Abstract
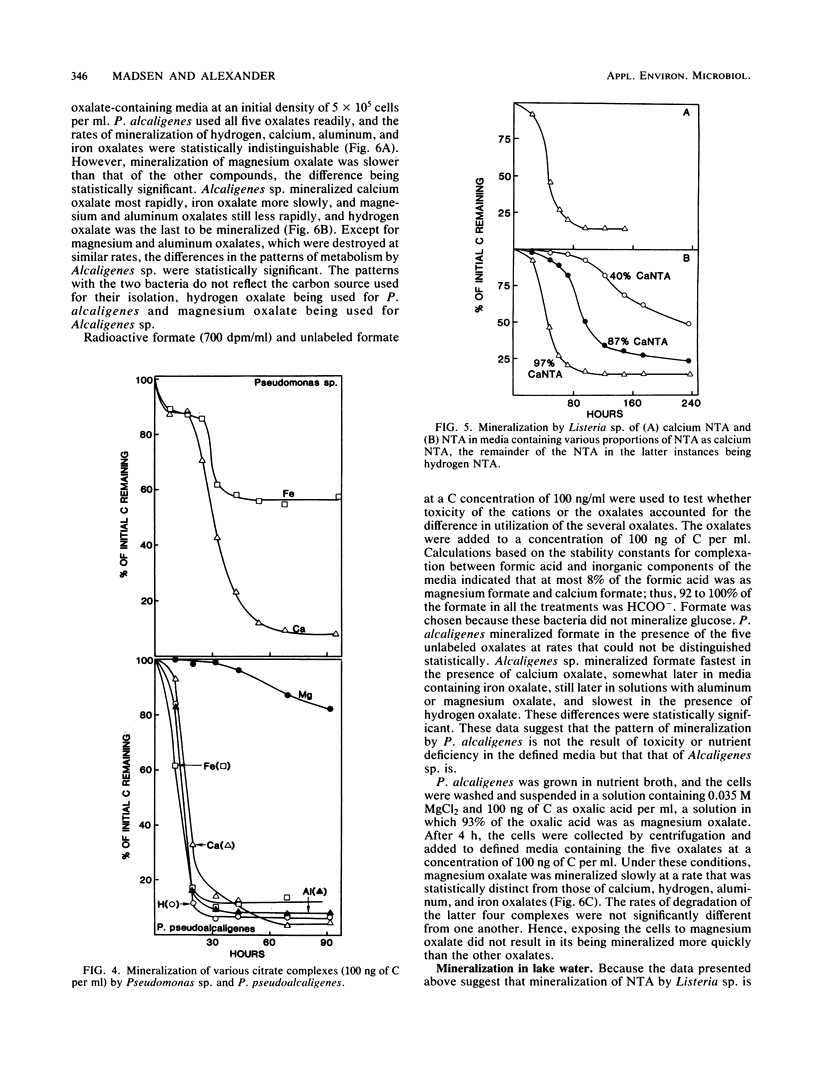
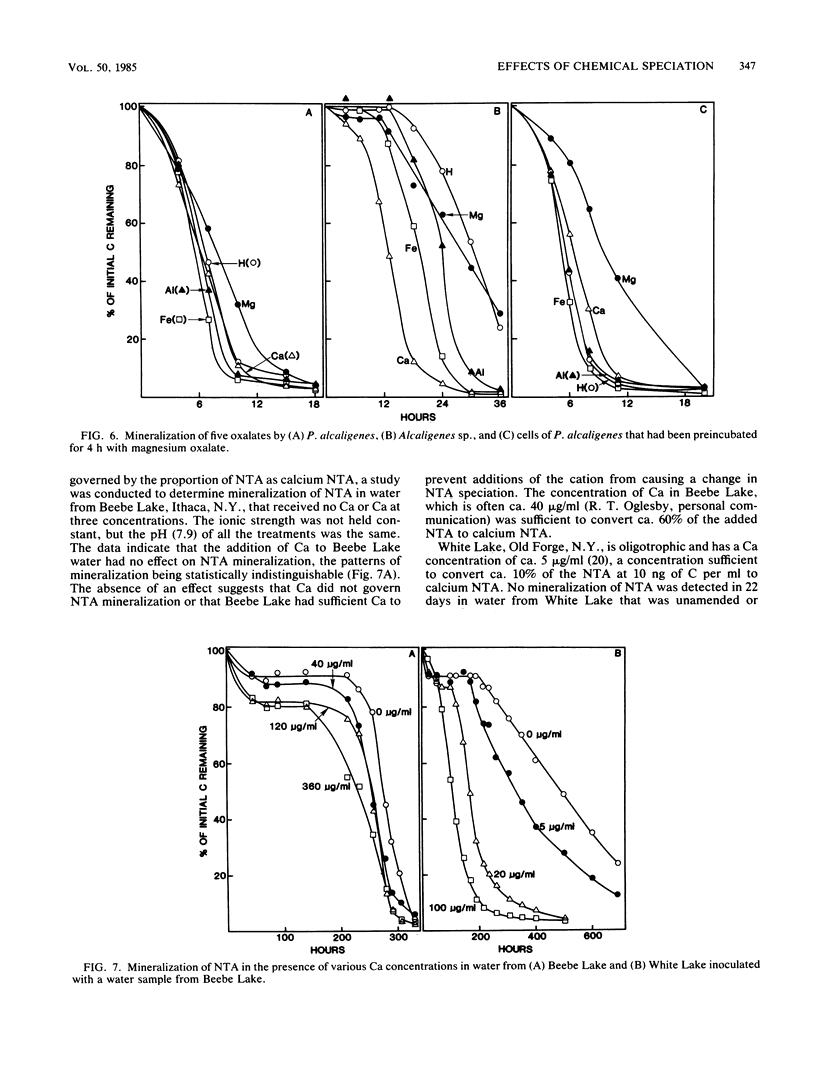
The mineralization of 1.0 to 100 ng each of four complexing compounds—oxalate, citrate, nitrilotriacetate (NTA), and EDTA—per ml was tested in media prepared in accordance with equilibrium calculations by a computer program so that the H, Ca, Mg, Fe, or Al complex (chemical species) was predominant. Sewage microorganisms mineralized calcium citrate more rapidly than iron, aluminum, or hydrogen citrate, and magnesium citrate was degraded slowest. Aluminum, hydrogen, and iron oxalates were mineralized more rapidly than calcium oxalate, and magnesium oxalate was decomposed slowest. Sewage microorganisms mineralized calcium NTA but not aluminum, magnesium, hydrogen, or iron NTA or any of the EDTA complexes. Pseudomonas sp. mineralized calcium and iron citrates but had no activity on hydrogen, aluminum, or magnesium citrate. Pseudomonas pseudoalcaligenes mineralized calcium, iron, hydrogen, and aluminum citrates but had little activity on magnesium citrate. Pseudomonas alcaligenes used calcium, iron, hydrogen, and aluminum oxalates readily, but it used magnesium oxalate at a slower rate. Listeria sp. destroyed calcium NTA but had no effect on hydrogen, iron, or magnesium NTA. Increasing the Ca concentration in the medium enhanced the breakdown of NTA by Listeria sp. The different activities of the bacterial isolates were not a result of the toxicity of the complexes or the lack of availability of a nutrient element. NTA mineralization was not enhanced by the addition of Ca to Beebe Lake water, but it was enhanced when Ca and an NTA-degrading inoculum were added to water from an oligotrophic lake. The data show that chemical speciation influences the mineralization of organic compounds by naturally occurring microbial communities and by individual bacterial populations.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belly R. T., Lauff J. J., Goodhue C. T. Degradation of ethylenediaminetetraacetic acid by mictobial populations from an aerated lagoon. Appl Microbiol. 1975 Jun;29(6):787–794. doi: 10.1128/am.29.6.787-794.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsma J., Konings W. N. The properties of citrate transport in membrane vesicles from Bacillus subtilis. Eur J Biochem. 1983 Jul 15;134(1):151–156. doi: 10.1111/j.1432-1033.1983.tb07545.x. [DOI] [PubMed] [Google Scholar]
- Dijkhuizen L., Groen L., Harder W., Konings W. N. Active transport of oxalate by Pseudomonas oxalaticus OX1. Arch Microbiol. 1977 Nov 18;115(2):223–227. doi: 10.1007/BF00406378. [DOI] [PubMed] [Google Scholar]
- Firestone M. K., Tiedje J. M. Biodegradation of metal-nitrilotriacetate complexes by a Pseudomonas species: mechanism of reaction. Appl Microbiol. 1975 Jun;29(6):758–764. doi: 10.1128/am.29.6.758-764.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder W., Dijkhuizen L. Physiological responses to nutrient limitation. Annu Rev Microbiol. 1983;37:1–23. doi: 10.1146/annurev.mi.37.100183.000245. [DOI] [PubMed] [Google Scholar]
- Subba-Rao R. V., Rubin H. E., Alexander M. Kinetics and extent of mineralization of organic chemicals at trace levels in freshwater and sewage. Appl Environ Microbiol. 1982 May;43(5):1139–1150. doi: 10.1128/aem.43.5.1139-1150.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiedje J. M. Microbial degradation of ethylenediaminetetraacetate in soils and sediments. Appl Microbiol. 1975 Aug;30(2):327–329. doi: 10.1128/am.30.2.327-329.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willecke K., Gries E. M., Oehr P. Coupled transport of citrate and magnesium in Bacillus subtilis. J Biol Chem. 1973 Feb 10;248(3):807–814. [PubMed] [Google Scholar]


