Abstract
Accelerated telomere shortening in lymphocytes has been associated with a variety of human pathologies, including HIV disease, Down syndrome, and cardiovascular disease. Recent findings indicate that reduced telomere length is also associated with chronic psychological stress and mood disorders. Telomerase, which prevents telomere shortening, can be upregulated in T lymphocytes in concert with activation, thereby retarding telomere shortening. Here, we demonstrate that exposure of human T lymphocytes to cortisol is associated with a significant reduction in telomerase activity both during primary stimulation of resting cells and secondary stimulation of previously activated cells. The effect is observed in both CD4 and CD8 T lymphocytes, and is associated with reduced transcription of hTERT, the telomerase catalytic component. These findings provide a potential mechanism for stress-associated telomere length attrition, and suggest that strategies to enhance T lymphocyte telomerase activity may provide beneficial effects on immune function in situations of chronic emotional stress.
Keywords: T lymphocytes, telomerase, telomeres, human, stress, cortisol
Introduction
Telomerase is a cellular ribonucleoprotein enzyme that is intimately involved in proliferation and senescence of human cells. The reverse transcriptase activity of telomerase functions to stabilize telomere length by adding hexomeric repeats to telomeric ends of linear chromosomes, thereby counteracting the end replication problem. Most normal somatic cells lack telomerase activity and lose approximately 50-100 base pairs (bp) of telomere sequence per cell division (Harley, Futcher, & Greider, 1990). However, human T lymphocytes are able to upregulate telomerase in concert with activation (Weng et al., 1997), thereby retarding telomere loss. Telomere length maintenance in antigen-specific human T lymphocytes has been demonstrated both in vivo and in vitro during periods of high telomerase activity (Valenzuela & Effros, 2002; Akbar & Vukmanovic-Stejic, 2007). Nonetheless, chronic and prolonged activation results in the loss of telomerase activity and telomere loss, ultimately leading to replicative senescence in human memory T lymphocytes (Akbar et al., 2007; Effros, et al., 2005).
Accelerated telomere shortening in peripheral blood lymphocytes has been documented in a variety of diseases, including Down syndrome, rheumatoid arthritis, and cardiovascular disease (Effros et al., 2005). Moreover, specific lymphocyte subpopulations have also been shown to undergo accelerated telomere shortening in Alzheimer's disease and chronic HIV-1 infection (Effros et al., 2005). Recently, reduced telomerase activity and telomere shortening in peripheral blood leukocytes were reported in mothers undergoing chronic emotional stress (Epel et al., 2004), and in caregivers of patients with Alzheimer's disease (Damjanovic et al., 2007). Mood disorders, which show abnormalities in stress-related biological systems, are also correlated with accelerated telomere shortening (Simon et al., 2006). Based on the well-characterized links between emotional stress and elevated cortisol levels (Yang & Glaser, 2000), we sought to determine whether cortisol might affect telomerase activity in immune cells. Our results demonstrate that activation of T lymphocytes in conjunction with exposure to hydrocortisone (at concentrations comparable to plasma cortisol levels that can be reached in vivo during stress) causes a significant reduction in telomerase activity. The effect is seen in both the CD4 and CD8 T lymphocyte subsets, and is associated with reduced levels of the transcript for hTERT, the telomerase catalytic component.
Methods
Blood samples
This study was approved by the UCLA Institutional Review Board. Blood donors (male and female, age 25-55, all in good health by self-report) were recruited according to UCLA Human Subjects guidelines and provided informed consent.
Cell cultures
Peripheral blood mononuclear cells (PBMC) were plated in AIM V serum-free medium (Gibco) and stimulated with phytohemagglutinin (PHA, 10ug/ml) in the presence of DMSO (diluent) or cortisol (hydrocortisone, Sigma). Cultures were incubated for 3 -4 days, at which time the concentration of viable blast-like cells was determined by microscopic evaluation (often validated by a second person), including trypan blue exclusion. In some experiments, immediately prior to lysing cells for the telomerase activity assay, T cells subsets were isolated, using magnetic beads (Milteny). For long-term cultures, cells were plated in RPMI/10% FCS and stimulated with anti-CD2/CD3/CD28 coated beads. Hydrocortisone or DMSO was added on day 0, or at the time of secondary stimulation.
Telomeric repeat amplification protocol (TRAP) assay
Cellular telomerase activity was measured, using the TRAPeze kit (Chemicon), as previously described (Valenzuela et al., 2002). Each cell lysate tested in the assay contained the equivalent of 10,000 blast cells, and each sample was run at least twice in the TRAP assay. Results of the TRAP assay are expressed as “Total Product Generated”, TPG, which reflects the telomerase activity in the sample, and is calculated according to a computer program which integrates signal intensity over the telomere length distribution on the gel as a function of molecular weight.
Quantitative real-time reverse transcription polymerase chain reaction (Q-RT-PCR)
RNA was extracted using RNeasy Mini Kit (Qiagen). RNA concentrations were determined using the Quant-iT Ribogreen RNA Assay Kit (Molecular Probes). 2ug of RNA were converted to cDNA with the iScript cDNA synthesis kit (BioRad). Q-RT-PCR was performed by using the iQ SYBR Green SuperMix and IQCycler (Bio-Rad). As an internal control, Q-RT-PCR for housekeeping gene GAPDH was performed. The sequences of the primers for amplification were GAPDH-F-429 (5′-CCT CAA GAT CAT CAG CAA TGC CTC CT-3′) and GAPDH-R-528 (5′-GGT CAT GAG TCC TTC CAC GAT ACC AA -3′). For the hTERT gene expression measurement, we used primers hTERT-F-1627 (5′-AAG TTC CTG CAC TGG CTG ATG AGT -3′), and hTERT-R-1758 (5′-GCT TTG CAA CTT GCT CCA GAC ACT -3′). Samples were run in triplicate in a 96-well plate in the I-CycleriQ Multicolor Real-Time Detection System (BioRad). Each well contained 10ul of cDNA (1:10 dilution of cDNA reaction), 12.5ul of iQ SYBR Green Super Mix, .75 ul of each primer (from 10 uM stock), and 1ul of H20, for a total of 25 ul. The IQcycler program consisted of initial denaturation at 95°C for 3 minutes, followed by 40 PCR cycles at 95°C for 15 seconds, 61°C for 30 seconds, and 72° for 30 seconds (single fluorescence measurement). Standard curves were created for both GAPDH and hTERT. The relative GAPDH and hTERT copy numbers and were calculated according to the standard curve method.
Statistical Analysis
TRAP assays for each sample were repeated at least twice. The TPG values in the presence of cortisol or DMSO (diluent control) were compared, and data are presented as the mean +/- SD of either the TPG value or the comparison between cortisol and control TPG (Relative Telomerase Activity). In comparing the degree of inhibition associated with cortisone exposure, significance was calculated using the Student t test, and p values < 0.05, are considered significant.
Results
Reduced telomerase activity in T cells exposed to hydrocortisone
In cultures established from 8 different donors, addition of hydrocortisone on day 0 was associated with a significant reduction (p=0.002) in the number of blast-like cells by day 3, with no evidence of cell death (data not shown). Despite diminished cell numbers in the cortisone-treated cultures, it was possible that those cells that did become activated would still show normal telomerase activity. Therefore, we compared equal numbers of blast-like cells from cortisol-treated and control cultures in a series of telomerase activity experiments. Figure 1 (top) shows a representative TRAP gel, illustrating the high telomerase activity in 10,000 cells from the control (DMSO diluent) culture, and the reduced telomerase activity in the same number of blast cells from the hydrocortisone-treated culture. To illustrate that telomerase activity is also subject to hormonal enhancement effects, the experiment also included cells that were exposed to estrogen rather than hydrocortisone, confirming our previous study, in which we showed a dose-dependent telomerase enhancement effect of estrogen (Effros et al., 2005). Figure 1 (bottom), shows representative results of 5 hydrocortisone titration experiments on PHA-activated PBMC, illustrating that the Total Product Generated (TPG), a measure of telomerase activity, is inhibited in a dose-dependent manner. Importantly, hydrocortisone in the physiological ranges of cortisol [i.e., 0.1 uM, (Gayrard, Alvinerie, & Toutain, 1996)] had no effect on the telomerase activity. By contrast, higher concentrations of hydrocortisone, comparable to those that might be reached in vivo during stress, reduced telomerase activity by as much as 50%.
Figure 1. Telomerase activity is inhibited by cortisol.

Top, Representative TRAP gel image for control (Con), cortisol (hydrocortisone, Hyd 30uM), Estrogen (E2) and loading buffer ( LB); bottom, PBMC were stimulated with 10ug/mL PHA in the presence of increasing concentrations of hydrocortisone. TRAP assay (day 3) was performed on 10,000 blast cells, with results presented as TPG (Total Product Generated), a measure of telomerase activity.
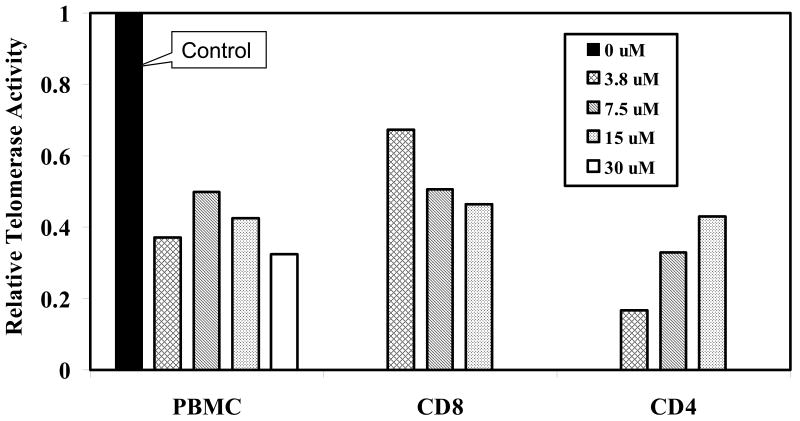
Hydrocortisone inhibits telomerase activity in both CD4 and CD8 T lymphocytes
Figure 2 summarizes results of 8 PBMC cultures, expressed as the relative telomerase activity for each hydrocortisone-treated culture compared to its control culture, which is arbitrarily set at 1.0. Since CD4 and CD8 T lymphocytes show divergent patterns in both telomerase activation in vitro and telomere shortening in vivo (Valenzuela et al., 2002; Akbar et al., 2007), we sought to determine whether subset differences also exist with respect to hydrocortisone effects on telomerase activity. Equal numbers of CD4 and CD8 blast cells, isolated by magnetic bead separation from 3 of the PBMC cultures immediately before the TRAP assay, were evaluated for telomerase activity. The data in Figure 2 indicate that the reduction in telomerase activity seen in activated PBMC exposed to hydrocortisone reflects the hydrocortisone effects on both CD4 and CD8 T cell subsets. Interestingly, for CD8 T cells, higher hydrocortisone concentrations exerted stronger telomerase inhibition, whereas the CD4 subset shows an opposite pattern, an observation that may relate to differences in the density and/or signaling of hormone receptors or the previously documented differences in telomerase regulation between the subsets (Effros et al., 2005).
Figure 2. Cortisol inhibits telomerase activity in both T cell subsets.
Cells were stimulated with PHA together with DMSO (diluent) or hydrocortisone Immediately prior to cell preparation for the TRAP assay, T cells or subsets thereof were isolated (Milteny magnetic beads). Results are expressed as relative telomerase activity for 10,000 blast cells from each cell population as compared to its corresponding DMSO control, which is arbitrarily set at 1.0. For PBMC (n=8), and for the CD8 & CD4 (n=3).
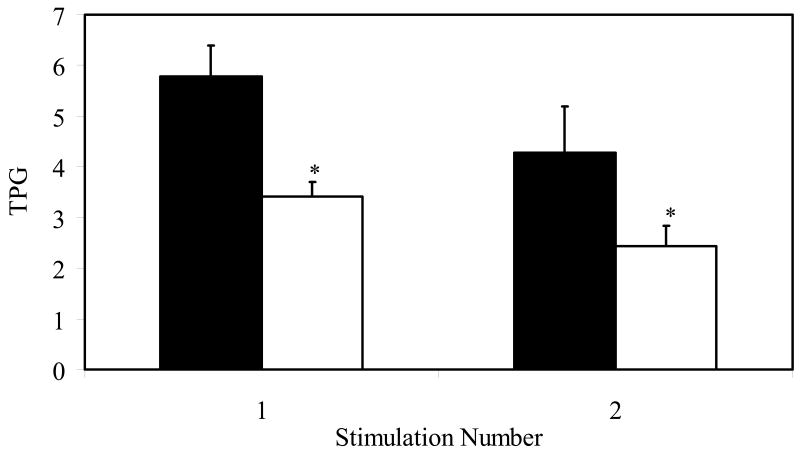
Hydrocortisone inhibits telomerase during both primary and secondary stimulation
Repeated stimulation of T cells in culture leads to progressive reduction in telomerase activity (Valenzuela & Effros, 2002). To determine if hydrocortisone would cause a further reduction in telomerase activity in cells that had been previously activated, PBMC were stimulated with anti-CD2/3/28-coated beads, and when the proliferative burst had subsided, identical cell numbers were restimulated with anti-CD2/3/28-coated beads. The data in Figure 3 show that exposure to hydrocortisone in concert with either primary or secondary TCR-mediated stimulation results in a significant reduction in telomerase activity (p< 0.05).
Figure 3. Inhibition of both primary and secondary stimulation-induced telomerase activity by cortisol.
PBMC (n=3) were stimulated with anti-CD2/CD3/CD28 coated beads (Miltenyi Biotech). Hydrocortisone (open bars) or DMSO (solid bars) was added on day 0 or at the time of secondary stimulation. (* p<0.05).
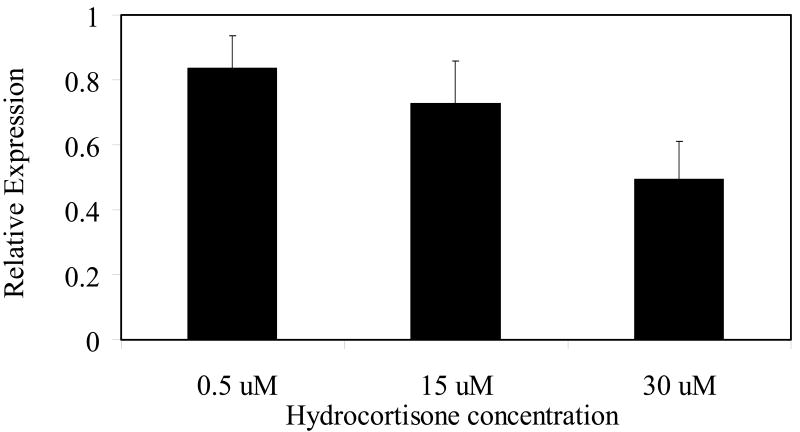
Telomerase inhibition by hydrocortisone is associated with reduced hTERT message
Telomerase activity is undetectable, or extremely low, in resting human T cells (Epel et al., 2004), although, hTERT, the catalytic component of the enzyme, is expressed (Liu et al., 2001; Plunkett et al., 2001). The induction of telomerase activity during T cell activation is associated with a significant increase in hTERT protein expression (Buchkovich & Greider, 1996). Figure 4 shows that exposure to hydrocortisone in concert with activation leads to a significant reduction in hTERT message. Although our data do not distinguish between reduced hTERT transcription versus message stability, our observation provides a possible mechanism for the observed reduced telomerase activity in the hydrocortisone-treated cells.
Figure 4. Hydrocortisone treatment decreases hTERT message in PBMC.
Q-RT-PCR was performed as described in Materials and Methods. Normalized hTERT message was calculated by dividing the hTERT value by the GAPDH value for each sample. Results are represented as the relative amount of normalized hTERT message in hydrocortisone-treated cells versus that in DMSO- treated cells ( the value of which value is arbitrarily set at 1.0). For the 15uM treatment, n=6; for the 0.5uM and 30uM treatment, n=2.
Discussion
The major finding of this study is that exposure to a major stress hormone is associated with the down-regulation of telomerase activity in activated human T lymphocytes. Importantly, the effect of hydrocortisone was observed not only during primary stimulation but also during subsequent stimulation of previously activated T cells. If cortisol exposure in vivo exerts the same effect as we observed in vitro, our data provide a potential mechanism for the reported association between psychological stress and shortened telomeres (Epel et al., 2004; Epel et al., 2006; Damjanovic et al., 2007). Glucocorticoids (GC) have been documented to attenuate T cell receptor signaling and to suppress cellular immunity (Van Laethem et al., 2001). The present study extends this work by demonstrating an important downstream outcome of one of the major GC hormones is the blunting of telomerase activity in human T lymphocytes. Although our study did not examine the long-term outcome of reduced telomerase, we have shown the converse, namely that enhanced telomerase activity retards telomere loss and extends proliferative potential of virus-specific T cells (Dagarag et al., 2004).
Telomere length in T lymphocytes reflects both cell division and telomerase activity. During primary and secondary stimulation of CD8 T lymphocytes, telomerase activity is high, resulting in telomere length stabilization even during extensive cell division. This phenomenon has been demonstrated both in vivo and in vitro. During acute mononucleosis, Epstein-Barr Virus (EBV) virus-specific CD8 T cells have high telomerase activity, and show telomere length maintenance. However, after one year, the virus-specific CD8 T cells have undetectable telomerase and shorter telomeres (Akbar et al., 2007). Similarly, CD8 T cells show high telomerase activity in concert with primary antigenic stimulation in cell culture, but by the third and all subsequent antigenic stimulations, telomerase activity is undetectable, and telomeres have shortened (Effros et al., 2005). Interesting, the in vitro studies on CD4 T cells from the same donor indicate that the pattern of telomerase regulation during the same antigenic stimulation differs from that of the CD8 T cells. The present study suggests that the two subsets may also differ in their responses to different doses of hydrocortisone.
Reduced telomerase activity, be it from repeated antigenic stimulation (Valenzuela & Effros, 2002) or as a down-stream effect of exposure to cortisol, could possibly be contributing to the generation of CD8 T cells with features of replicative senescence. Such cells, which increase during normal aging, are associated with a variety of deleterious effects, including early mortality in the very old (Pawelec et al., 2004) and reduced responses to vaccines (Goronzy et al., 2001). In persons chronically infected with HIV, more rapid progression to clinically-defined AIDS correlates with the abundance of senescent CD8 T cells (Cao et al., 2007).
In the elderly, the clonally expanded CD8 T cells with features of replicative senescence have been proposed to reflect the ongoing work required to maintain control over persistent viruses, such as cytomegalovirus ( CMV) and EBV (Pawelec et al., 2004). Although none of the studies on latent infections in the elderly have addressed the potential role of stress, several reports on younger persons have, in fact, shown an association between reduced control over latent viruses and concomitant increases in stress and/or elevated levels of serum cortisol. For example, in astronauts, reactivation of latent cytomegalovirus (CMV) and Epstein-Barr virus (EBV) are correlated with increased stress hormone levels associated with space flight (Stowe et al., 2001; Mehta et al., 2000). Stress is associated with re-emergence of varicella zoster (shingles) and herpes labialis (Haynes & Swain, 2006). Finally, in HIV disease, progression to AIDS, which is associated with increased proportions of CD8 T cells with features of replicative senescence ( Cao et al., 2007) is associated with elevated serum cortisol (Leserman et al., 2002).
Telomerase activity in immune cells is subject to modulation by a variety of hormones and cytokines. Both estradiol and TNF-alpha function to enhance telomerase activity in normal human T lymphocytes (Effros et al., 2005), whereas Interferon alpha and Transforming growth factor-beta have opposite effects. These findings, as well as reports showing the role of the autonomic nervous system and hypothalamic-pituitary-adrenal axis hormones in the reactivation of latent infections (Cacioppo et al., 2002), underscore the intricate balance of physiological systems involved in host-pathogen interactions.
In sum, our results may help elucidate the complex interactions between the endocrine, nervous and immune systems, which can affect both mental and physical health. The link between mental stress and shortened telomeres has recently been documented in studies on mothers of chronically ill children (Epel et al., 2004) and in caregivers of patients with Alzheimer's disease (Damjanovic et al., 2007). Earlier experiments in humans involving both vaccinations (Kiecolt-Glaser et al., 1996) and responses to infections have shown that immunity is blunted during periods of psychological stress (Yang et al., 2000). Our study provides a possible mechanism for these effects, and also suggests that therapeutic strategies that enhance telomerase activity in virus-specific CD8 T lymphocytes may augment immunity in situations of chronic emotional stress.
Acknowledgments
We thank Drs. Calvin Harley and Graham Pawelec for helpful comments on the manuscript.
Footnotes
This research was supported by the following NIH grants: AG 023720 & AI 060362 (RBE) and AI 52031 (SRF).
Disclosures: The authors have no financial conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akbar AN, Vukmanovic-Stejic M. Telomerase in T lymphocytes: use it and lose it? J Immunol. 2007;178:6689–6694. doi: 10.4049/jimmunol.178.11.6689. [DOI] [PubMed] [Google Scholar]
- Buchkovich KJ, Greider CW. Telomerase regulation during entry into the cell cycle in normal human T cells. Mol Biol Cell. 1996;7:1443–1454. doi: 10.1091/mbc.7.9.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Kiecolt-Glaser JK, Malarkey WB, Laskowski BF, Rozlog LA, Poehlmann KM, et al. Autonomic and glucocorticoid associations with the steady-state expression of latent Epstein-Barr virus. Horm Behav. 2002;42:32–41. doi: 10.1006/hbeh.2002.1801. [DOI] [PubMed] [Google Scholar]
- Cao W. PhD Thesis. 2007. Immunological Factors Associated with HIV-1 Disease Progression to AIDS and Death. [Google Scholar]
- Dagarag MD, Evazyan T, Rao N, Effros RB. Genetic manipulation of telomerase in HIV-specific CD8+ T cells:enhanced anti-viral functions accompany the increased proliferative potential and telomere length stabilization. J Immunol. 2004;173:6303–6311. doi: 10.4049/jimmunol.173.10.6303. [DOI] [PubMed] [Google Scholar]
- Damjanovic AK, Yang Y, Glaser R, Kiecolt-Glaser JK, Nguyen H, Laskowski B, Zou Y, Beversdorf DQ, Weng NP. Accelerated telomere erosion is associated with a declining immune function of caregivers of Alzheimer's disease patients. J Immunol. 2007;179:4249–4254. doi: 10.4049/jimmunol.179.6.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effros RB, Dagarag MD, Spaulding CC, Man J. The role of CD8 T cell replicative senescence in human aging. Immunological Reviews. 2005;205:147–157. doi: 10.1111/j.0105-2896.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101:17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, Lin J, Wilhelm FH, Wolkowitz OM, Cawthon R, Adler NE, et al. Cell aging in relation to stress arousal and cardiovascular disease risk factors. Psychoneuroendocrinology. 2006;31:277–287. doi: 10.1016/j.psyneuen.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Gayrard V, Alvinerie M, Toutain PL. Interspecies variations of corticosteroid-binding globulin parameters. Domest Anim Endocrinol. 1996;13:35–45. doi: 10.1016/0739-7240(95)00042-9. [DOI] [PubMed] [Google Scholar]
- Goronzy JJ, Fulbright JW, Crowson CS, Poland GA, O'Fallon WM, Weyand CM. Value of immunological markers in predicting responsiveness to influenza vaccination in elderly individuals. J Virol. 2001;75:12182–12187. doi: 10.1128/JVI.75.24.12182-12187.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley C, Futcher AB, Greider C. Telomeres shorten during ageing of human fibroblasts. Int Immunol. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- Haynes L, Swain SL. Why aging T cells fail: implications for vaccination. Immunity. 2006;24:663–666. doi: 10.1016/j.immuni.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Glaser R, Gravenstein S, Malarkey WB, Sheridan J. Chronic stress alters the immune response to influenza virus vaccine in older adults. Proc Natl Acad Sci U S A. 1996;93:3043–3047. doi: 10.1073/pnas.93.7.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leserman J, Petitto JM, Gu H, Gaynes BN, Barroso J, Golden RN, et al. Progression to AIDS, a clinical AIDS condition and mortality: psychosocial and physiological predictors. Psychol Med. 2002;32:1059–1073. doi: 10.1017/s0033291702005949. [DOI] [PubMed] [Google Scholar]
- Liu K, Hodes RJ, Weng N. Cutting edge: telomerase activation in human T lymphocytes does not require increase in telomerase reverse transcriptase (hTERT) protein but is associated with hTERT phosphorylation and nuclear translocation. J Immunol. 2001;166:4826–4830. doi: 10.4049/jimmunol.166.8.4826. [DOI] [PubMed] [Google Scholar]
- Mehta SK, Stowe RP, Feiveson AH, Tyring SK, Pierson DL. Reactivation and shedding of cytomegalovirus in astronauts during spaceflight. J Infect Dis. 2000;182:1761–1764. doi: 10.1086/317624. [DOI] [PubMed] [Google Scholar]
- Pawelec G, Akbar A, Caruso C, Effros RB, Grubeck-Loebenstein B, Wikby A. Is immunosenescence infectious? Trends Immunol. 2004;25:406–410. doi: 10.1016/j.it.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Plunkett FJ, Soares MV, Annels N, Hislop A, Ivory K, Lowdell M, et al. The flow cytometric analysis of telomere length in antigen-specific CD8+ T cells during acute Epstein-Barr virus infection. Blood. 2001;97:700–707. doi: 10.1182/blood.v97.3.700. [DOI] [PubMed] [Google Scholar]
- Simon NM, Smoller JW, McNamara KL, Maser RS, Zalta AK, Pollack MH, et al. Telomere shortening and mood disorders: preliminary support for a chronic stress model of accelerated aging. Biol Psychiatry. 2006;60:432–435. doi: 10.1016/j.biopsych.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Stowe RP, Mehta SK, Ferrando AA, Feeback DL, Pierson DL. Immune responses and latent herpesvirus reactivation in spaceflight. Aviat Space Environ Med. 2001;72:884–891. [PubMed] [Google Scholar]
- Valenzuela HF, Effros RB. Divergent telomerase and CD28 expression patterns in human CD4 and CD8 T cells following repeated encounters with the same antigenic stimulus. Clin Immunol. 2002;105:117–125. doi: 10.1006/clim.2002.5271. [DOI] [PubMed] [Google Scholar]
- Van Laethem F, Baus E, Smyth LA, Andris F, Bex F, Urbain J, et al. Glucocorticoids attenuate T cell receptor signaling. J Exp Med. 2001;193:803–814. doi: 10.1084/jem.193.7.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng NP, Palmer LD, Levine BL, Lane HC, June CH, Hodes RJ. Tales of tails: regulation of telomere length and telomerase activity during lymphocyte development, differentiation, activation, and aging. Immunological Reviews. 1997;160:43–54. doi: 10.1111/j.1600-065x.1997.tb01026.x. [DOI] [PubMed] [Google Scholar]
- Yang EV, Glaser R. Stress-induced immunomodulation: impact on immune defenses against infectious disease. Biomed Pharmacother. 2000;54:245–250. doi: 10.1016/S0753-3322(00)80066-9. [DOI] [PubMed] [Google Scholar]