thoracostomy has changed considerably since its initial description by Hippocrates.7 In 1876, Hewitt was the first to use a completely closed intercostal drainage system,8 but it was not until World War II that tube thoracostomy became common in the treatment of injured patients.9
As a consequence of its clinical utility, chest tube insertion has been classified as a mandatory skill for all physicians involved in the care of injured patients, including general surgeons, intensivists and emergency medicine specialists.10–12 Unfortunately, this potentially life-saving procedure also continues to be a significant source of preventable morbidity. In general, chest tube complications are categorized as insertional, positional or infective.13 More specifically, pain, vascular injury, improper positioning of the tube, inadvertent tube removal, postremoval complications, longer hospital stays, empyema and pneumonia have been reported in up to 30% of cases.14–20 This is not surprising because major vascular and visceral structures typically reside in close proximity to chest tubes and their varied insertion sites.21
Although polytrauma, mechanical ventilation, hypotension, intensive care admission, prehospital placement, blunt chest injury and inappropriate training have each been identified as risk factors for chest tube–related morbidity, there are no available data specific to the insertion abilities of resident physicians.22–26 Further, many of these studies use partial or complete insertion with the trocar puncture technique, a method associated with a greater incidence of lung and other thoracic injuries,27–29 compared with the more widely accepted blunt dissection technique.
As a result, our primary goal was to define the complication rate and corresponding risk factors for chest tubes inserted by resident physicians in severely injured patients at our level 1 trauma centre. Our secondary goal was to identify the incidence of chest tube complications occult to the initial postinsertional supine chest radiograph (CXR), as determined by CT imaging.
Material and methods
The overall study population consisted of all adult trauma patients with an injury severity score (ISS) ≥ 12 who presented to our level 1 trauma centre between June 30, 2002, and July 1, 2003, and had a chest tube inserted. Each patient had a complete (chest, abdomen and pelvis) CT scan and corresponding computed radiograph of their post– chest tube insertional supine anteroposterior (AP) CXR. We excluded patients with pre-existing thoracic drainage at the time of arrival to our institution as well as patients with chest tubes placed by attending staff physicians or surgeons. Research ethics board approval was obtained before commencement of the study.
Chest tubes were routinely inserted by a resident physician-in-training between the posterior and anterior axillary lines with a blunt dissection technique using a curved clamp. Most residents (72%) had undergone advanced trauma life support training (ATLS).3 No trocar insertions were performed. Once inserted, the tube was connected to a standard underwater-seal drainage system (Pleur-evac, Teleflex, UK) with low continuous suction. Tube size, method of securing the tube and the precise site of placement were determined by the operator. No prophylactic antibiotics were employed specifically for chest tube thoracostomy. Direct supervision by an attending staff physician or surgeon was not mandatory, but assistance was available on request.
After placement, chest tube position was subsequently evaluated by the treating team with a standard supine AP CXR. All tubes were eventually removed by the trauma team as indicated by the rate of drainage and clinical status of the patient.
Complications were defined as insertional (visceral or parietal injuries of the intercostal artery or intraparenchymal lung), positional (extrathoracic placement or atypical intrathoracic placement resulting in tube failure and replacement) or infectious (wound infection or empyema). We obtained data from retrospective examination of the corresponding dictated CT and CXR reports of board-certified radiologists experienced in trauma imaging as well as from patient charts.
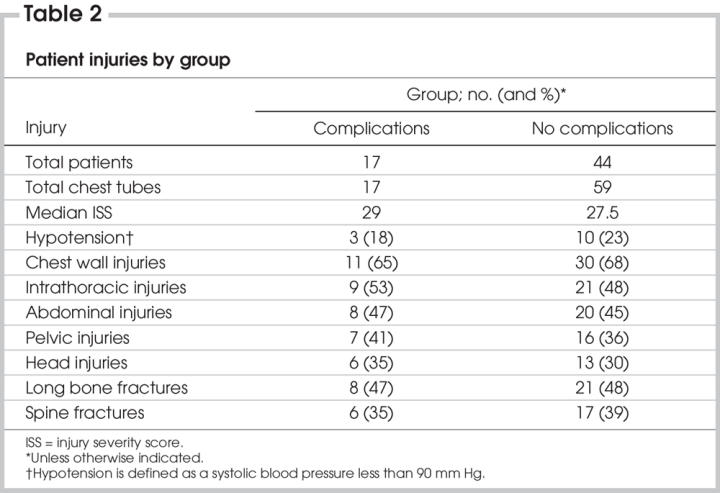
We obtained patient demographics, injuries and outcomes from the trauma and intensive care unit (ICU) registries as well as from patient charts. These data included patient age, sex, ISS, hemodynamic stability at presentation (heart rate, systolic blood pressure, respiratory rate and Glasgow Coma Scale score), discharge status (alive or dead), type of trauma (blunt or penetrating), type of injury (Box 1) and pre-existing pulmonary comorbidities. Site of chest tube placement (midaxillary or midclavicular), seniority of the operator (junior resident, PGY 1–3; senior resident, PGY 4–5; ICU postgraduate fellow), time of day (6 am–6 pm or 6 pm–6 am), location of placement (trauma bay, ICU or operating room), size of chest tube and specialty of the resident operator (general surgery, other surgical residents, emergency medicine, ICU or other residents rotating in the ICU at the time of placement) were also identified. Outcomes of interest included chest tube–related complications, length of hospital stay, length of ICU stay, length of endotracheal intubation and pulmonary complications in ventilated patients such as ventilator-associated pneumonia or acute respiratory distress syndrome (ARDS).
Box 1.
We used Stata Version 8.0 (StataCorp, College Station, Tex.) to perform the analysis. Normally or near-normally distributed variables were reported as means and standard deviations (SDs) and nonnormally distributed variables as medians with interquartile ranges. We used Student's t test to compare means and the Mann–Whitney U test to compare medians. Differences in proportions among categorical data were assessed with Fisher's exact test. A p value less than 0.05 was considered to represent statistical significance for all comparisons.
Results
Of 761 trauma patients, 338 (44%) had a paired CT scan and supine AP CXR. Seventy-six chest tubes were placed in 61 patients by resident physicians. Among these 61 (18%) patients, 77% were male (Table 1) and 99% were injured by blunt trauma. Chest tubes were indicated on the basis of pneumothoraces alone in 47 (62%) patients, hemothoraces alone in 6 (8%) patients and both in 23 (30%) patients.
Table 1
There was no difference across study groups in median age, sex, ISS or length of stay, except that patients without chest tubes had a lower median ISS than those with chest tubes (p = 0.04) (Table 1). Injury patterns and hypotension at presentation were also similar across groups (Table 2).
Table 2
Chest tube–related complications were divided into insertional, positional and infective (Table 3). No patient required a thoracotomy to treat any complication. Intercostal artery lacerations were treated with direct suture repair (“figure of eight”). Malpositioned chest tubes were replaced whether they had been inserted into the lung parenchyma (Fig. 1) or other misdirected intrathoracic (Fig. 2 and Fig. 3) or extrathoracic (Fig. 4 and Fig. 5) locations. Replacement was based on clinical or radiologic grounds, or both. No replacement tube led to a complication. Infections were treated with intravenous or oral antibiotics, or both.
Table 3
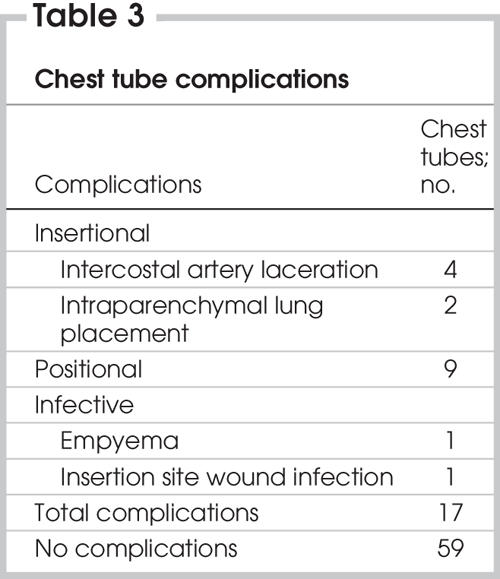
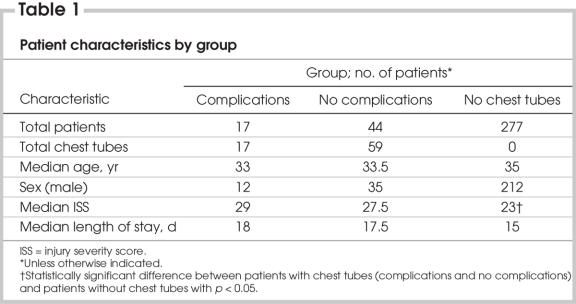
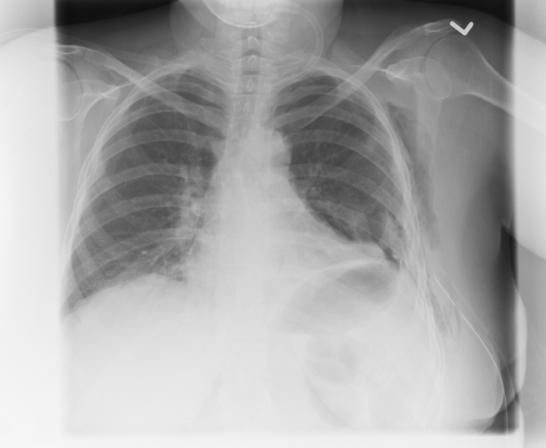
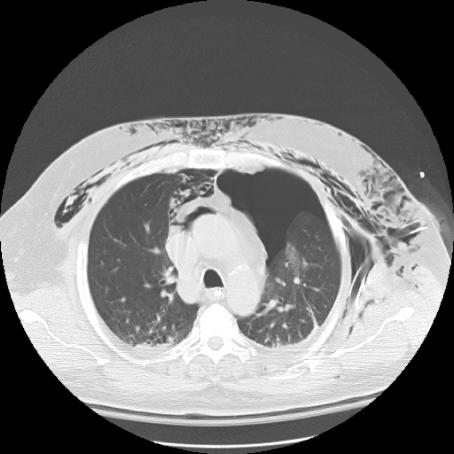
FIG. 1. Left: Computed radiograph of chest (anteroposterior supine) shows chest tube after insertion. Right: Axial CT scan shows a chest tube placed into the lung parenchyma and its associated hemorrhage.
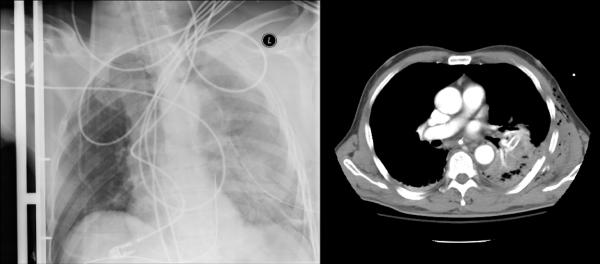
FIG. 2. Left: Computed radiograph of chest (anteroposterior supine) shows a tube malposition. Right: Axial CT scan shows a chest tube abutting the right atrium along the posterior sternum.
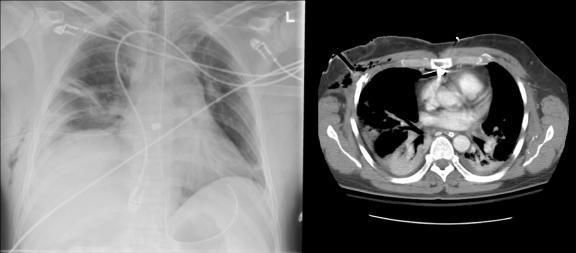
FIG. 3. Computed radiograph of chest (supine anteroposterior) shows a kinked and malpositioned chest tube.
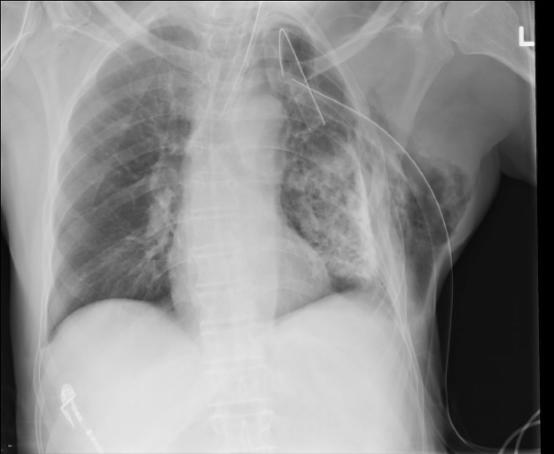
FIG. 4. Computed radiograph of chest (supine antero-posterior) shows an extrathoracic chest tube malposition.
FIG. 5. Axial CT scan showing an extrathoracic chest tube malposition.
Significant associations between chest tube thoracostomy complications and resident specialty were observed (Table 4). Surgical residents were significantly less likely to have a complication than nonsurgical residents (risk ratio 0.4, 95% confidence interval [CI] 0.16–0.96). There was also a trend toward emergency medicine residents having more insertional complications when compared with all other residents (p = 0.057). Overall, their complication rate was 40% of all tubes placed, compared with 7.1% among general surgery residents, 25% among other surgical residents and 12.5% among family medicine and internal medicine residents during their ICU rotations. Emergency medicine residents were more than twice as likely to have a complication (risk ratio 2.49, 95% CI 1.11–5.56), compared with all other residents. Further, there was no difference between the emergency medicine residents trained over 5 years (FRCP) and those with 1 year of training. The complication rate among ICU postgraduate fellows was 50% (n = 4).
Table 4
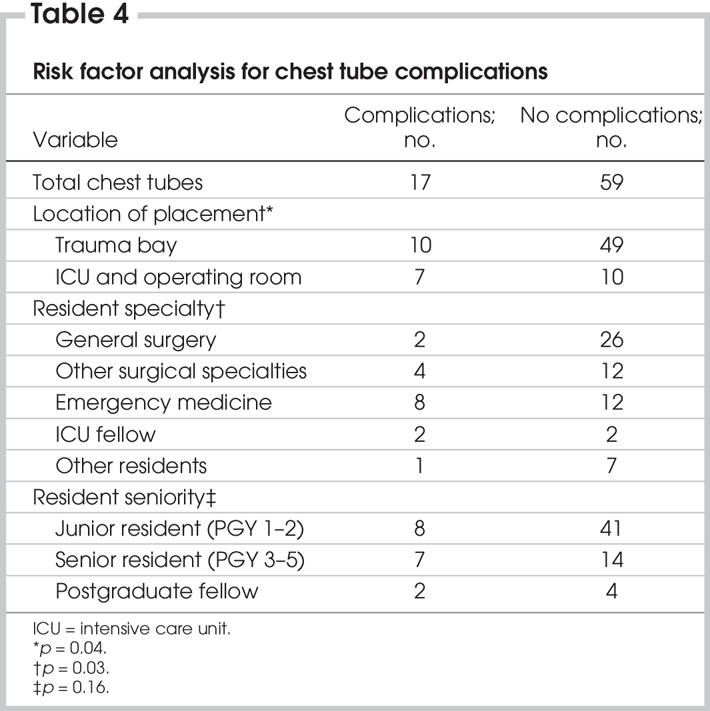
Location of chest tube placement was also independently predictive of complications (Table 4). When tube thoracostomy was performed in the trauma bay, there were fewer complications than in other locations (ICU and operating room; p = 0.04).
There were no associations between chest tube complications and age, sex, ISS, seniority of resident operator, ATLS training status, time of day, tube size, tube position (posterior v. anterior axillary lines), mechanism of injury, intubation status at the time of insertion, injuries, pre-existing pulmonary comorbidities, hypotension or Glasgow Coma Scale status. Further, length of hospital stay, length of ICU stay, length of intubation, discharge status, ventilator-associated pneumonia and ARDS were not affected by chest tube complications.
Complications caused as a result of chest tube insertions were frequently occult to postinsertional computed radiographs. Of the 11 chest tube insertions with complications observed on postinsertional thoracic CT (malpositions and lung intraparenchymal placements), only 5 (45%) were evident on the preceding postinsertional supine AP CXR. Both lung intraparenchymal tube placements were occult to plain radiographs (Fig. 1).
Discussion
Chest tube thoracostomy is often lifesaving in the treatment of severely injured patients. It serves to monitor thoracic blood loss, evacuate blood in the pleural cavity, prevent tension pneumothoraces and increase lung re-expansion, thereby tamponading low-pressure pulmonary vessels and improving respiratory compromise.30–32
Unfortunately, this procedure is also associated with significant morbidity and occasional mortality.22 Published complications in adult patients include lacerations of the lung, intercostal artery, esophagus, stomach, liver, spleen, diaphragm, pulmonary artery and atrium as well as right ventricular compression.27,33–36 In our study, the overall complication rate was 28% per patient, with a corresponding value of 22.4% per procedure. Although each tube was placed exclusively by a resident physician, these values still fit well within the previously published range of up to 30% for all operators.19,20,22,26,30,37–42 Our observed category-specific rates were 7.9% for insertional, 11.8% for positional and 2.6% for infective complications (Table 3). Although the precise definitions of similar complications vary widely across the literature, our rates for positional and infective complications are within the commonly quoted ranges of 1.2%–15.0%19,20,22,25,30 and 1.1%–4.0%,19,20,22,23,30–32,37,41,42 respectively. In our resident cohort, however, nearly 8% (6/76) of all chest tube insertions had postinsertional complications. Most studies approximate 0%–3% in a mixture of resident and staff physician operators.13,19,20,22,23,30,43 Our findings are of particular concern because each of these studies employed the trocar insertion technique, at least in part. This method is known to have a much higher incidence of complications than the blunt dissection technique used exclusively by our resident operators.27–29 Our insertional complications comprised 4 intercostal artery lacerations and 2 intraparenchymal lung placements. Although none of these complications resulted in a thoracotomy or statistical worsening of the clinical outcome measures, individual patients were not completely free of morbidity. In one patient with a chest tube inserted into the lung parenchyma, the complication was associated with 10 additional days of mechanical ventilation and 13 days in the ICU.
Although residents at our institution are instructed to perform a finger sweep within the thoracic cavity to ensure the lung is not adherent to the chest wall before tube placement and to place the chest tube on the superior rib margin to avoid injuring the inferior intercostal neurovascular bundle, it is unclear how often these techniques were actually employed. This is currently being addressed as a quality-control issue.
As a secondary objective, we aimed to define the incidence of injuries that were occult to the initial postinsertional supine AP CXR. Although postinsertional x-rays should ideally include both AP and lateral views, this is frequently impractical in the acutely injured trauma patient because of clinical concerns and cervical spine immobilization.43–45 In our study, only 5 of 11 (45%) tube malpositions and intraparenchymal lung placements were evident on the supine AP CXR that preceded the corresponding thoracic CT. Although the superiority of CT imaging over supine AP CXR in detecting thoracostomy complications is not unique to our study,25,26,37,46,47 this observation further supports the rationale for complete (chest, abdomen and pelvis) imaging in all severely injured blunt trauma patients. Chest tube malpositions can have a significant cardiopulmonary impact if drainage of a tension pneumothorax or severe hemothorax is insufficient. Only with CT and, more recently, ultrasound imaging can all chest injuries including pneumothoraces, and the chest tubes often required to treat them, be accurately defined.48–50
Although hypotension, admission to the ICU, mechanical ventilation, polytrauma, blunt trauma and prehospital tube placement have been shown to be univariate risk factors for chest tube complications, only tube insertion by emergency physicians has proven to be a truly independent predictor.19,22–26 Although we evaluated 14 unique factors, including resident level of training, time of day, size of chest tube, mechanism of injury, concurrent injuries and others, only location of insertion and resident specialty were predictive of complications in our study (Table 4). Chest tubes inserted in the trauma bay were less likely to have a complication than those inserted in the ICU or operating room. This observation was surprising because the acute circumstances of placement would have been expected to be higher in the trauma bay. We postulate that there is likely more supervision in the trauma bay.
Residents training in surgical specialties were also significantly less likely to have a complication than other resident operators. This was primarily because the complication rate among emergency medicine residents was 40%. This cohort had a risk ratio of 2.49, and was responsible for 5 of the 6 (83%) most serious complications (intercostal artery laceration and intraparenchymal lung placements). Further, complications were spread across various emergency medicine operators, regardless of whether they had 1 or 5 years of training. Patient ISS or instability and resident seniority did not act as confounding variables. This is particularly concerning because chest tube insertion has been classified as a mandatory skill for all physicians involved in the care of injured patients, including emergency medicine specialists.10–12 Although this observation has been previously reported among non–resident-specific studies,19,22 it was somewhat surprising to our group because emergency medicine residents are commonly believed to have well-developed technical skills based on increased exposure and training. It is not surprising, however, that internal medicine residents had a low complication rate because many of them had rotated through the pulmonary medicine service where they commonly placed chest tubes for medical indications under the direct supervision of the attending pulmonologist. We also postulate that both internal and family medicine residents seek out closer supervision and feedback for procedures outside their “typical” scope of practice. Despite the infrequency of tube insertions, emergency medicine residents are typically assumed to have already acquired this skill and, as such, are less likely to be intimately supervised. It should also be noted that patient chart review easily explained the 50% complication rate of the ICU fellows, in that a limited number of tubes were placed and the cases were difficult and complex.
Clearly, given such a discrepancy in the complication rates between various resident specialties, the manner in which residents are trained must be further examined. Whereas surgical residents are trained in a technical manner on a daily basis, the availability of supervised training in tube thoracostomy while in the emergency department is more opportunistic. It is also dangerous and irresponsible to suggest that chest tube thoracostomy should be reserved solely for those with surgical training. Although we tend to discourage the use of “inappropriate training” to describe this issue,22 we recognize that perhaps better training models do exist for the cohort of residents that need to perform critical interventions infrequently. Some authors recommend that a “given number” of procedures should be observed for minimal competence21; however, most successful instructional formats for teaching and retaining vital invasive techniques include a skill performance component along with didactic teaching.51–53 The most promising example is Custalow and colleagues' study51 outlining retention and improvement in competency and speed of chest tube thoracostomy by emergency medicine residents 6 months after completion of an interactive training course. We have proposed this formal teaching method for all residents, especially those with infrequent exposure to a given skill, as a method of addressing the high insertional complication rate observed in our study. All residents rotating through our emergency department, ICU or trauma service will have access to the animal laboratory where chest tube insertion skills can be practised and updated. Because of the poor fidelity in inanimate models, live models are preferable in the near future. On a long-term basis, our emergency physician group is planning a formal training course based on Custalow's; it will incorporate not only chest tube insertion skills but also central line placement.
While this study is unique in scope, it has several limitations. First, it is retrospective in nature and has a relatively small sample size for subgroup analysis. This was most clearly displayed among the ICU postgraduate fellows. Second, data outlining the level of supervision by an attending staff physician or surgeon during chest tube insertion were not always available. This limited our conclusions regarding the underlying reasons for the observed differences across resident specialty.
In conclusion, chest tube thoracostomy placement outside the trauma bay and by residents without surgical training are each predictors of complications. In our study, these factors accounted for most insertional and serious complications. Resident seniority did not have a significant impact. Interactive training models incorporating both didactic and skill performance components, as well as increased supervision, are required to address these events in an intuitively simple but technically challenging procedure such as tube thoracostomy. Equally important, all severely injured patients undergoing chest tube placement should have a thoracic CT scan to rule out positional and insertional complications.
This manuscript was presented at the Canadian Association of General Surgeons Surgical Forum in Montréal, Quebec, on September 8, 2005.
Contributors: Drs. Ball, Lord, Gmora, Ng and Kirkpatrick designed the study. Drs. Ball and Schiemann acquired the data, which Drs. Ball, Laupland, Mulloy and Kirkpatrick analyzed. Drs. Ball and Kirkpatrick wrote the aricle, and all authors revised it. All authors gave final approval for the article to be published.
Competing interests: None declared.
Accepted for publication Apr. 13, 2006
Correspondence to: Dr. Andrew Kirkpatrick, Foothills Medical Centre, Rm. EG23, 1403 – 29th St. N.W., Calgary AB T2N 2T9; fax 403 944-1277; Andrew.Kirkpatrick@calgaryhealthregion.ca
References
- 1.Shorr RM, Crittenden M, Indeck M, et al. Blunt thoracic trauma: analysis of 515 patients. Ann Surg 1987;206:200-5. [DOI] [PMC free article] [PubMed]
- 2.Livingston DH, Hauser CJ. Trauma to the chest wall and lung. In: Moore EE, Feliciano DV, Mattox KL, editors. Trauma. 5th ed. New York: McGraw-Hill; 2004. p. 507-37.
- 3.American College of Surgeons, Committee on Trauma. Advanced trauma life support course for doctors instructor course manual. Chicago: American College of Surgeons; 2004.
- 4.Blaisdale WF. Pneumothorax and hemothorax. In: Blaisdale WF, Trunkey DD, editors. Cervicothoracic trauma. 3rd ed. New York: Thieme; 1986. p. 150-65.
- 5.Richardson JD, Miller FB. Injury to the lung and pleura. In: Feliciano DV, Moore EE, Mattox KL, editors. Trauma. 3rd ed. Stamford (CT): Appleton & Lange; 1996. p. 387-407.
- 6.Stocchetti N, Pagliarini G, Gennari M, et al. Trauma care in Italy: evidence of in-hospital preventable deaths. J Trauma 1994;36:401-5. [PubMed]
- 7.Hippocrates. Writings. In: Hutchins RM, ed. Great books of the western world. Vol. 10. Chicago: Encyclopedia Brittanica; 1952. p. 142.
- 8.Lilienthal H. Resection of the lung for suppurative infections with a report based on 31 operative cases in which resection was done or intended. Ann Surg 1922;75:257-320. [DOI] [PMC free article] [PubMed]
- 9.Bretts RH, Lees WM. Military thoracic surgery in the forward area. J Thorac Surg 1946;15:44-63. [PubMed]
- 10.Emergency medicine condition/skills list. JACEP 1976;5:599-604. [PubMed]
- 11.Ramoska EA, Sacchetti AD, Warden TM. Credentialing of emergency medicine physicians: support for delineation of privileges in invasive procedures. Am J Emerg Med 1988;6:278-81. [DOI] [PubMed]
- 12.Sanders AB, Criss E, Witzke D. Core content survey of undergraduate education in emergency medicine. Ann Emerg Med 1986;15:6-11. [DOI] [PubMed]
- 13.Collop NA, Kim SK, Sahn SA. Analysis of tube thoracostomy performed by pulmonologists at a teaching hospital. Chest 1997;112:709-13. [DOI] [PubMed]
- 14.Wall SD, Federle MP, Jeffrey RB, et al. CT diagnosis of unsuspected pneumothorax after blunt abdominal trauma. AJR Am J Roentgenol 1983;141:919-21. [DOI] [PubMed]
- 15.Tocino IM, Miller MH, Frederick PR, et al. CT detection of occult pneumothoraces in head trauma. AJR Am J Roentgenol 1984;143:987-90. [DOI] [PubMed]
- 16.Bridges KG, Welch G, Silver M, et al. CT diagnosis of occult pneumothoraces in multiple trauma patients. J Emerg Med 1993;11:179-86. [DOI] [PubMed]
- 17.Collins JC, Levine G, Waxman K. Occult traumatic pneumothorax: immediate tube thoracostomy versus expectant management. Am Surg 1992;58:743-6. [PubMed]
- 18.Enderson BL, Abdalla R, Frame SB, et al. Tube thoracostomy for occult pneumothorax: a prospective randomized study of its use. J Trauma 1993;35:726-30. [PubMed]
- 19.Etoch SW, Bar-Natan MF, Miller FB, et al. Tube thoracostomy: Factors related to complications. Arch Surg 1995;130:521-6. [DOI] [PubMed]
- 20.Bailey RC. Complications of tube thoracostomy in trauma. J Accid Emerg Med 2000;17:111-4. [DOI] [PMC free article] [PubMed]
- 21.Gilbert TB, McGrath BJ, Soberman M. Chest tubes: indications, placement, management and complications. J Intensive Care Med 1993;8:73-86. [DOI] [PubMed]
- 22.Deneuville M. Morbidity of percutaneous tube thoracostomy in trauma patients. Eur J Cardiothorac Surg 2002;22:673-8. [DOI] [PubMed]
- 23.Grover FL, Richardson JD, Fewel JG, et al. Prophylactic antibiotics in the treatment of penetrating chest wounds. A prospective double-blind study. J Thorac Cardiovasc Surg 1977;74:528-36. [PubMed]
- 24.Groskin SA. Selected topics in chest trauma. Radiology 1992;183:605-17. [DOI] [PubMed]
- 25.Mirvis SE, Tobin KD, Kostrubiak I, et al. Thoracic CT in detecting occult disease in critically ill patients. AJR Am J Roentgenol 1987;148:685-9. [DOI] [PubMed]
- 26.Helling TS, Nicholad RG, Einstein CL, et al. Complications following bunt and penetrating injuries in 216 victims of chest trauma requiring tube thoracostomy. J Trauma 1989;29:1367-70. [DOI] [PubMed]
- 27.Meisel S, Ram Z, Priel I, et al. Another complication of tube thoracostomy: perforation of the right atrium. Chest 1990;98:772-3. [DOI] [PubMed]
- 28.Fraser RS. Lung perforation complicating tube thoracostomy: pathologic description of three cases. Hum Pathol 1988;19:518-23. [DOI] [PubMed]
- 29.Jung AL, Minton SD, Roan Y. Pulmonary hemorrhage secondary to chest placement for pneumothorax in neonates. Clin Pediatr (Phila) 1980;19:624-7. [DOI] [PubMed]
- 30.Daly RC, Mucha P, Pairolero PC, et al. The risk of percutaneous chest tube thoracostomy for blunt thoracic trauma. Ann Emerg Med 1985;14:865-70. [DOI] [PubMed]
- 31.Beall AC Jr, Bricker DL, Crawford HW, et al. Considerations in the management of penetrating thoracic trauma. J Trauma 1968;8:408-17. [DOI] [PubMed]
- 32.Griffith GL, Todd EP, McMillin RD, et al. Acute traumatic hemothorax. Ann Thorac Surg 1978;26:204-7. [DOI] [PubMed]
- 33.Kollef MH, Dothager DW. Reversible cardiogenic shock due to chest tube compression of the right ventricle. Chest 1991;99:976-80. [DOI] [PubMed]
- 34.McFadden PM, Jones JW. Tube thoracostomy: anatomical considerations, overview of complications, and a proposed technique to avoid complications. Mil Med 1985;150:681-5. [PubMed]
- 35.Shapira OM, Aldea GS, Kupferschmidt J, et al. Delayed perforation of the esophagus by a closed thoracostomy tube. Chest 1993;104:1897-8. [DOI] [PubMed]
- 36.Singh KJ, Newman MA. Pulmonary artery catheterization-an unusual complication of chest drain insertion. Aust N Z J Surg 1994;64:513-4. [DOI] [PubMed]
- 37.Millikan JS, Moore EE, Steiner E, et al. Complications of tube thoracostomy for acute trauma. Am J Surg 1980;140:738-41. [DOI] [PubMed]
- 38.Martino K, Merrit S, Boyakye K, et al. Prospective randomized trial of thoracostomy removal algorithms. J Trauma 1999;46:369-71. [DOI] [PubMed]
- 39.Schermer CR, Metteson BD, Demarest GB III, et al. A prospective evaluation of video-assisted surgery for persistent air leak due to trauma. Am J Surg 1999;177:480-4. [DOI] [PubMed]
- 40.Hirshberg A, Thompson SR, Bade PG, et al. Pitfalls in the management of penetrating chest trauma. Am J Surg 1989;157:372-6. [DOI] [PubMed]
- 41.Chan L, Reilly K, Henderson C, et al. Complication rates of tube thoracostomy. Am J Emerg Med 1997;15:368-70. [DOI] [PubMed]
- 42.Mucha p Jr, Farnell MB, Czech JM, et al. A rural regional trauma center. J Trauma 1983;23:337-40. [DOI] [PubMed]
- 43.Maurer JR, Friedman PJ, Wing VW. Thoracostomy tube in an interlobar fissure: radiologic recognition of a potential problem. AJR Am J Roentgenol 1982;139:1155-61. [DOI] [PubMed]
- 44.Conces DJ Jr, Tarver RD, Gray WC, et al. Treatment of pneumothoraces utilizing small caliber chest tubes. Chest 1988;94:55-7. [DOI] [PubMed]
- 45.Webb WR, LaBerge JM. Radiographic recognition of chest tube malposition in the major fissure. Chest 1984;85:81-3. [DOI] [PubMed]
- 46.Stark DD, Federle MP, Goodman P. CT and radiographic assessment of tube thoracostomy. AJR Am J Roentgenol 1983;141:253-8. [DOI] [PubMed]
- 47.Baldt MM, Bankier AA, Germann PS, et al. Complications after emergency tube thoracostomy: assessment with CT. Radiology 1995;195:539-43. [DOI] [PubMed]
- 48.Ball CG, Kirkpatrick AW, Laupland KB, et al. Incidence, risk factors and outcomes for occult pneumothoraces in victims of major trauma. J Trauma 2005;59:917-24. [DOI] [PubMed]
- 49.Ball CG, Kirkpatrick AW, Laupland KB, et al. Factors related to the failure of radiographic recognition of occult posttraumatic pneumothoraces. Am J Surg 2005;189:541-6. [DOI] [PubMed]
- 50.Kirkpatrick AW, Sirois M, Laupland KB, et al. Hand-held sonography for detecting post-traumatic pneumothoraces: the extended focused assessment with sonography for trauma (EFAST). J Trauma 2004;57:288-95. [DOI] [PubMed]
- 51.Custalow CB, Kline JA, Marx JA, et al. Emergency department resuscitative procedures: animal laboratory training improves procedural competency. Acad Emerg Med 2002;9:575-86. [DOI] [PubMed]
- 52.Martin M, Scalabrini B, Rioux A, et al. Training fourth-year medical students in critical invasive skills improves subsequent patient safety. Am Surg 2003;69:437-40. [PubMed]
- 53.Martin M, Vashisht B, Frezza E, et al. Competency-based instruction in critical invasive skill improves both resident performance and patient safety. Surgery 1998;124:313-7. [PubMed]