Abstract
Previous work in the endocrine and neuroendocrine fields has viewed androgen receptors (AR) as a transcription factor activated by testosterone or one of its many metabolites. The bound androgen receptor acts as transcription factor and binds to specific DNA response elements in target gene promoters, causing activation or repression of transcription and subsequently protein synthesis. Over the past two decades evidence has begun to accumulate to implicate androgens, dependent or independent of the AR, in rapid actions at the cellular and organism level. Androgen’s rapid time course of action; effects in the absence or inhibition of the cellular machinery necessary for transcription/translation; and/or the effects of androgens not able to translocate to the nucleus suggest a method of androgen action not initially dependent on genomic mechcanisms (i.e. non-genomic in nature). In the present paper the non-genomic effects of androgens are reviewed, along with a discussion of the possible role non-genomic androgen actions have on animal physiology and behavior.
Introduction
Sex steroid hormones, including androgens, mediate biological effects on all manner of cellular mechanisms including proliferation, differentiation, and homeostasis. Historically, the dogma of hormonal regulation of biological functions centered around gene transcription and protein synthesis [1]. This classic genomic model for steroid hormone action presumes that steroid hormones can freely cross the plasma membrane, enter the cytoplasm, and bind to and activate specific intracellular steroid receptor proteins. The bound steroid receptors act as transcription factors and bind as homodimers or heterodimers to specific DNA response elements in target gene promoters, causing activation or repression of transcription and subsequently protein synthesis (Figure 1) [2; 3; 4; 5; 6].
Figure 1.
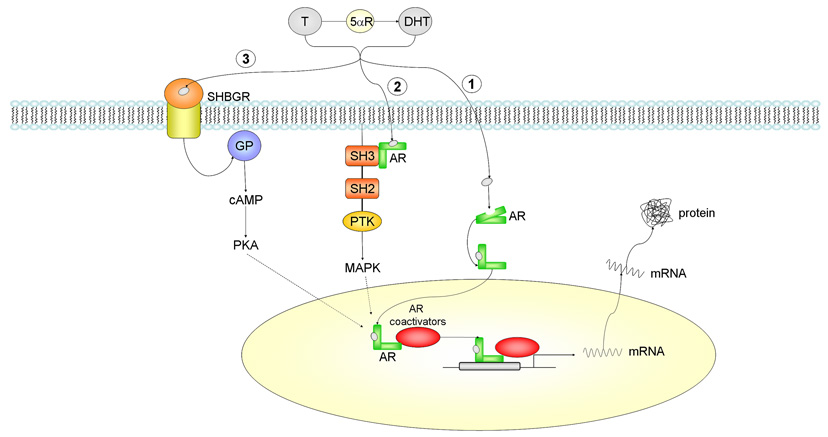
Androgen actions via intracellular androgen receptor mediated pathways. Testosterone (T) can be converted to dihydrotestosterone (DHT) by the 5αR enzyme. 1) In the classical pathway, androgen freely passes through the membrane bi-layer and binds cytoplasmic androgen receptor (AR). Bound AR translocates to the nucleus, binds to a DNA response element on a promoter of an androgen responsive gene and stimulates transcription. 2) Bound AR interacts with the SH3 domain of the tyrosine kinase c-Src to activate the MAPK pathway and influence AR-mediated transcription via phosphorylation of coactivator/receptor complexes. 3) Androgen bound to steroid hormone binding globulin (SHBG) can activate SHBG receptor (SHBGR) and lead to an increase in PKA activity. PKA may influence AR-mediated transcription via alteration of phosphorylation status of AR and AR coregulators. Abbreviations: T = testosterone, DHT = dihydrotestosterone, 5αR = 5 alpha reductase enzyme, AR = androgen receptor, PKA = protein kinase A, GP = g-protein, SH2 = Src homology domain 2, SH3 = Src homology domain 3, PTK = protein tyrosine kinase, MAPK = mitogen-activated protein kinase, SHBGR = steroid hormone binding globulin receptor, cAMP = cyclic adenosine monophosphate.
There is little doubt that the classical genomic model for steroid action accurately describes the molecular mechanisms for many responses to steroid hormones. However, over the past two decades numerous experiments lend support to the conclusion that some steroid responses, but not all, involve non-classical, and initially non-genomic mechanisms. Studies in a variety of in vitro and in vivo models have shown that steroid hormones can affect cellular processes in a non-genomic fashion. For instance, hormone-bound/activated nuclear receptors are able to interact with other transcription factors on target gene promoters without direct binding to DNA [7; 8]. Steroid receptors are able to activate intracellular signaling molecules, such as the mitogen-activated protein kinase (MAPK) family, ERK1/2, by transcription-independent mechanisms [9; 10; 11]. Steroids have also been shown to elicit cellular responses in a rapid fashion even when prevented from entering the cell.
Perhaps the most conserved cellular response to steroid hormones indicating a non-genomic action is the rapid rise of intracellular calcium concentration ([Ca2+]i), observed in a variety of cell types [12; 13; 14; 15; 16; 17]. These effects appear within seconds to minutes and have been described for all classes of steroids [18; 19; 20].
While the vast majority of work examining non-genomic actions of steroid hormones has focused on rapid estrogen effects, the present review will focus on potential non-genomic actions of androgens. Similar to the non-genomic actions of other steroids, there are certain basic criteria/categories for an androgen induced response to be considered non-genomic in nature. 1) Speed: the effects should occur in a time frame (seconds to minutes) not sufficiently long enough to allow gene transcription/translation. The classical genomic model predicts that the latency between steroid exposure and observed responses can be no shorter than the time it takes for the steroid to trigger gene transcription followed by protein synthesis. Typically, gene transcription peaks several hours after steroid exposure [21], although the latency for transcription events has been reported to be as short as 7.5min [22]. However, it then takes additional time for mRNA to be translated into proteins and for the proteins to be processed and induce measurable responses. Typically, cellular responses, which meet this requirement, are changes in free intracellular calcium, and activation of second messenger pathways. 2) Membrane mediated: the response may involve membrane embedded or associated receptors or binding proteins, and with an action that can be induced even when the steroid is conjugated to molecules that prohibit it from entering deep into the cytoplasm or from translocating to the nucleus when bound to a receptor. The most common example is the use of testosterone (T) conjugated to large molecules such as bovine serum albumin (BSA). 3) Lacking transcription/translation machinery activation: experiments using either cell lines that lack the necessary machinery for a genomic response or identify androgen effects which are insensitive to inhibitors of transcription and translation, thereby demonstrate that certain steroid responses can be elicited in systems where gene transcription or protein synthesis is unlikely or impossible.
As stated above we, and others, have chosen to term these novel, non-classical actions of hormones non-genomic. This terminology although widely used is somewhat flawed in that some of the non-genomic actions of hormones previously outlined may lead to genomic responses (i.e. second messenger activation), however this has not been exstensively studied in all cases and therefore still remains unknown. Consequently, for this discussion, the term non-genomic will be used for androgen actions that meet the above criteria, with the caveat that genomic effects could be identified as one of the many downstream end points.
The non-genomic actions of androgens will be reviewed and possible mechanisms discussed. Evidence will be presented that androgens can bind to receptors in or around the plasma membrane, activate cell-signaling pathways, and regulate responses on a time scale of seconds or minutes. The existence of these alternative regulatory pathways for steroid hormones has already begun to challenge endocrinologists and neurobiologists to shift their thinking about how steroid hormones work to regulate cell function. It is no longer valid to assume that minute-to-minute changes in steroid concentrations are not regulating biologically important, short-term responses.
Androgens can interact with intracellular calcium regulatory mechanisms
Although the data demonstrating non-genomic androgen action are limited, the most consistent non-genomic effect of androgen exposure is a rapid change in [Ca2+]i [23; 24; 25; 26; 27; 28]. Because calcium modulation is a fairly rapid response, occurring within seconds to minutes, it has been presumed that the androgen must bind to some sort of receptor at the surface of the cell to achieve this result (Figure 2). Interestingly, not all cell types that demonstrate a rapid androgen response express the classic nuclear androgen receptor (AR) or are blocked by AR antagonists. Therefore, it is not yet known whether the receptor located at the cell surface is the classic intracellular AR coupled to other signal transduction machinery located in the membrane or a unique protein, capable of binding androgen and initiating signal transduction cascades [24; 25; 26; 28].
Figure 2.
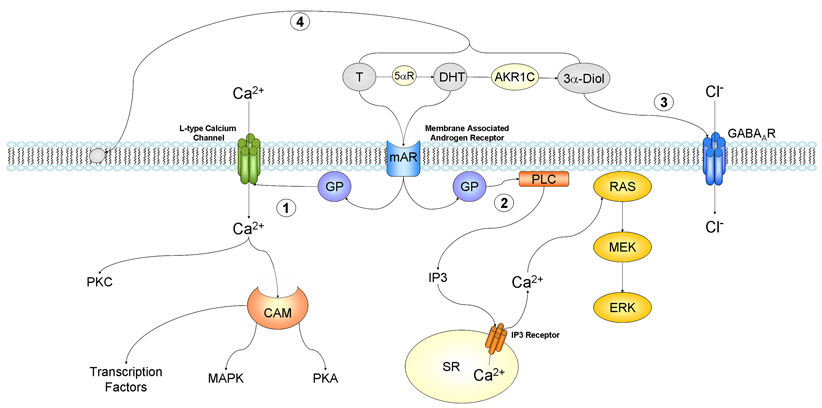
Non-genomic androgen actions via changes in intracellular ion concentrations and membrane fluidity. 1) Androgen interacts with a membrane associated androgen receptor (mAR) leading to the activation of L-type calcium channels through some type of inhibitory g-protein (GP). This increase in intracellular calcium can lead to activation of PKC, and via calmodulin (CAM) activate PKA and MAPK pathways, ultimately influencing gene transcription through phosphorylation. 2) Androgen interacts with a membrane associated androgen receptor (mAR) leading to modulation of g-protein activity and subsequent activation of phospholipase C (PLC). Resulting increases in IP3 lead to the release of intracellular calcium stores from the sarcoplasmic reticulum (SR), and consequently the activation of the RAS/MEK/ERK pathway. 3) Dihydrotestosterone (DHT) metabolite 3α-Diol may interact with the GABAA receptor and lead to increases in intracellular calcium and thus membrane potential. 4) Testosterone and its metabolites can interact with phospholipids in the membrane bilayer to change membrane flexibility and subsequently alter the function of sodium/potassium ATPase and calcium ATPase. Abbreviations: T = testosterone, DHT = dihydrotestosterone, 5αR = 5 alpha reductase enzyme, AKR1C = aldo-keto reductase, 3α-Diol = 3α-androstanediol, GABA = gamma-aminobutyric acid, GP = g-protein, PKA = protein kinase A, PKC = protein kinase C, CAM = calmodulin, MAPK = mitogen-activated protein kinase, PLC = phospholipase C, IP3 = inositol 1,4,5-triphosphate, SR = sarcoplasmic reticulum, MEK = MAPK/ERK kinase, ERK = extracellular-signal regulated kinase.
For over two decades a group of researchers led by Frank Wunderlich have demonstrated [Ca2+]i changes due to androgen treatment on a number of cell types including murine macrophages [29]. The macrophages used in these experiments lacked measurable expression of the intracellular AR. However, treatment with T increased intracellular calcium by binding to a unique androgen-binding unit, and involved non-voltage-gated calcium channels, an inhibitory G-protein, and activation of the phospholipase C pathway (Figure 2). The calcium mobilization was also inducible by plasma membrane-impermeable T-BSA. T actions were not affected by the AR antagonists, cyproterone acetate or flutamide, whereas it was completely inhibited by the phospholipase C inhibitor, U-73122, and G-protein inhibitor, pertussis toxin. Furthermore, putative T binding sites were discovered on the surface of these macrophages [30]. These binding sites were found to internalize in caveolae and clathrin-coated vesicles after agonist stimulation [26; 27; 29]. These findings suggest the involvment of a novel membrance AR in the androgen dependent [Ca2+]i response.
A rapid androgen receptor-independent effects of T on intracellular [Ca2+]i in neuroblastoma cells has alsobeen recently shown by Estrada et al (2006). The initial transient rise in [Ca2+]i was dependent upon production of inositol 1,4,5-trisphosphate [Ins(1,4,5)P3], but propagation of the calcium rise required both Ca2+ influx from extracellular sources as well as Ca2+ release from intracellular stores [31].
A similar response is found in rat osteoblasts, where T induced both the influx of extracellular Ca2+ via Ca2+ channels and Ca2+ release from internal stores through G-protein-coupled receptors activating phospholipase C [23]. In contrast to macrophages, calcium influx in osteoblasts was sensitive to verapamil, a phenylalkylamine calcium channel blocker, indicating the involvement of voltage-gated calcium channels. Of note, the ability of T or T conjugated to BSA (T-BSA) to increase Ca2+ in primary osteoblasts was found to be sexually dimorphic. T was only able to induce Ca2+ influx in male primary osteoblasts but not those cells derived from females [23]. In contrast, Ca2+ influx in female osteoblasts was affected by estradiol, in a non-genomic fashion, whereas male osteoblasts were estradiol insensitive [23]. The basis of this sexual dimorphism is not known, but it is possible that different membrane receptors for estradiol and T exist.
Androgens have also been shown to have profound effects on the cells of the cardiovascular system. Androgens can induce relaxation of the aorta and coronary arteries [32; 33; 34; 35], but it can also facilitate vasoconstriction [36; 37]. Perfusion of T to isolated rat hearts has been shown to cause an acute increase in vascular resistance and block the effects of vasodilatory agents. These effects were presumed to be of non-genomic origin as they were exerted at the cell membrane [38; 39]. In cultured cardiac myocytes, T has been found to induce a rapid [Ca2+]i increase through activation of a plasma membrane androgen receptor associated with the PTX-sensitive G protein-PLC/IP3 signaling pathway (Figure 2). The androgen induced rise in [Ca2+]i in cardiac myocytes appears to dependent on Ca2+ release from internal stores by a PLC/IP3-dependent mechanism [40; 41].
The ability of T to induce a rapid influx of Ca2+ has also been reported in primary cultures of rat Sertoli cells [24]. The [Ca2+]i increases in Sertoli cells occur within 4 min of T treatment and can be inhibited by the androgen receptor antagonist, flutamide [42]. Similarly, the non-aromatizable androgen, 5α-dihydrotestosterone (DHT) has been shown to increase [Ca2+]i in human prostate cancer cells (LNCaP) and in primary hippocampal neurons, a response that, in both cases, can also be blocked by flutamide [17; 25]. However, in primary hippocampal neurons it remains unknown if the androgen-induced increase is due to non-genomic actions [17].
In the case of androgen induction of Ca2+ mobilization, relatively little is known about the ultimate cellular effect. Ca2+ functions as a ubiquitous second messenger molecule and modulation of intracellular Ca2+ levels impacts a wide range of cellular processes, including cell proliferation, apoptosis, necrosis, motility, and gene expression [43]. The elevation of [Ca2+]i is detected by specific Ca2+ sensor molecules (including PKC and calmodulin) to induce signal transduction cascades and modulation of transcription [44]. Androgen induced [Ca2+]i rise may itself be able to regulate AR activation since increased [Ca2+]i levels stimulate the binding of androgens to AR [45]. In addition, androgens can activate calcium-dependent kinase pathways, such as ERK or Src, which could phosphorylate the AR and enhance its activity [46; 47]. However, the sustained elevation of [Ca2+]i following treatment with Ca2+ ionophores or inhibitors of Ca2+-ATPase have also been found to reduce AR expression [48]. Therefore, the functional importance of androgen-induced non-genomic Ca2+ signaling, in particular with regard to gene expression and cell function, is not yet fully understood.
The most intriguing conclusion which can be made from the numerous observations of non-genomic androgen-mediated [Ca2+]i increases is that the mechanisms which produce the increases appear to be different in different cell types (e.g. Ca2+ release from internal stores verses influx from extracellular space or both, may or may not be blocked by AR antagonists)[49]. It has yet to be determined if this represents the function of different androgen-binding proteins with a distinct cell type-dependent distribution or if there is one membrane androgen-binding protein that conveys its signal in a manner subject to other cell type-specific differences. Regardless, by altering [Ca2+]i in unique ways the androgen exposed cell can use the same signaling molecule to specifically regulate different cellular functions [50].
Androgen induces changes in membrane “flexibility”
Independent of receptors, channels or second messenger pathways, androgens may mediate some non-genomic actions via their structural properties. Androgen metabolites have been found to acquire additional charges from sulfate residues and in turn achieve the necessary charge to penetrate into the lipid/protein complex of the cell membrane, thereby decreasing the membrane flexibility and modulating the actions of enzymes required for ATP hydrolysis [51]. For example, Verbist et al (1991) demonstrated a direct interaction of negatively charged phospholipids with membrane ATPase calcium pumps via fluorescent resonance energy transfer [52]. These observations may have physiological consequences, because local steroid synthesis could allow permanent, calcium-independent regulation of Ca2+-ATPase activity in neuronal plasma membranes. In support of this hypothesis, hydrophobic steroids, including T and DHT, have been shown to interact with membrane phospholipids to influence membrane fluidity (Figure 2) [53; 54].
Likewise, molecules that interact with the lipid bilayer, such as cholesterol, have been shown to decrease the activity of both cation-activated adenosine 5 triphosphatases, such as the sodium/potassium adenosine 5 triphosphatase (Na/K-ATPase) and the calcium-dependent adenosine 5 triphosphatase (Ca2+-ATPase)[55; 56]. Testosterone appears to increase the activity of both Na/K-ATPase and Ca2+-ATPase [57]. Acute T treatment causes a dose-dependent increase in the hydrolytic ability of Ca2+-ATPase purified from synaptosomal membranes of rat cortex [51].
Similarly, T has been shown to attenuate lipopolysaccharide signaling. Testosterone reduces lipopolysaccharide induced activation of the c-fos promoter and nitric oxide production, which is abolished by the intracellular Ca2+ chelator BAPTA (1,2-bis(o-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid) [58]. Testosterone also attenuates the lipopolysaccharide activation of p38 but not that of ERK1/2 and JNK/SAPK, and this inhibition was also abolished in the presence of BAPTA [58].
Testosterone has also been shown to interfere with the direct cell-to-cell exchange of ions and molecules that occurs through gap junctions in Sertoli and cardiac cells of young rats. Gap junctions are specialized membrane channels built by the interaction of two half channels, termed connexons. Testosterone treatment caused a rapid impairment of the intercellular coupling. This interruption of the cell-to-cell communication through gap junction channels was dose-dependent, and was progressively reversed after T withdrawal. Pretreatment with the AR antagonist, cyproterone acetate, failed to prevent the uncoupling action of the T. The mechanism of the identified uncoupling is unknown but could be the result of the direct interaction of T with the membrane to alter the conformation of the gap junction channels and thereby change their functional state [59].
Androgens activate second messenger pathways
As well as calcium, Testosterone has been shown rapidly activate other second messenger pathways. Testosterone has been shown to reduce potassium influx in Xenopus oocytes overexpressing G-protein inward rectifying potassium channels, further implicating androgens in the attenuation Gβγ activity. Interestingly, reduction of AR expression by RNA interference reduced T’s effects on potassium channel activity at low, but not high, T concentrations [60].
Androgen receptors have been shown to activate second messenger pathways independent of their classical transcriptional activity. Consistent with this mode of action, AR has been found to interact with and activate the tyrosine kinase c-Src [47; 61; 62]. c-Src is normally targeted to the inner surface of the plasma membrane suggesting the necessity of a membrane or membrane associated AR. The tyrosine kinase activity of c-Src is auto-inhibited by the interaction between the tyrosine kinase domain and the Src homology 2 (SH2) and Src homology 3 (SH3) domains. Disruption of these interactions by proteins binding to the SH2 or SH3 domains results in activation of the c-Src kinase. Androgen receptor bound to an androgen has been shown to interact with the SH3 domain of c-Src (Figure 1) [47; 61]. The association of AR with c-Src results in stimulation of c-Src kinase activity within 1 min in the LNCaP prostate cancer cell line [47]. Additionally, AR has been shown to complex with Src and the MNAR (modulator of non-genomic action of estrogen receptor) protein [63]. In prostate cancer cells, AR/Src/MNAR association and the resulting MAPK activation has been shown to be both androgen dependent and independent [63]. One of the targets of c-Src is the adapter protein Shc, an upstream regulator of the MAPK pathway. The c-Src-mediated activation of MAPK is involved in multiple cellular processes, including migration, proliferation, and differentiation [64; 65].
Androgen treatment also results in stimulation of two members of the MAPK signaling cascade, Raf-1 and ERK-2 within 5 min [61]. Androgen induction of c-Src/Raf/ERK signaling is stopped by inhibition of c-Src kinase activity or treatment with AR antagonists [47; 61; 62]. In AR-negative COS-1 cells, transfection of AR is necessary to induce the androgen dependent increase in activity of c-Src/Raf/ERK [47]. These results suggest that androgens are working through the known AR to activate a non-genomic second messenger pathway. Interestingly, AR can also function cooperatively with estrogen receptors (ER) to induce c-Src kinase activity as part of a complex composed of c-Src, ERs, and AR [47; 61].
One of the non-genomic actions of AR ultimately might be to influence AR mediated transcriptional activity. The activity of AR and AR coactivators are modulated by direct phosphorylation by MAPK. AR phosphorylation by ERK-2 is associated with enhanced AR transcriptional activity and an increased ability to recruit coactivators [65]. The steroid receptor coactivator (SRC) family of transcriptional coactivators is a target of MAPK phosphorylation. Phosphorylation of SRCs results in an increased ability of these coactivators to recruit additional coregulatory complexes to the DNA-bound receptor [66] [67; 68]. It is possible that androgens can induce an autocrine type of feed-forward loop in which androgen-bound AR stimulates the MAPK pathway through direct interaction with c-Src kinase, resulting in phosphorylation of AR and AR coactivators and enhancement of AR transcriptional activity [69; 70; 71].
Androgens can also activate cAMP and PKA through the sex hormone binding globulin (SHBG) receptor (Figure 1). Sex hormone-binding globulin (SHBG) is a liver derived glycoprotein that binds to sex hormones, specifically T, DHT and estradiol [72]. The majority of serum T and DHT (approximately 60%) is complexed with SHBG, with the remainder bound to albumin [73; 74]. A cell surface receptor for SHBG has been functionally identified in a number of tissues including the prostate, testis, breast, and liver [75; 76] [77]. The induction of cAMP by SHBG results in the activation of PKA in both prostate and breast cancer cells [78; 79]. Androgen receptor transcriptional activity is enhanced by PKA stimulation [69; 70; 80]. However, AR does not contain a consensus PKA phosphorylation site and is not directly phosphorylated by PKA [70]. The enhancement of AR transcription by PKA may be through increased activity of AR coregulators. As discussed above, phosphorylation of coregulatory proteins has been found to influence their ability to interact with the steroid receptor and affect the recruitment of other coregulatory proteins [68; 81]. Therefore, it is possible that androgen-SHBG stimulation of PKA may result in alteration of the phosphorylation of AR and AR coregulators and thus modulates AR transcriptional activity (Figure 1).
Treatment of LNCaP prostate cells with T-BSA has been shown to cause phosphorylation and activation of focal adhesion kinase (FAK) and increase secretion of prostate-specific antigen (PSA) within minutes. The activated FAK in turn phosphorylates and activates the phosphatidylinositol-3 (PI-3) kinase, and the subsequent activation of the latter results in the activation of the small guanosine triphosphatases Cdc42/Rac1 and alterations of the cytoskeletal protein, actin. Pretreatment of cells with the specific PI-3 kinase inhibitor, wortmannin, abolished both the activation of Cdc42/Rac1 and the alterations of actin cytoskeleton, whereas it did not affect the phosphorylation of FAK. These findings indicate that PI-3 kinase is activated downstream of FAK and upstream of Cdc42/Rac1, to subsequently regulate actin dynamics [82]. The T-BSA induced PSA secretion requires a reorganization of the actin cytoskeleton, since coincubation of cells with the actin disrupting agent blocked the PSA secretion [82; 83].
Membrane receptors
The existence of a novel membrane-bound AR has been postulated by a number of authors based on the detection of specific androgen binding to plasma membranes in different cell types including endothelial cells [84], breast cancer cells [85], prostate cancer cells [83], osteoblasts [86], macrophages [58], and T-lymphocytes [26; 28; 87]. The ability of androgens to rapidly modulate the activity of ion channels and [Ca2+]i has been observed in several cell types. However, it has not yet been determined whether these non-genomic effects are mediated through a specific membrane androgen receptor or are acting through another signaling pathway such as SHBG or an c-Src kinase-AR complex (Figure 1, 2). Unfortunately, the putative membrane AR has not yet been purified or cloned, preventing further definitive characterization. An example of such a receptor candidate is AR45, a splice variant of the AR lacking part of the n-terminal domain encoded by exon 1, which was discovered in heart and skeletal muscle [88]. Whether this truncated AR plays a significant role in androgen’s actions in these tissues is unclear.
The identification of distinct membrane receptors for other steroid hormones suggests a novel membrane receptor for androgens may also exist. Androgen receptor-immunoreactivity (AR-IR) has been found in dendritic spines, many arising from pyramidal and granule cell dendrites. Androgen receptor-IR has also been associated with clusters of small, synaptic vesicles within preterminal axons and axon terminals [89]. Androgen receptors, like the other steroid receptor proteins, appear to have a conserved amino acid sequence for possible membrane trafficing. Mutational analysis established that a 9 amino acid motif in the ligand-binding domain of the AR mediates membrane translocation via palmitoylation. Mutations of these amino acids significantly reduced membrane localization, MAPK and PI3 kinase activation [90].
As with the SHBG receptor, other known receptors may play a role in the non-genomic actions of androgens. Androgens have a specific binding site on neurotransmitter receptors, in particular the gamma-aminobutyric acid A (GABAA) receptor. Binding to GABAA receptors alters neuronal activity through changes in postsynaptic inhibition [91; 92]. In particular, DHT’s metabolite, 5α-androstane-3α, 17β-diol(3α-Diol), has been shown to alter recombinant rat GABAA receptor function in oocytes and it’s effect on sexual receptivity has been postulated to be via promotion of GABA stimulated chloride flux (Figure 2) [93; 94; 95]. Hormone treatments also result in rapid negative and positive actions on other ion channel plasma membrane receptors, including N-methyl-D-aspartate (NMDA) receptors, glycine and nicotinic receptors as well as G protein-coupled receptors [96; 97; 98; 99; 100; 101]. It is also well established that neuronal excitability mediated by ion channels is modulated by the local neuroactive steroids [102]. Thus, the rapid induction by neuroactive steroids at the plasma membrane could play an important role in the regulation of the brain function. It remains to be determined if the modulation induced by other steroids will be the same for androgens as in seen in the GABAA receptor.
Rapid effects of androgens in the regulation of GnRH release
Of course androgens are known to have rapid effects on biological systems. Androgens are intimately involved in the reproductive system, more specificly the neuroendocrine control of the gonadotropin releasing hormone (GnRH). Androgens have long been known to inhibit luteinizing hormone secretion, which is under the direct control of the hypothalamus through secretion of GnRH. While androgens are known to effect pituitary sensitivity to GnRH, there are findings, which strongly suggest a neuronal component for androgen regulation of LH secretion, the specific neural site(s) of action of androgens remains largely unknown [103; 104; 105]. For example, castration has no effect on GnRH mRNA levels at least within the first 7 weeks post-castration [106; 107]. However, some studies have shown that T increases GnRH mRNA levels when administered to castrated male rats [103; 108; 109; 110; 111; 112; 113]. In addition, T has been shown to increase GnRH protein levels in the median eminence of castrated rats [114] and monkeys [115]. However, since T can be aromatized to estrogenic metabolites, and these findings have not been replicated with the non aromatizable androgen, DHT alone, or in the absence of estrogen receptor activation, it is unknown if an estrogenic metabolite of testosterone is working to increase GnRH expression. Likewise, androgen effects on GnRH secretion may be indirectly mediated by opioids, since naloxone, a general opioid receptor antagonist, can block androgen induced negative feedback in vivo [116]. Perhaps most importantly GnRH neurons do not contain AR. Therefore androgens are widely thought to be working through a transsynaptic pathway involving interneurons to affect GnRH secretion [117; 118].
A possible direct action of androgens on GnRH neurons comes from the results of studies demonstrating the expression and function of the classic nuclear AR in the GnRH-secreting GT1 hypothalamic cell line [119; 120; 121]. Furthermore, Shakil et al (2002) have demonstrated what appears to be the classic AR in plasma membrane fractions of GT1-7 cells. They observed androgen binding activity with a cell-impermeable T-BSA and were able to detect a 110-kDa protein, recognized by a monoclonal antibody (MA150) targeted to the intracellular AR, in the plasma membrane fraction of the GT1-7 cells by Western analysis. These studies further demonstrated that when GT1-7 cells were transfected with the chimeric AR protein tagged with green fluorescent protein, the receptor translocated to the plasma membrane, and the membrane trafficking of AR was increased in the presence of DHT.
In addition, these studies identified non-genomic actions of AR in GT1-7 neurons. While treatment with DHT alone has no effect on cAMP, DHT inhibited forskolin-stimulated accumulation of cAMP, through a pertussis toxin-sensitive G protein. The inhibition of forskolin-stimulated cAMP accumulation by DHT was blocked by the AR antagonist, hydroxyflutamide. Interestingly, GnRH mRNA levels were down regulated by DHT and T, but not by treatment with T-BSA suggesting different genomic/non-genomic actions in this cell line [122].
Dihydrotestosterone, T, and T-BSA, all caused significant elevations in [Ca2+]i in GT1-7 cells within 200 seconds of treatment similar to findings in other cell lines. Testosterone-BSA also stimulated a 2-fold increase in GnRH secretion [122]. This is not surprising, considering [Ca2+]i changes have long been postulated to be a key factor controlling pulsatile GnRH secretion [123; 124; 125; 126]. Due to the presence of the AR and the apparent positive role androgens have on GnRH release in GT1-7 cells, these data question the suitability of GT1-7 cells for studying androgen’s role in GnRH regulation in adulthood. An artifact of the immortalization process that produced these cells or remnants of the embryonic origin of the cell line may account for the differences. However, GT1-7 cells may still be a useful model in identifying the mechanisms by which androgens activate non-genomic pathways.
Rapid effects of androgen and androgen metabolites on behavior
Androgen treatment has been shown to cause rapid changes in animal behavior, although the exact mechanism of action remains difficult to discern. Using the female lordosis reflex as an end point, researchers have shown that androgens can rapidly modulate sexual receptivity in female rodents [127]. Dihydrotestosterone and its metabolite, 3α-Diol, have been shown to be important for the termination of sexual receptivity in rodents and this effect is observed in the absence of functional intracellular androgens receptors [127]. Similarly, altering GABAA receptor function in the hypothalamus can abolish 3α-Diol’s inhibition of sexual receptivity [94; 95; 128]. These findings suggest that 3α-Diol can influences lordosis via non-classical actions. Jorge-Rivera et al. (2000) reported that 3α-Diol potentiated the peak amplitude of spontaneous IPSCs (sIPSCs) recorded from the medial preoptic area (mPOA) and the ventromedial nucleus (VMN) of female rats suggesting, alone with GABAA receptor mediation, the beginnings of a mechanism of 3α-Diol on sexual behavior [129].
Similarly, DHT inhibits estrogen-induced sexual receptivity in several rodent species [130; 131; 132; 133; 134; 135; 136; 137], but the precise mechanisms by which DHT inhibits reproductive processes are still unclear. It has been proposed that DHT may inhibit sexual receptivity by interfering with the binding of E to its receptor [132] or by its before mentioned metabolite, 3α-Diol. Dihydrotestosterone is readily and reversibly [138] metabolized to 3α-Diol by the enzyme 3-α-hydroxysteroid dehydrogenase (3α-HSD or Aldo-Keto Reductase 1C, a member of the AKR superfamily of proteins), in many tissues, including the brain [138; 139; 140; 141; 142; 143],while conversion also occurs in astrocytes [144; 145; 146]. Both 3α-Diol and DHT inhibit E-induced lordosis behavior [131; 135; 137]. However, 3α-Diol is three times more potent than DHT, and DHT is behaviorally effective only at dosages that produce circulating levels of 3α-Diol sufficient to inhibit sexual behavior [147].
3α-androstanediol also alters the affective components of behavior in a sex-dependant manner. Infusion of 3α-Diol into the basolateral amygdala alters behavior in the conditioned place preference test and Vogel conflict test in female rats, but has no effect in males [148]. Additionally, 3α-Diol is rewarding and reinforcing when given to ovariectomized female rats via a self-administration paradigm [149; 150].
In cats, 3α-Diol rapidly and reversibly inhibits brain electrical activity [151]. 3α-Diol has membrane actions as evidenced by the ability of 3α-Diol conjugated to BSA to alter sexual receptivity following central administration in a fashion similar to that seen following systemic 3α-Diol administration [95; 152]. Likewise, 3α-Diol may affect lordosis behavior by acting through GABAA receptors since the 5α-reduced, 3-hydroxylated structure of 3α-Diol meets the requirements for steroid modulators of GABAA receptors [153; 154]. GABA-stimulated chloride flux is increased in receptive animals compared to that of unreceptive ovariectomized controls, whereas GABAergic activity is further elevated in rats with inhibited receptivity [127].
Rapid actions of androgens on behavior are not confined to mammalian species. In toadfish, males call to attract females to their nesting sites. Field experiments have demonstrated that when males are faced with a territorial challenge it produces rapid elevations in calling behavior and circulating levels of the teleost-specific androgen 11-ketotestosterone (11kT). Treatment with 11kT has been shown to induce elevation in calling behavior of males within 10 min. Electrophysiological experiments revealed that intramuscular injections of 11kT induces increases in the output of the vocal pattern generator in males within 5 min of treatment. Together, these experiments provide strong support for the hypothesis that surges in circulating androgen levels play a role in shaping rapid changes in social behavior through non-genomic actions on neural circuits resulting in behavioral changes [155; 156; 157; 158; 159].
Neuroprotective verses Neuroendangering - Genomic verses Non-genomic?
In the central nervous system, androgens can reportedly have either neuroprotective or neuroendangering effects. Dihydrotestosterone has been found to regulate cellular growth, differentiation, survival, or death through both genomic and non-genomic signaling pathways [160]. Androgens, including T and DHT, can protect neurons from various insults in culture, including kainic acid toxicity [161], β-amyloid toxicity [162; 163] and serum deprivation [164], and have been shown to rapidly activate the cytoprotection-associated ERK/MAPK pathway [161; 163]. The receptor mediating these protective effects is thought to be AR due to the fact that AR antagonists block the neuroprotective effects [163; 164]. In contrast, supraphysiological levels of T have been found to increase neuronal apoptosis [165]. In fact, the damage-promoting effects of T have been observed in several experimental models. For example, T was shown to exacerbate the size of a lesion resulting from middle cerebral artery occlusion in male rats [166; 167]. Furthermore, T treatment increased kainic acid-induced cell death of cultured oligodendrocytes [168]. Thus, androgens can be protective or damage promoting, but the mechanism underlying this duality is still unclear. However several hypotheses have been proposed to explain these disparate effects of androgens [85; 169; 170].
The intracellular pathways underlying androgens protective or endangering action can be traced to the fact that androgens elicit opposing actions on the ERK/MAPK and Akt signaling pathways. This contrasting action depends on whether a membrane AR or intracellular AR is activated. Gatson et al (2007) hypothesized that androgens may affect cell viability differently depending on which receptor is activated. Dihydrotestosterone has been shown to protect primary cortical astrocytes from the metabolic and oxidative insult associated with iodoacetic acid-induced toxicity, whereas DHT-BSA suppressed Akt signaling, increased caspase 3/7 activity, and enhanced iodoacetic acid-induced cell death [170; 171]. The stimulatory effects of DHT on intracellular signaling and cell survival were blocked by the AR antagonist, flutamide, whereas both DHT-BSA-induced suppression of ERK and Akt phosphorylation, and DHT-BSA-induced increase in cell death, were insensitive to flutamide [170; 171]. Similarly, in T47D breast cancer cells and LNCaP prostate cancer cells, the cell death-promoting effects of androgens were attributed to a membrane AR due to the fact that T-BSA mimicked the death-promoting effects of T [85; 169]. In the brain, it has been shown that DHT-BSA suppresses the phosphorylation of two key effectors of cell survival (MAPK and PI-3K/Akt pathways) [170]. In the presence of the anionic alkylating agent, iodoacetate, it was determined that activation of the membrane AR by DHT-BSA enhances cell death of primary cortical astrocytes [171]. Collectively, these data support the existence of two, potentially competing, pathways for androgens in a given cell or tissue [170; 171]. In the role of androgens in cell survival there appears to be through two competing pathways, one that is associated with brain protection and another that is associated with the promotion of cell death.
Summary
Whether through androgen membrane or membrane associated receptors/binding proteins, changes in membrane flexibility, changes in [Ca2+]i, activation of second messenger pathway or a combination of some or all of these mechanisms, the known non-genomic actions of androgens can be mediated via multiple pathways. The physiological effect of the majority of identified non-genomic stimulation in vivo remains largely unclear. In addition, non-genomic androgen effects can function independently, or in tandem, with the other genomic effects, to initiate steroid responses. The genomic and non-genomic pathways of androgen action are likely inter-linked and can act in concert to effect normal cell function.
Acknowledgments
Work supported by: USPHS 1 R01 NS033951 for R.J.H., 5F32NS049892 and the Lalor Foundation Fellowship for C.D.F.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Evans RM. The steroid and thyroid hormone receptor superfamily. Science. 1988;240:889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beato M. Gene regulation by steroid hormones. Cell. 1989;56:335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- 3.Roy AK, Lavrovsky Y, Song CS, Chen S, Jung MH, Velu NK, Bi BY, Chatterjee B. Regulation of androgen action. Vitam Horm. 1999;55:309–352. doi: 10.1016/s0083-6729(08)60938-3. [DOI] [PubMed] [Google Scholar]
- 4.Heinlein CA, Chang C. Androgen receptor (AR) coregulators: an overview. Endocr Rev. 2002;23:175–200. doi: 10.1210/edrv.23.2.0460. [DOI] [PubMed] [Google Scholar]
- 5.Zhou ZX, Wong CI, Sar M, Wilson EM. The androgen receptor: an overview. Recent Prog Horm Res. 1994;49:249–274. doi: 10.1016/b978-0-12-571149-4.50017-9. [DOI] [PubMed] [Google Scholar]
- 6.Quigley CA, De Bellis A, Marschke KB, el-Awady MK, Wilson EM, French FS. Androgen receptor defects: historical, clinical, and molecular perspectives. Endocr Rev. 1995;16:271–321. doi: 10.1210/edrv-16-3-271. [DOI] [PubMed] [Google Scholar]
- 7.Gottlicher M, Heck c, Herrlich P. Transcriptional cross-talk, the second mode of steroid hormone receptor action. J Mol Med. 1998;76:480–489. doi: 10.1007/s001090050242. [DOI] [PubMed] [Google Scholar]
- 8.Beato M, Klug J. Steroid hormone receptors: an update. Hum Reprod Update. 2000;6:225–236. doi: 10.1093/humupd/6.3.225. [DOI] [PubMed] [Google Scholar]
- 9.Migliaccio A, Di Domenico M, Castoria G, de Falco A, Bontempo P, Nola E, Auricchio F. Tyrosine kinase/p21ras/MAP-kinase pathway activation by estradiol-receptor complex in MCF-7 cells. Embo J. 1996;15:1292–1300. [PMC free article] [PubMed] [Google Scholar]
- 10.Castoria G, Barone MV, Di Domenico M, Bilancio A, Ametrano D, Migliaccio A, Auricchio F. Non-transcriptional action of oestradiol and progestin triggers DNA synthesis. Embo J. 1999;18:2500–2510. doi: 10.1093/emboj/18.9.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Migliaccio A, Piccolo D, Castoria G, Di Domenico M, Bilancio A, Lombardi M, Gong W, Beato M, Auricchio F. Activation of the Src/p21ras/Erk pathway by progesterone receptor via cross-talk with estrogen receptor. Embo J. 1998;17:2008–2018. doi: 10.1093/emboj/17.7.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lieberherr M, Grosse B, Kachkache M, Balsan S. Cell signaling and estrogens in female rat osteoblasts: a possible involvement of unconventional nonnuclear receptors. J Bone Miner Res. 1993;8:1365–1376. doi: 10.1002/jbmr.5650081111. [DOI] [PubMed] [Google Scholar]
- 13.Audy MC, Vacher P, Duly B. 17 beta-estradiol stimulates a rapid Ca2+ influx in LNCaP human prostate cancer cells. Eur J Endocrinol. 1996;135:367–373. doi: 10.1530/eje.0.1350367. [DOI] [PubMed] [Google Scholar]
- 14.Benten WP, Lieberherr M, Giese G, Wunderlich F. Estradiol binding to cell surface raises cytosolic free calcium in T cells. FEBS Lett. 1998;422:349–353. doi: 10.1016/s0014-5793(98)00039-8. [DOI] [PubMed] [Google Scholar]
- 15.Benten WP, Stephan C, Lieberherr M, Wunderlich F. Estradiol signaling via sequestrable surface receptors. Endocrinology. 2001;142:1669–1677. doi: 10.1210/endo.142.4.8094. [DOI] [PubMed] [Google Scholar]
- 16.Azenabor AA, Hoffman-Goetz L. 17 beta-estradiol increases Ca(2+) influx and down regulates interleukin-2 receptor in mouse thymocytes. Biochem Biophys Res Commun. 2001;281:277–281. doi: 10.1006/bbrc.2001.4341. [DOI] [PubMed] [Google Scholar]
- 17.Foradori CD, Werner SB, Sandau US, Clapp TR, Handa RJ. Activation of the androgen receptor alters the intracellular calcium response to glutamate in primary hippocampal neurons and modulates sarco/endoplasmic reticulum calcium ATPase 2 transcription. Neuroscience. 2007;149:155–164. doi: 10.1016/j.neuroscience.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 18.Moriarty K, Kim KH, Bender JR. Minireview: estrogen receptor-mediated rapid signaling. Endocrinology. 2006;147:5557–5563. doi: 10.1210/en.2006-0729. [DOI] [PubMed] [Google Scholar]
- 19.Wehling M, Schultz A, Losel R. Nongenomic actions of estrogens: exciting opportunities for pharmacology. Maturitas. 2006;54:321–326. doi: 10.1016/j.maturitas.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 20.Baulieu EE, Robel P. Non-genomic mechanisms of action of steroid hormones. Ciba Found Symp. 1995;191:24–37. doi: 10.1002/9780470514757.ch3. discussion 37–42. [DOI] [PubMed] [Google Scholar]
- 21.Cato AC, Skroch P, Weinmann J, Butkeraitis P, Ponta H. DNA sequences outside the receptor-binding sites differently modulate the responsiveness of the mouse mammary tumour virus promoter to various steroid hormones. Embo J. 1988;7:1403–1410. doi: 10.1002/j.1460-2075.1988.tb02957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Groner B, Ponta H, Beato M, Hynes NE. The proviral DNA of mouse mammary tumor virus: its use in the study of the molecular details of steroid hormone action. Mol Cell Endocrinol. 1983;32:101–116. doi: 10.1016/0303-7207(83)90075-8. [DOI] [PubMed] [Google Scholar]
- 23.Lieberherr M, Grosse B. Androgens increase intracellular calcium concentration and inositol 1,4,5-trisphosphate and diacylglycerol formation via a pertussis toxin-sensitive G-protein. J Biol Chem. 1994;269:7217–7223. [PubMed] [Google Scholar]
- 24.Gorczynska E, Handelsman DJ. Androgens rapidly increase the cytosolic calcium concentration in Sertoli cells. Endocrinology. 1995;136:2052–2059. doi: 10.1210/endo.136.5.7720654. [DOI] [PubMed] [Google Scholar]
- 25.Steinsapir J, Socci R, Reinach P. Effects of androgen on intracellular calcium of LNCaP cells. Biochem Biophys Res Commun. 1991;179:90–96. doi: 10.1016/0006-291x(91)91338-d. [DOI] [PubMed] [Google Scholar]
- 26.Benten WP, Lieberherr M, Stamm O, Wrehlke C, Guo Z, Wunderlich F. Testosterone signaling through internalizable surface receptors in androgen receptor-free macrophages. Mol Biol Cell. 1999;10:3113–3123. doi: 10.1091/mbc.10.10.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benten WP, Lieberherr M, Sekeris CE, Wunderlich F. Testosterone induces Ca2+ influx via non-genomic surface receptors in activated T cells. FEBS Lett. 1997;407:211–214. doi: 10.1016/s0014-5793(97)00346-3. [DOI] [PubMed] [Google Scholar]
- 28.Benten WP, Lieberherr M, Giese G, Wrehlke C, Stamm O, Sekeris CE, Mossmann H, Wunderlich F. Functional testosterone receptors in plasma membranes of T cells. Faseb J. 1999;13:123–133. doi: 10.1096/fasebj.13.1.123. [DOI] [PubMed] [Google Scholar]
- 29.Wunderlich F, Benten WP, Lieberherr M, Guo Z, Stamm O, Wrehlke C, Sekeris CE, Mossmann H. Testosterone signaling in T cells and macrophages. Steroids. 2002;67:535–538. doi: 10.1016/s0039-128x(01)00175-1. [DOI] [PubMed] [Google Scholar]
- 30.Liu L, Zhao Y, Wang Y, Li Q, Wang Z, Wang L, Qiao Z. Testosterone induced Ca2+ influx in bone marrow-derived macrophages via surface binding sites. Methods Find Exp Clin Pharmacol. 2005;27:623–628. doi: 10.1358/mf.2005.27.9.939336. [DOI] [PubMed] [Google Scholar]
- 31.Estrada M, Uhlen P, Ehrlich BE. Ca2+ oscillations induced by testosterone enhance neurite outgrowth. J Cell Sci. 2006;119:733–743. doi: 10.1242/jcs.02775. [DOI] [PubMed] [Google Scholar]
- 32.Yue P, Chatterjee K, Beale C, Poole-Wilson PA, Collins P. Testosterone relaxes rabbit coronary arteries and aorta. Circulation. 1995;91:1154–1160. doi: 10.1161/01.cir.91.4.1154. [DOI] [PubMed] [Google Scholar]
- 33.Costarella CE, Stallone JN, Rutecki GW, Whittier FC. Testosterone causes direct relaxation of rat thoracic aorta. J Pharmacol Exp Ther. 1996;277:34–39. [PubMed] [Google Scholar]
- 34.Chou TM, Sudhir K, Hutchison SJ, Ko E, Amidon TM, Collins P, Chatterjee K. Testosterone induces dilation of canine coronary conductance and resistance arteries in vivo. Circulation. 1996;94:2614–2619. doi: 10.1161/01.cir.94.10.2614. [DOI] [PubMed] [Google Scholar]
- 35.Rosano GM, Leonardo F, Pagnotta P, Pelliccia F, Panina G, Cerquetani E, della Monica PL, Bonfigli B, Volpe M, Chierchia SL. Acute anti-ischemic effect of testosterone in men with coronary artery disease. Circulation. 1999;99:1666–1670. doi: 10.1161/01.cir.99.13.1666. [DOI] [PubMed] [Google Scholar]
- 36.Masuda A, Mathur R, Halushka PV. Testosterone increases thromboxane A2 receptors in cultured rat aortic smooth muscle cells. Circ Res. 1991;69:638–643. doi: 10.1161/01.res.69.3.638. [DOI] [PubMed] [Google Scholar]
- 37.Schror K, Morinelli TA, Masuda A, Matsuda K, Mathur RS, Halushka PV. Testosterone treatment enhances thromboxane A2 mimetic induced coronary artery vasoconstriction in guinea pigs. Eur J Clin Invest. 1994;24(Suppl 1):50–52. doi: 10.1111/j.1365-2362.1994.tb02428.x. [DOI] [PubMed] [Google Scholar]
- 38.Ceballos G, Figueroa L, Rubio I, Gallo G, Garcia A, Martinez A, Yanez R, Perez J, Morato T, Chamorro G. Acute and nongenomic effects of testosterone on isolated and perfused rat heart. J Cardiovasc Pharmacol. 1999;33:691–697. doi: 10.1097/00005344-199905000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Rubio-Gayosso I, Garcia-Ramirez O, Gutierrez-Serdan R, Guevara-Balcazar G, Munoz-Garcia O, Morato-Cartajena T, Zamora-Garza M, Ceballos-Reyes G. Testosterone inhibits bradykinin-induced intracellular calcium kinetics in rat aortic endothelial cells in culture. Steroids. 2002;67:393–397. doi: 10.1016/s0039-128x(01)00192-1. [DOI] [PubMed] [Google Scholar]
- 40.Vicencio JM, Ibarra C, Estrada M, Chiong M, Soto D, Parra V, Diaz-Araya G, Jaimovich E, Lavandero S. Testosterone induces an intracellular calcium increase by a nongenomic mechanism in cultured rat cardiac myocytes. Endocrinology. 2006;147:1386–1395. doi: 10.1210/en.2005-1139. [DOI] [PubMed] [Google Scholar]
- 41.Gerasimenko O, Gerasimenko J. New aspects of nuclear calcium signalling. J Cell Sci. 2004;117:3087–3094. doi: 10.1242/jcs.01295. [DOI] [PubMed] [Google Scholar]
- 42.Lyng FM, Jones GR, Rommerts FF. Rapid androgen actions on calcium signaling in rat sertoli cells and two human prostatic cell lines: similar biphasic responses between 1 picomolar and 100 nanomolar concentrations. Biol Reprod. 2000;63:736–747. doi: 10.1095/biolreprod63.3.736. [DOI] [PubMed] [Google Scholar]
- 43.Berridge MJ, Bootman MD, Lipp P. Calcium--a life and death signal. Nature. 1998;395:645–648. doi: 10.1038/27094. [DOI] [PubMed] [Google Scholar]
- 44.Mellstrom B, Naranjo JR. Mechanisms of Ca(2+)-dependent transcription. Curr Opin Neurobiol. 2001;11:312–319. doi: 10.1016/s0959-4388(00)00213-0. [DOI] [PubMed] [Google Scholar]
- 45.Cabeza M, Flores M, Bratoeff E, de la Pena A, Mendez E, Ceballos G. Intracellular Ca2+ stimulates the binding to androgen receptors in platelets. Steroids. 2004;69:767–772. doi: 10.1016/j.steroids.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 46.Estrada M, Espinosa A, Muller M, Jaimovich E. Testosterone stimulates intracellular calcium release and mitogen-activated protein kinases via a G protein-coupled receptor in skeletal muscle cells. Endocrinology. 2003;144:3586–3597. doi: 10.1210/en.2002-0164. [DOI] [PubMed] [Google Scholar]
- 47.Migliaccio A, Castoria G, Di Domenico M, de Falco A, Bilancio A, Lombardi M, Barone MV, Ametrano D, Zannini MS, Abbondanza C, Auricchio F. Steroid-induced androgen receptor-oestradiol receptor beta-Src complex triggers prostate cancer cell proliferation. Embo J. 2000;19:5406–5417. doi: 10.1093/emboj/19.20.5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gong Y, Blok LJ, Perry JE, Lindzey JK, Tindall DJ. Calcium regulation of androgen receptor expression in the human prostate cancer cell line LNCaP. Endocrinology. 1995;136:2172–2178. doi: 10.1210/endo.136.5.7720667. [DOI] [PubMed] [Google Scholar]
- 49.Falkenstein E, Tillmann HC, Christ M, Feuring M, Wehling M. Multiple actions of steroid hormones--a focus on rapid, nongenomic effects. Pharmacol Rev. 2000;52:513–556. [PubMed] [Google Scholar]
- 50.Carafoli E, Santella L, Branca D, Brini M. Generation, control, and processing of cellular calcium signals. Crit Rev Biochem Mol Biol. 2001;36:107–260. doi: 10.1080/20014091074183. [DOI] [PubMed] [Google Scholar]
- 51.Zylinska L, Gromadzinska E, Lachowicz L. Short-time effects of neuroactive steroids on rat cortical Ca2+-ATPase activity. Biochim Biophys Acta. 1999;1437:257–264. doi: 10.1016/s1388-1981(99)00015-3. [DOI] [PubMed] [Google Scholar]
- 52.Verbist J, Gadella TW, Jr, Raeymaekers L, Wuytack F, Wirtz KW, Casteels R. Phosphoinositide-protein interactions of the plasma-membrane Ca2(+)-transport ATPase as revealed by fluorescence energy transfer. Biochim Biophys Acta. 1991;1063:1–6. doi: 10.1016/0005-2736(91)90345-9. [DOI] [PubMed] [Google Scholar]
- 53.Duval D, Durant S, Homo-Delarche F. Non-genomic effects of steroids. Interactions of steroid molecules with membrane structures and functions. Biochim Biophys Acta. 1983;737:409–442. doi: 10.1016/0304-4157(83)90008-4. [DOI] [PubMed] [Google Scholar]
- 54.Van Bommel T, Marsen T, Bojar H. Effects of high-dose medroxyprogesterone acetate and various other steroid hormones on plasma membrane lipid mobility in CAMA-1 mammary cancer cells. Anticancer Res. 1987;7:1217–1223. [PubMed] [Google Scholar]
- 55.Madden TD, Chapman D, Quinn PJ. Cholesterol modulates activity of calcium-dependent ATPase of the sarcoplasmic reticulum. Nature. 1979;279:538–541. doi: 10.1038/279538a0. [DOI] [PubMed] [Google Scholar]
- 56.Yeagle PL, Young J, Rice D. Effects of cholesterol on (Na+,K+)-ATPase ATP hydrolyzing activity in bovine kidney. Biochemistry. 1988;27:6449–6452. doi: 10.1021/bi00417a037. [DOI] [PubMed] [Google Scholar]
- 57.Deliconstantinos G. Structure activity relationship of cholesterol and steroid hormones with respect to their effects on the Ca2+-stimulated ATPase and lipid fluidity of synaptosomal plasma membranes from dog and rabbit brain. Comp Biochem Physiol B. 1988;89:585–594. doi: 10.1016/0305-0491(88)90178-2. [DOI] [PubMed] [Google Scholar]
- 58.Guo Z, Benten WP, Krucken J, Wunderlich F. Nongenomic testosterone calcium signaling Genotropic actions in androgen receptor-free macrophages. J Biol Chem. 2002;277:29600–29607. doi: 10.1074/jbc.M202997200. [DOI] [PubMed] [Google Scholar]
- 59.Pluciennik F, Verrecchia F, Bastide B, Herve JC, Joffre M, Deleze J. Reversible interruption of gap junctional communication by testosterone propionate in cultured Sertoli cells and cardiac myocytes. J Membr Biol. 1996;149:169–177. doi: 10.1007/s002329900017. [DOI] [PubMed] [Google Scholar]
- 60.Evaul K, Jamnongjit M, Bhagavath B, Hammes SR. Testosterone and progesterone rapidly attenuate plasma membrane Gbetagamma-mediated signaling in Xenopus laevis oocytes by signaling through classical steroid receptors. Mol Endocrinol. 2007;21:186–196. doi: 10.1210/me.2006-0301. [DOI] [PubMed] [Google Scholar]
- 61.Kousteni S, Bellido T, Plotkin LI, O'Brien, CA, Bodenner DL, Han L, Han K, DiGregorio GB, Katzenellenbogen JA, Katzenellenbogen BS, Roberson PK, Weinstein RS, Jilka RL, Manolagas SC. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell. 2001;104:719–730. [PubMed] [Google Scholar]
- 62.Cheng J, Watkins SC, Walker WH. Testosterone activates mitogen-activated protein kinase via Src kinase and the epidermal growth factor receptor in sertoli cells. Endocrinology. 2007;148:2066–2074. doi: 10.1210/en.2006-1465. [DOI] [PubMed] [Google Scholar]
- 63.Unni E, Sun S, Nan B, McPhaul MJ, Cheskis B, Mancini MA, Marcelli M. Changes in androgen receptor nongenotropic signaling correlate with transition of LNCaP cells to androgen independence. Cancer Res. 2004;64:7156–7168. doi: 10.1158/0008-5472.CAN-04-1121. [DOI] [PubMed] [Google Scholar]
- 64.Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 65.Schlessinger J. New roles for Src kinases in control of cell survival and angiogenesis. Cell. 2000;100:293–296. doi: 10.1016/s0092-8674(00)80664-9. [DOI] [PubMed] [Google Scholar]
- 66.Font de Mora J, Brown M. AIB1 is a conduit for kinase-mediated growth factor signaling to the estrogen receptor. Mol Cell Biol. 2000;20:5041–5047. doi: 10.1128/mcb.20.14.5041-5047.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lopez GN, Turck CW, Schaufele F, Stallcup MR, Kushner PJ. Growth factors signal to steroid receptors through mitogen-activated protein kinase regulation of p160 coactivator activity. J Biol Chem. 2001;276:22177–22182. doi: 10.1074/jbc.M010718200. [DOI] [PubMed] [Google Scholar]
- 68.Rowan BG, Garrison N, Weigel NL, O'Malley BW. 8-Bromo-cyclic AMP induces phosphorylation of two sites in SRC-1 that facilitate ligand-independent activation of the chicken progesterone receptor and are critical for functional cooperation between SRC-1 and CREB binding protein. Mol Cell Biol. 2000;20:8720–8730. doi: 10.1128/mcb.20.23.8720-8730.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nazareth LV, Weigel NL. Activation of the human androgen receptor through a protein kinase A signaling pathway. J Biol Chem. 1996;271:19900–19907. doi: 10.1074/jbc.271.33.19900. [DOI] [PubMed] [Google Scholar]
- 70.Ikonen T, Palvimo JJ, Kallio PJ, Reinikainen P, Janne OA. Stimulation of androgen-regulated transactivation by modulators of protein phosphorylation. Endocrinology. 1994;135:1359–1366. doi: 10.1210/endo.135.4.7925097. [DOI] [PubMed] [Google Scholar]
- 71.Culig Z, Steiner H, Bartsch G, Hobisch A. Mechanisms of endocrine therapy-responsive and -unresponsive prostate tumours. Endocr Relat Cancer. 2005;12:229–244. doi: 10.1677/erc.1.00775a. [DOI] [PubMed] [Google Scholar]
- 72.Mean F, Pellaton M, Magrini G. Study on the binding of dihydrotestosterone, testosterone and oestradiol with sex hormone binding globulin. Clin Chim Acta. 1977;80:171–180. doi: 10.1016/0009-8981(77)90276-5. [DOI] [PubMed] [Google Scholar]
- 73.Mendel CM. The free hormone hypothesis: a physiologically based mathematical model. Endocr Rev. 1989;10:232–274. doi: 10.1210/edrv-10-3-232. [DOI] [PubMed] [Google Scholar]
- 74.Siiteri PK, Murai JT, Hammond GL, Nisker JA, Raymoure WJ, Kuhn RW. The serum transport of steroid hormones. Recent Prog Horm Res. 1982;38:457–510. doi: 10.1016/b978-0-12-571138-8.50016-0. [DOI] [PubMed] [Google Scholar]
- 75.Fortunati N. Sex hormone-binding globulin: not only a transport protein What news is around the corner? J Endocrinol Invest. 1999;22:223–234. doi: 10.1007/BF03343547. [DOI] [PubMed] [Google Scholar]
- 76.Hryb DJ, Khan MS, Rosner W. Testosterone-estradiol-binding globulin binds to human prostatic cell membranes. Biochem Biophys Res Commun. 1985;128:432–440. doi: 10.1016/0006-291x(85)91697-3. [DOI] [PubMed] [Google Scholar]
- 77.Krupenko SA, Krupenko NI, Danzo BJ. Interaction of sex hormone-binding globulin with plasma membranes from the rat epididymis and other tissues. J Steroid Biochem Mol Biol. 1994;51:115–124. doi: 10.1016/0960-0760(94)90122-8. [DOI] [PubMed] [Google Scholar]
- 78.Fortunati N, Fissore F, Fazzari A, Becchis M, Comba A, Catalano MG, Berta L, Frairia R. Sex steroid binding protein exerts a negative control on estradiol action in MCF-7 cells (human breast cancer) through cyclic adenosine 3',5'-monophosphate and protein kinase A. Endocrinology. 1996;137:686–692. doi: 10.1210/endo.137.2.8593818. [DOI] [PubMed] [Google Scholar]
- 79.Nakhla AM, Romas NA, Rosner W. Estradiol activates the prostate androgen receptor and prostate-specific antigen secretion through the intermediacy of sex hormone-binding globulin. J Biol Chem. 1997;272:6838–6841. doi: 10.1074/jbc.272.11.6838. [DOI] [PubMed] [Google Scholar]
- 80.Sadar MD. Androgen-independent induction of prostate-specific antigen gene expression via cross-talk between the androgen receptor and protein kinase A signal transduction pathways. J Biol Chem. 1999;274:7777–7783. doi: 10.1074/jbc.274.12.7777. [DOI] [PubMed] [Google Scholar]
- 81.Wang X, Yang Y, Guo X, Sampson ER, Hsu CL, Tsai MY, Yeh S, Wu G, Guo Y, Chang C. Suppression of androgen receptor transactivation by Pyk2 via interaction and phosphorylation of the ARA55 coregulator. J Biol Chem. 2002;277:15426–15431. doi: 10.1074/jbc.M111218200. [DOI] [PubMed] [Google Scholar]
- 82.Papakonstanti EA, Kampa M, Castanas E, Stournaras C. A rapid, nongenomic, signaling pathway regulates the actin reorganization induced by activation of membrane testosterone receptors. Mol Endocrinol. 2003;17:870–881. doi: 10.1210/me.2002-0253. [DOI] [PubMed] [Google Scholar]
- 83.Kampa M, Papakonstanti EA, Hatzoglou A, Stathopoulos EN, Stournaras C, Castanas E. The human prostate cancer cell line LNCaP bears functional membrane testosterone receptors that increase PSA secretion and modify actin cytoskeleton. Faseb J. 2002;16:1429–1431. doi: 10.1096/fj.02-0131fje. [DOI] [PubMed] [Google Scholar]
- 84.Figueroa-Valverde L, Luna H, Castillo-Henkel C, Munoz-Garcia O, Morato-Cartagena T, Ceballos-Reyes G. Synthesis and evaluation of the cardiovascular effects of two, membrane impermeant, macromolecular complexes of dextran-testosterone. Steroids. 2002;67:611–619. doi: 10.1016/s0039-128x(02)00011-9. [DOI] [PubMed] [Google Scholar]
- 85.Hatzoglou A, Kampa M, Kogia C, Charalampopoulos I, Theodoropoulos PA, Anezinis P, Dambaki C, Papakonstanti EA, Stathopoulos EN, Stournaras C, Gravanis A, Castanas E. Membrane androgen receptor activation induces apoptotic regression of human prostate cancer cells in vitro and in vivo. J Clin Endocrinol Metab. 2005;90:893–903. doi: 10.1210/jc.2004-0801. [DOI] [PubMed] [Google Scholar]
- 86.Armen TA, Gay CV. Simultaneous detection and functional response of testosterone and estradiol receptors in osteoblast plasma membranes. J Cell Biochem. 2000;79:620–627. [PubMed] [Google Scholar]
- 87.Konoplya EF, Popoff EH. Identification of the classical androgen receptor in male rat liver and prostate cell plasma membranes. Int J Biochem. 1992;24:1979–1983. doi: 10.1016/0020-711x(92)90294-b. [DOI] [PubMed] [Google Scholar]
- 88.Ahrens-Fath I, Politz O, Geserick C, Haendler B. Androgen receptor function is modulated by the tissue-specific AR45 variant. Febs J. 2005;272:74–84. doi: 10.1111/j.1742-4658.2004.04395.x. [DOI] [PubMed] [Google Scholar]
- 89.Tabori NE, Stewart LS, Znamensky V, Romeo RD, Alves SE, McEwen BS, Milner TA. Ultrastructural evidence that androgen receptors are located at extranuclear sites in the rat hippocampal formation. Neuroscience. 2005;130:151–163. doi: 10.1016/j.neuroscience.2004.08.048. [DOI] [PubMed] [Google Scholar]
- 90.Pedram A, Razandi M, Sainson RC, Kim JK, Hughes CC, Levin ER. A conserved mechanism for steroid receptor translocation to the plasma membrane. J Biol Chem. 2007 doi: 10.1074/jbc.M611877200. [DOI] [PubMed] [Google Scholar]
- 91.Lambert JJ, Belelli D, Hill-Venning C, Peters JA. Neurosteroids and GABAA receptor function. Trends Pharmacol Sci. 1995;16:295–303. doi: 10.1016/s0165-6147(00)89058-6. [DOI] [PubMed] [Google Scholar]
- 92.Peters JA, Kirkness EF, Callachan H, Lambert JJ, Turner AJ. Modulation of the GABAA receptor by depressant barbiturates and pregnane steroids. Br J Pharmacol. 1988;94:1257–1269. doi: 10.1111/j.1476-5381.1988.tb11646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rahman M, Lindblad C, Johansson IM, Backstrom T, Wang MD. Neurosteroid modulation of recombinant rat alpha5beta2gamma2L and alpha1beta2gamma2L GABA(A) receptors in Xenopus oocyte. Eur J Pharmacol. 2006;547:37–44. doi: 10.1016/j.ejphar.2006.07.039. [DOI] [PubMed] [Google Scholar]
- 94.Frye CA, Duncan JE, Basham M, Erskine MS. Behavioral effects of 3 alpha-androstanediol. II: Hypothalamic and preoptic area actions via a GABAergic mechanism. Behav Brain Res. 1996;79:119–130. doi: 10.1016/0166-4328(96)00005-8. [DOI] [PubMed] [Google Scholar]
- 95.Frye CA, Van Keuren KR, Erskine MS. Behavioral effects of 3 alpha-androstanediol. I: Modulation of sexual receptivity and promotion of GABA-stimulated chloride flux. Behav Brain Res. 1996;79:109–118. doi: 10.1016/0166-4328(96)00004-6. [DOI] [PubMed] [Google Scholar]
- 96.Mellon SH. Neurosteroids: biochemistry, modes of action, and clinical relevance. J Clin Endocrinol Metab. 1994;78:1003–1008. doi: 10.1210/jcem.78.5.8175951. [DOI] [PubMed] [Google Scholar]
- 97.Irwin RP, Maragakis NJ, Rogawski MA, Purdy RH, Farb DH, Paul SM. Pregnenolone sulfate augments NMDA receptor mediated increases in intracellular Ca2+ in cultured rat hippocampal neurons. Neurosci Lett. 1992;141:30–34. doi: 10.1016/0304-3940(92)90327-4. [DOI] [PubMed] [Google Scholar]
- 98.Baulieu EE, Robel P. Neurosteroids: a new brain function? J Steroid Biochem Mol Biol. 1990;37:395–403. doi: 10.1016/0960-0760(90)90490-c. [DOI] [PubMed] [Google Scholar]
- 99.Wu FS, Gibbs TT, Farb DH. Pregnenolone sulfate: a positive allosteric modulator at the N-methyl-D-aspartate receptor. Mol Pharmacol. 1991;40:333–336. [PubMed] [Google Scholar]
- 100.Majewska MD, Mienville JM, Vicini S. Neurosteroid pregnenolone sulfate antagonizes electrophysiological responses to GABA in neurons. Neurosci Lett. 1988;90:279–284. doi: 10.1016/0304-3940(88)90202-9. [DOI] [PubMed] [Google Scholar]
- 101.ffrench-Mullen JM, Danks P, Spence KT. Neurosteroids modulate calcium currents in hippocampal CA1 neurons via a pertussis toxin-sensitive G-proteincoupled mechanism. J Neurosci. 1994;14:1963–1977. doi: 10.1523/JNEUROSCI.14-04-01963.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ffrench-Mullen JM, Spence KT. Neurosteroids block Ca2+ channel current in freshly isolated hippocampal CA1 neurons. Eur J Pharmacol. 1991;202:269–272. doi: 10.1016/0014-2999(91)90303-8. [DOI] [PubMed] [Google Scholar]
- 103.Kalra SP, Kalra PS. Do testosterone and estradiol-17 beta enforce inhibition or stimulation of luteinizing hormone-releasing hormone secretion? Biol Reprod. 1989;41:559–570. doi: 10.1095/biolreprod41.4.559. [DOI] [PubMed] [Google Scholar]
- 104.Levine JE, Bauer-Dantoin AC, Besecke LM, Conaghan LA, Legan SJ, Meredith JM, Strobl FJ, Urban JH, Vogelsong KM, Wolfe AM. Neuroendocrine regulation of the luteinizing hormone-releasing hormone pulse generator in the rat. Recent Prog Horm Res. 1991;47:97–151. doi: 10.1016/b978-0-12-571147-0.50008-1. discussion 151-3. [DOI] [PubMed] [Google Scholar]
- 105.Phelps CP, Kalra SP, Kalra PS. In vivo pulsatile LHRH release into the anterior pituitary of the male rat: effects of castration. Brain Res. 1992;569:159–163. doi: 10.1016/0006-8993(92)90384-l. [DOI] [PubMed] [Google Scholar]
- 106.Wiemann JN, Clifton DK, Steiner RA. Gonadotropin-releasing hormone messenger ribonucleic acid levels are unaltered with changes in the gonadal hormone milieu of the adult male rat. Endocrinology. 1990;127:523–532. doi: 10.1210/endo-127-2-523. [DOI] [PubMed] [Google Scholar]
- 107.Spratt DP, Herbison AE. Regulation of preoptic area gonadotrophin-releasing hormone (GnRH) mRNA expression by gonadal steroids in the long-term gonadectomized male rat. Brain Res Mol Brain Res. 1997;47:125–133. doi: 10.1016/s0169-328x(97)00037-5. [DOI] [PubMed] [Google Scholar]
- 108.Kalra PS. Further studies on the effects of testosterone on hypothalamic LH-RH and serum LH levels: castration-induced delayed response. Neuroendocrinology. 1985;41:219–223. doi: 10.1159/000124188. [DOI] [PubMed] [Google Scholar]
- 109.Cicero TJ, Schainker BA, Meyer ER. Endogenous opioids participate in the regulation of the hypothalamus-pituitary-luteinizing hormone axis and testosterone's negative feedback control of luteinizing hormone. Endocrinology. 1979;104:1286–1291. doi: 10.1210/endo-104-5-1286. [DOI] [PubMed] [Google Scholar]
- 110.Shin SH, Howitt CJ. Effect of testosterone on hypothalamic LH-RH content. Neuroendocrinology. 1976;21:165–174. doi: 10.1159/000122523. [DOI] [PubMed] [Google Scholar]
- 111.Kalra PS, Kalra SP. Steroidal modulation of the regulatory neuropeptides: luteinizing hormone releasing hormone, neuropeptide Y and endogenous opioid peptides. J Steroid Biochem. 1986;25:733–740. doi: 10.1016/0022-4731(86)90302-x. [DOI] [PubMed] [Google Scholar]
- 112.Steger RW, Bartke A. Neuroendocrine control of reproduction. Adv Exp Med Biol. 1995;377:15–32. doi: 10.1007/978-1-4899-0952-7_2. [DOI] [PubMed] [Google Scholar]
- 113.Kalra PS, Kalra SP. Discriminative effects of testosterone on hypothalamic luteinizing hormone-releasing hormone levels and luteinizing hormone secretion in castrated male rats: analyses of dose and duration characteristics. Endocrinology. 1982;111:24–29. doi: 10.1210/endo-111-1-24. [DOI] [PubMed] [Google Scholar]
- 114.Kalra PS, Simpkins JW, Kalra SP. Testosterone raises LHRH levels exclusively in the median eminence of castrated rats. Neuroendocrinology. 1984;39:45–48. doi: 10.1159/000123953. [DOI] [PubMed] [Google Scholar]
- 115.Roselli CE, Resko JA. Regulation of hypothalamic luteinizing hormone-releasing hormone levels by testosterone and estradiol in male rhesus monkeys. Brain Res. 1990;509:343–346. doi: 10.1016/0006-8993(90)90563-q. [DOI] [PubMed] [Google Scholar]
- 116.Van Vugt DA, Sylvester PW, Aylsworth CF, Meites J. Counteraction of gonadal steroid inhibition of luteinizing hormone release by naloxone. Neuroendocrinology. 1982;34:274–278. doi: 10.1159/000123312. [DOI] [PubMed] [Google Scholar]
- 117.Huang X, Harlan RE. Absence of androgen receptors in LHRH immunoreactive neurons. Brain Res. 1993;624:309–311. doi: 10.1016/0006-8993(93)90094-4. [DOI] [PubMed] [Google Scholar]
- 118.Herbison AE, Skinner DC, Robinson JE, King IS. Androgen receptor-immunoreactive cells in ram hypothalamus: distribution and co-localization patterns with gonadotropin-releasing hormone, somatostatin and tyrosine hydroxylase. Neuroendocrinology. 1996;63:120–131. doi: 10.1159/000126948. [DOI] [PubMed] [Google Scholar]
- 119.Belsham DD, Evangelou A, Roy D, Duc VL, Brown TJ. Regulation of gonadotropin-releasing hormone (GnRH) gene expression by 5alpha-dihydrotestosterone in GnRH-secreting GT1-7 hypothalamic neurons. Endocrinology. 1998;139:1108–1114. doi: 10.1210/endo.139.3.5846. [DOI] [PubMed] [Google Scholar]
- 120.Poletti A, Melcangi RC, Negri-Cesi P, Maggi R, Martini L. Steroid binding and metabolism in the luteinizing hormone-releasing hormone-producing neuronal cell line GT1-1. Endocrinology. 1994;135:2623–2628. doi: 10.1210/endo.135.6.7988451. [DOI] [PubMed] [Google Scholar]
- 121.Poletti A, Rampoldi A, Piccioni F, Volpi S, Simeoni S, Zanisi M, Martini L. 5Alpha-reductase type 2 and androgen receptor expression in gonadotropin releasing hormone GT1-1 cells. J Neuroendocrinol. 2001;13:353–357. doi: 10.1046/j.1365-2826.2001.00635.x. [DOI] [PubMed] [Google Scholar]
- 122.Shakil T, Hoque AN, Husain M, Belsham DD. Differential regulation of gonadotropin-releasing hormone secretion and gene expressin by androgen: membrane versus nuclear receptor activation. Mol Endocrinol. 2002;16:2592–2602. doi: 10.1210/me.2002-0011. [DOI] [PubMed] [Google Scholar]
- 123.Wetsel WC, Valenca MM, Merchenthaler I, Liposits Z, Lopez FJ, Weiner RI, Mellon PL, Negro-Vilar A. Intrinsic pulsatile secretory activity of immortalized luteinizing hormone-releasing hormone-secreting neurons. Proc Natl Acad Sci U S A. 1992;89:4149–4153. doi: 10.1073/pnas.89.9.4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Martinez de la Escalera G, Choi AL, Weiner RI. Generation and synchronization of gonadotropin-releasing hormone (GnRH) pulses: intrinsic properties of the GT1-1 GnRH neuronal cell line. Proc Natl Acad Sci U S A. 1992;89:1852–1855. doi: 10.1073/pnas.89.5.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Krsmanovic LZ, Stojilkovic SS, Merelli F, Dufour SM, Virmani MA, Catt KJ. Calcium signaling and episodic secretion of gonadotropin-releasing hormone in hypothalamic neurons. Proc Natl Acad Sci U S A. 1992;89:8462–8466. doi: 10.1073/pnas.89.18.8462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nunez L, Villalobos C, Boockfor FR, Frawley LS. The relationship between pulsatile secretion and calcium dynamics in single living gonadotropin-releasing hormone neurons. Endocrinology. 2000;141:2012–2017. doi: 10.1210/endo.141.6.7491. [DOI] [PubMed] [Google Scholar]
- 127.Frye CA. The role of neurosteroids and non-genomic effects of progestins and androgens in mediating sexual receptivity of rodents. Brain Res Brain Res Rev. 2001;37:201–222. doi: 10.1016/s0165-0173(01)00119-9. [DOI] [PubMed] [Google Scholar]
- 128.Erskine MS. Serum 5 alpha-androstane-3 alpha, 17 beta-diol increases in response to paced coital stimulation in cycling female rats. Biol Reprod. 1987;37:1139–1148. doi: 10.1095/biolreprod37.5.1139. [DOI] [PubMed] [Google Scholar]
- 129.Jorge-Rivera JC, McIntyre KL, Henderson LP. Anabolic steroids induce region- and subunit-specific rapid modulation of GABA(A) receptor-mediated currents in the rat forebrain. J Neurophysiol. 2000;83:3299–3309. doi: 10.1152/jn.2000.83.6.3299. [DOI] [PubMed] [Google Scholar]
- 130.Baum MJ, Sodersten P, Vreeburg JT. Mounting and receptive behavior in the ovariectomized female rat: influence of estradiol, dihydrotestosterone, and genital anesthetization. Horm Behav. 1974;5:175–190. doi: 10.1016/0018-506x(74)90042-7. [DOI] [PubMed] [Google Scholar]
- 131.Baum MJ, Vreeburg JT. Differential effects of the anti-estrogen MER-25 and of three 5alpha-reduced androgens on mounting and lordosis behavior in the rat. Horm Behav. 1976;7:87–104. doi: 10.1016/0018-506x(76)90007-6. [DOI] [PubMed] [Google Scholar]
- 132.DeBold JF, Morris JL, Clemens LG. The inhibitory actions of progesterone: effects on male and female sexual behavior of the hamster. Horm Behav. 1978;11:28–41. doi: 10.1016/0018-506x(78)90056-9. [DOI] [PubMed] [Google Scholar]
- 133.Dohanich GP, Clemens LG. Inhibition of estrogen-activated sexual behavior by androgens. Horm Behav. 1983;17:366–373. doi: 10.1016/0018-506x(83)90046-6. [DOI] [PubMed] [Google Scholar]
- 134.Erskine MS. Effects of an anti-androgen and 5 alpha-reductase inhibitors on estrus duration in the cycling female rat. Physiol Behav. 1983;30:519–524. doi: 10.1016/0031-9384(83)90214-7. [DOI] [PubMed] [Google Scholar]
- 135.Erskine MS. Effect of 5 alpha-dihydrotestosterone and flutamide on the facilitation of lordosis by LHRH and naloxone in estrogen-primed female rats. Physiol Behav. 1989;45:753–759. doi: 10.1016/0031-9384(89)90290-4. [DOI] [PubMed] [Google Scholar]
- 136.Erskine MS, MacLusky NJ, Baum MJ. Effect of 5 alpha-dihydrotestosterone on sexual receptivity and neural progestin receptors in ovariectomized rats given pulsed estradiol. Biol Reprod. 1985;33:551–559. doi: 10.1095/biolreprod33.3.551. [DOI] [PubMed] [Google Scholar]
- 137.Luttge WG, Jasper TW, Gray HE, Sheets CS. Estrogen-induced sexual receptivity and localization of 3H-estradiol in brains of female mice: effects of 5 alpha-reduced androgens, progestins and cyproterone acetate. Pharmacol Biochem Behav. 1977;6:521–528. doi: 10.1016/0091-3057(77)90111-3. [DOI] [PubMed] [Google Scholar]
- 138.Van Doorn EJ, Burns B, Wood D, Bird CE, Clark AF. In vivo metabolism of 3H-dihydrotestosterone and 3H-androstanediol in adult male rats. J Steroid Biochem. 1975;6:1549–1554. doi: 10.1016/0022-4731(75)90213-7. [DOI] [PubMed] [Google Scholar]
- 139.Denef C, Magnus C, McEwen BS. Sex differences and hormonal control of testosterone metabolism in rat pituitary and brain. J Endocrinol. 1973;59:605–621. doi: 10.1677/joe.0.0590605. [DOI] [PubMed] [Google Scholar]
- 140.Denef C, Magnus C, McEwen BS. Sex-dependent changes in pituitary 5alpha-dihydrotestosterone and 3alpha-androstanediol formation during postnatal development and puberty in the rat. Endocrinology. 1974;94:1265–1274. doi: 10.1210/endo-94-5-1265. [DOI] [PubMed] [Google Scholar]
- 141.Deviche P, Delville Y, Balthazart J. Central and peripheral metabolism of 5 alpha-dihydrotestosterone in the male Japanese quail: biochemical characterization and relationship with reproductive behavior. Brain Res. 1987;421:105–116. doi: 10.1016/0006-8993(87)91280-7. [DOI] [PubMed] [Google Scholar]
- 142.Mani SK, Fienberg AA, O'Callaghan JP, Snyder GL, Allen PB, Dash PK, Moore AN, Mitchell AJ, Bibb J, Greengard P, O'Malley BW. Requirement for DARPP-32 in progesterone-facilitated sexual receptivity in female rats and mice. Science. 2000;287:1053–1056. doi: 10.1126/science.287.5455.1053. [DOI] [PubMed] [Google Scholar]
- 143.Pilven A, Thieulant ML, Ducouret B, Samperez S, Jouan P. Rapid and intensive conversion of 5alpha-androstane-3alpha, 17beta-diol into 5alpha-dihydrotestosterone in the male rat anterior pituitary: in vivo and in vitro studies. Steroids. 1976;28:349–358. doi: 10.1016/0039-128x(76)90045-3. [DOI] [PubMed] [Google Scholar]
- 144.Martini L, Melcangi RC, Maggi R. Androgen and progesterone metabolism in the central and peripheral nervous system. J Steroid Biochem Mol Biol. 1993;47:195–205. doi: 10.1016/0960-0760(93)90075-8. [DOI] [PubMed] [Google Scholar]
- 145.Melcangi RC, Celotti F, Castano P, Martini L. Differential localization of the 5 alpha-reductase and the 3 alpha-hydroxysteroid dehydrogenase in neuronal and glial cultures. Endocrinology. 1993;132:1252–1259. doi: 10.1210/endo.132.3.8440186. [DOI] [PubMed] [Google Scholar]
- 146.Melcangi RC, Celotti F, Martini L. Progesterone 5-alpha-reduction in neuronal and in different types of glial cell cultures: type 1 and 2 astrocytes and oligodendrocytes. Brain Res. 1994;639:202–206. doi: 10.1016/0006-8993(94)91731-0. [DOI] [PubMed] [Google Scholar]
- 147.Erskine MS, Hippensteil M, Kornberg E. Metabolism of dihydrotestosterone to 3 alpha-androstanediol in brain and plasma: effect on behavioural activity in female rats. J Endocrinol. 1992;134:183–195. doi: 10.1677/joe.0.1340183. [DOI] [PubMed] [Google Scholar]
- 148.Perez-Acevedo NL, Lathroum L, Jorge JC. The Neurosteroid 3αDIOL Modulates Place Preference When Infused in the Basolateral Amygdala According to Sex. Behav Neurosci. 2006;120:632–640. doi: 10.1037/0735-7044.120.3.632. [DOI] [PubMed] [Google Scholar]
- 149.Jorge JC, Velazquez KT, Ramos-Ortolaza DL, Lorenzini I, Marrero J, Maldonado-Vlaar CS. A testosterone metabolite is rewarding to ovariectomized female rats. Behav Neurosci. 2005;119:1222–1226. doi: 10.1037/0735-7044.119.5.1222. [DOI] [PubMed] [Google Scholar]
- 150.Frye CA. Some rewarding effects of androgens may be mediated by actions of its 5alpha-reduced metabolite 3alpha-androstanediol. Pharmacol Biochem Behav. 2007;86:354–367. doi: 10.1016/j.pbb.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kubli-Garfias C, Canchola E, Arauz-Contreras J, Feria-Velasco A. Depressant effect of androgens on the cat brain electrical activity and its antagonism by ruthenium red. Neuroscience. 1982;7:2777–2782. doi: 10.1016/0306-4522(82)90100-2. [DOI] [PubMed] [Google Scholar]
- 152.Frye CA, Van Keuren KR, Rao PN, Erskine MS. Progesterone and 3 alpha-androstanediol conjugated to bovine serum albumin affects estrous behavior when applied to the MBH and POA. Behav Neurosci. 1996;110:603–612. doi: 10.1037//0735-7044.110.3.603. [DOI] [PubMed] [Google Scholar]
- 153.Belelli D, Lan NC, Gee KW. Anticonvulsant steroids and the GABA/benzodiazepine receptor-chloride ionophore complex. Neurosci Biobehav Rev. 1990;14:315–322. doi: 10.1016/s0149-7634(05)80041-7. [DOI] [PubMed] [Google Scholar]
- 154.Beyer C, Gonzalez-Mariscal G, Eguibar JR, Gomora P. Lordosis facilitation in estrogen primed rats by intrabrain injection of pregnanes. Pharmacol Biochem Behav. 1988;31:919–926. doi: 10.1016/0091-3057(88)90405-4. [DOI] [PubMed] [Google Scholar]
- 155.Remage-Healey L, Bass AH. Rapid, hierarchical modulation of vocal patterning by steroid hormones. J Neurosci. 2004;24:5892–5900. doi: 10.1523/JNEUROSCI.1220-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Remage-Healey L, Bass AH. Rapid elevations in both steroid hormones and vocal signaling during playback challenge: a field experiment in Gulf toadfish. Horm Behav. 2005;47:297–305. doi: 10.1016/j.yhbeh.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 157.Remage-Healey L, Bass AH. A rapid neuromodulatory role for steroid hormones in the control of reproductive behavior. Brain Res. 2006;1126:27–35. doi: 10.1016/j.brainres.2006.06.049. [DOI] [PubMed] [Google Scholar]
- 158.Remage-Healey L, Bass AH. From social behavior to neural circuitry: steroid hormones rapidly modulate advertisement calling via a vocal pattern generator. Horm Behav. 2006;50:432–441. doi: 10.1016/j.yhbeh.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 159.Remage-Healey L, Bass AH. Plasticity in brain sexuality is revealed by the rapid actions of steroid hormones. J Neurosci. 2007;27:1114–1122. doi: 10.1523/JNEUROSCI.4282-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ramsden M, Shin TM, Pike CJ. Androgens modulate neuronal vulnerability to kainate lesion. Neuroscience. 2003;122:573–578. doi: 10.1016/j.neuroscience.2003.08.048. [DOI] [PubMed] [Google Scholar]
- 162.Ramsden M, Nyborg AC, Murphy MP, Chang L, Stanczyk FZ, Golde TE, Pike CJ. Androgens modulate beta-amyloid levels in male rat brain. J Neurochem. 2003;87:1052–1055. doi: 10.1046/j.1471-4159.2003.02114.x. [DOI] [PubMed] [Google Scholar]
- 163.Nguyen TV, Yao M, Pike CJ. Androgens activate mitogen-activated protein kinase signaling: role in neuroprotection. J Neurochem. 2005;94:1639–1651. doi: 10.1111/j.1471-4159.2005.03318.x. [DOI] [PubMed] [Google Scholar]
- 164.Hammond J, Le Q, Goodyer C, Gelfand M, Trifiro M, LeBlanc A. Testosterone-mediated neuroprotection through the androgen receptor in human primary neurons. J Neurochem. 2001;77:1319–1326. doi: 10.1046/j.1471-4159.2001.00345.x. [DOI] [PubMed] [Google Scholar]
- 165.Estrada M, Varshney A, Ehrlich BE. Elevated testosterone induces apoptosis in neuronal cells. J Biol Chem. 2006;281:25492–25501. doi: 10.1074/jbc.M603193200. [DOI] [PubMed] [Google Scholar]
- 166.Yang SH, Perez E, Cutright J, Liu R, He Z, Day AL, Simpkins JW. Testosterone increases neurotoxicity of glutamate in vitro and ischemia-reperfusion injury in an animal model. J Appl Physiol. 2002;92:195–201. doi: 10.1152/jappl.2002.92.1.195. [DOI] [PubMed] [Google Scholar]
- 167.Yang SH, Liu R, Wen Y, Perez E, Cutright J, Brun-Zinkernagel AM, Singh M, Day AL, Simpkins JW. Neuroendocrine mechanism for tolerance to cerebral ischemia-reperfusion injury in male rats. J Neurobiol. 2005;62:341–351. doi: 10.1002/neu.20103. [DOI] [PubMed] [Google Scholar]
- 168.Caruso A, Di Giorgi Gerevini V, Castiglione M, Marinelli F, Tomassini V, Pozzilli C, Caricasole A, Bruno V, Caciagli F, Moretti A, Nicoletti F, Melchiorri D. Testosterone amplifies excitotoxic damage of cultured oligodendrocytes. J Neurochem. 2004;88:1179–1185. doi: 10.1046/j.1471-4159.2004.02284.x. [DOI] [PubMed] [Google Scholar]
- 169.Kampa M, Nifli AP, Charalampopoulos I, Alexaki VI, Theodoropoulos PA, Stathopoulos EN, Gravanis A, Castanas E. Opposing effects of estradiol-and testosterone-membrane binding sites on T47D breast cancer cell apoptosis. Exp Cell Res. 2005;307:41–51. doi: 10.1016/j.yexcr.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 170.Gatson JW, Kaur P, Singh M. Dihydrotestosterone differentially modulates the mitogen-activated protein kinase and the phosphoinositide 3-kinase/Akt pathways through the nuclear and novel membrane androgen receptor in C6 cells. Endocrinology. 2006;147:2028–2034. doi: 10.1210/en.2005-1395. [DOI] [PubMed] [Google Scholar]
- 171.Gatson JW, Singh M. Activation of a membrane-associated androgen receptor promotes cell death in primary cortical astrocytes. Endocrinology. 2007;148:2458–2464. doi: 10.1210/en.2006-1443. [DOI] [PubMed] [Google Scholar]


