Abstract
Mortality from sepsis has remained high despite recent advances in supportive and targeted therapies. Toll-like receptors (TLRs) sense bacterial products and stimulate pathogenic innate immune responses. Mice deficient in the common adapter protein MyD88, downstream from most TLRs, have reduced mortality and acute kidney injury (AKI) from polymicrobial sepsis. However, the identity of the TLR(s) responsible for the host response to polymicrobial sepsis is unknown. Here, we show that chloroquine, an inhibitor of endocytic TLRs (TLR3, 7, 8, 9), improves sepsis-induced mortality and acute kidney injury in a clinically relevant polymicrobial sepsis mouse model, even when administered 6h after the septic insult. Chloroquine administration attenuated the decline in renal function, splenic apoptosis, serum markers of damage to other organs, and prototypical serum pro- and anti-inflammatory cytokines TNF-alpha and IL-10. An oligodeoxynucleotide inhibitor (H154) of TLR9 and TLR9-deficient mice mirror the actions of chloroquine in all functional parameters that we tested. In addition, chloroquine decreased TLR9 protein abundance in spleen, further suggesting that TLR9 signaling may be a major target for the protective actions of chloroquine. Our findings indicate that chloroquine improves survival by inhibiting multiple pathways leading to polymicrobial sepsis, and that chloroquine and TLR9 inhibitors represent viable broad-spectrum and targeted therapeutic strategies, respectively, that are promising candidates for further clinical development.
Keywords: chloroquine, TLR9, sepsis, acute kidney injury
Introduction
Despite advances in supportive care and specific therapeutic interventions, the mortality rate of sepsis is still unacceptably high. Death frequently follows the development of multiple organ failure (MOF) (42, 47). Invasion of bacteria elicits a complex interplay of local and systemic responses that seek to control the infection. The innate immune system recognizes the presence of invading bacteria and bacterial products, and initiates a local host response by releasing cytokines that recruit inflammatory cells, enhance bacterial clearance, and prime adaptive immune cells to generate pathogen-specific antibodies (27). But if the infection worsens, the local host response becomes amplified, resulting in a systemic “counter-regulatory” anti-inflammatory response syndrome (CARS) that aids local recruitment of inflammatory cells and prevents it from spreading systemically. However, over time, this can lead to immune paralysis (29, 35) as evidenced by impaired leukocyte, T and B cell function, bacterial clearance (“hypo-responsiveness”) and lymphocyte apoptosis in GI tract, lymph nodes, and spleen (7, 9, 20, 23, 25) that together is thought to be a major contributor to the morbidity and mortality of sepsis (8). Indeed, blockade of pro-inflammatory pathways has not been effective clinically, and may have increased mortality in some subpopulations. This immunosuppressive environment increases the susceptibility to subsequent sepsis episodes and other infections (45), and may account for the high mortality rate of sepsis.
Innate immunity is triggered by toll-like receptors (TLRs), which recognize invasion of microbial pathogens and initiate the immune response. Most TLR signals are activated via MyD88, which is an essential downstream adaptor molecule that activates signaling pathways such as mitogen-activated protein kinases and NF-kappaB with subsequent induction of cytokines. Weighardt et al. demonstrated that MyD88-deficient mice were resistant to polymicrobial sepsis produced by a colon ascendens stent peritonitis (CASP), although TLR2 and TLR4-deficient mice were not resistant (44). We have recently shown that a cecal ligation and puncture (CLP)-induced AKI was attenuated in MyD88-deficient mice but not in TLR4-deficient mice (11).
The Toll-like receptors that recognize bacterial and viral nucleic acids (3, 7, 8, and 9) are found in the endosomal compartment (4, 26) and appear to be trafficking between endosomes and lysosomes (1, 14, 29). When trafficking and/or acidification is disrupted by chloroquine or bafilomycin A1, Toll-like receptor signaling is inhibited (1, 14, 30, 32, 37). In addition to these studies in vitro, a few reports suggest that chloroquine can inhibit innate immune responses in vivo: in a 2-hit model of hemorrhage then CLP (13), after CpG/LPS administration (18), and in a mouse cryptococcosis infection model (31).
We sought to determine the therapeutic potential of the clinically well-tolerated TLR inhibitor chloroquine, in CLP-induced poly-microbial sepsis in elderly mice treated with fluid and antibiotics, a clinically relevant model of sepsis and sepsis-induced AKI. Further, we examined TLR9 as a potential target for chloroquine action by using an oligodeoxynucleotide TLR9 inhibitor H154 and TLR9-deficient mice.
Materials and Methods
Animals
Animal care followed the National Institutes of Health (NIH) criteria for the care and use of laboratory animals in research. C57BL/6 mice (32 – 40 weeks of age) were obtained from NIH (Frederick, MD, USA). TLR9-deficient mice were obtained from S. Akira (Osaka University, Osaka, Japan) (17). The TLR9-deficient mice were backcrossed with C57BL/6 mice for >8 generations before TLR9-deficient mice were reestablished.
Surgery
The CLP procedure used for induction of septic peritonitis was described in detail previously (49).
Drug treatments
Chloroquine (50 mg/kg; Sigma-Aldrich Inc., St. Louis, MO, USA) or an equal volume of saline was administered orally at either 3 hr before or 6 hr after CLP surgery.
Phosphorothioate-containing oligodeoxynucleotides were synthesized as described previously (40, 48) and administered (3 mg/kg) i.p. immediately after cecal ligation puncture surgery. A suppressive ODN H154 (5′-CCTCAAGCTTGAGGGG-3′) acts specifically through TLR9 (48), whereas a control suppressive ODN A151 (5′-TTAGGGTTAGGGTTAGGGTTAGGG -3′) does not act through TLR9 (40).
Survival study
Survival was assessed every ∼6-12 hr after surgery. Antibiotic injection and fluid resuscitation were started 6 hr after surgery by subcutaneous injection, and then repeated every 12 hr for 4 days. Animals exhibiting extreme morbidity were euthanized.
Renal pathology
Tissue was fixed in 10% formalin and embedded in paraffin. 4 μm sections were stained with periodic acid-Schiff (PAS) reagent. Tubular damage was assessed by counting vacuolized tubules at 400X magnification using >100 randomly selected tubules from each animal.
Splenic apoptosis
Active caspase 3 staining, a marker of splenic apoptosis, was examined in > 5 randomly chosen 400X fields of white pulp and expressed as positive cells per high power field (HPF) as described previously (11).
Measurements BUN, serum creatinine and cytokines
BUN and serum creatinine were measured as previously described (34, 50). Serum TNF-alpha and IL-10 were determined by ELISA (R&D Systems, Minneapolis, MN, USA).
Statistical analysis
All the data are expressed as mean ± SE. Differences between groups were examined for statistical significance by analysis of variance (ANOVA) with a multiple comparison correction or t-test (Prism 4, GraphPad Software, San Diego, CA, USA, or SigmaStat, Systat Software, Point Richmond, CA). A P value < 0.05 was accepted as statistically significant.
Results
Chloroquine improves survival and acute kidney injury after polymicrobial sepsis
We determined whether chloroquine alters sepsis-induced mortality and renal dysfunction in aged mice treated with fluid and antibiotics. Chloroquine given 3 hr before CLP surgery significantly improved survival: At 96 hr after CLP, the survival was 15% for mice treated with vehicle, and 69% for mice treated with chloroquine (Fig. 1A). Delaying chloroquine administration to start at 6 hr after CLP, when clinical symptoms first appear, also improved survival; at 96 hr after CLP, the survival was 24% for mice treated with vehicle, and 55% for mice treated with chloroquine (Fig. 1B). These results suggest that chloroquine can function both as a preventative and as a therapeutic agent in polymicrobial sepsis.
Figure 1. Chloroquine decreases mortality after polymicrobial sepsis, even when administered after 6 hr.
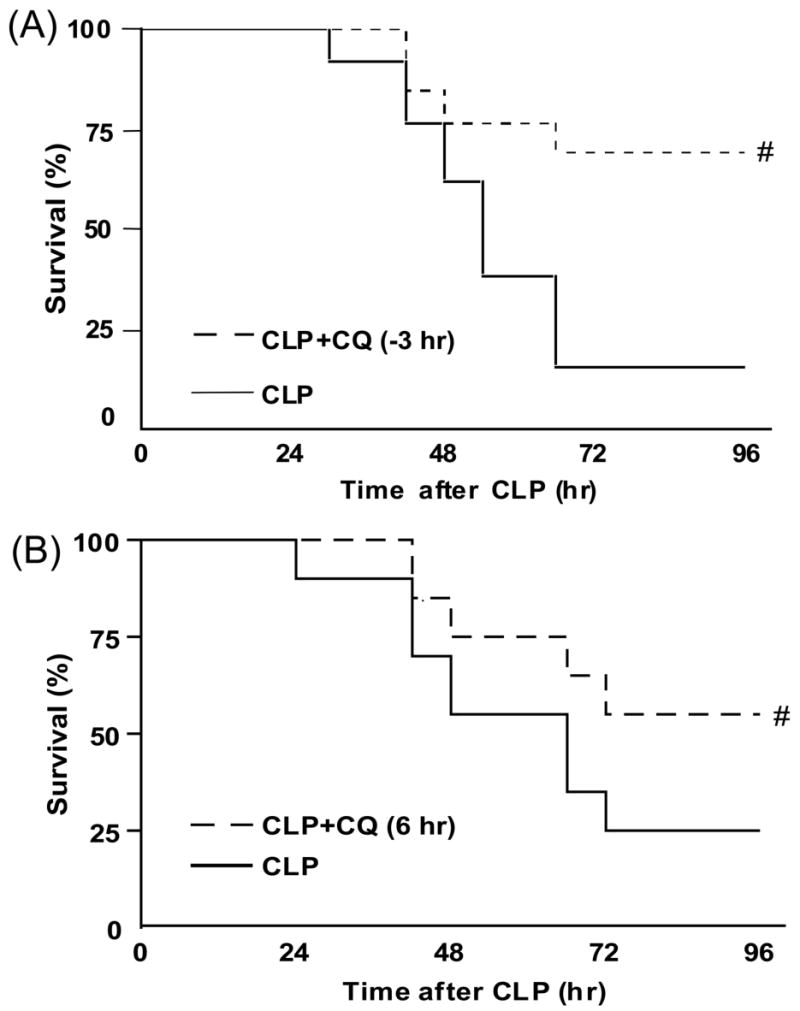
(A) Survival curves from mice given vehicle (dashed line, N = 13) or chloroquine (50 mg/kg, p.o.) 3 hr before CLP surgery (solid line, N = 13). (B) Survival curves from mice given vehicle (dashed line, N = 20) or chloroquine (50 mg/kg, p.o.) 6 hr after CLP surgery (solid line, N = 20). #, P < 0.05 vs. CLP plus vehicle.
Chloroquine administration, either 3 hr before or 6 hr after CLP, significantly improved both sepsis-induced increases in serum creatinine and BUN (Fig. 2A) and tubular damage in both the outer stripe of the outer medulla and the cortex (Fig. 2B, Supplement Fig. 1A,B). To assess injury to other organs, ALT, AST, amylase, CK, and LDH were measured in serum. As previously reported (34), all of these enzymes were significantly elevated 24 hr after CLP. Treatment with chloroquine 3 hr before CLP resulted in a significant decrease in each of these parameters. Treatment with chloroquine 6 hr after CLP also improved each parameter; however, only ALT was significantly decreased (Fig 3).
Figure 2. Chloroquine improves renal dysfunction, and tubular damage from polymicrobial sepsis.
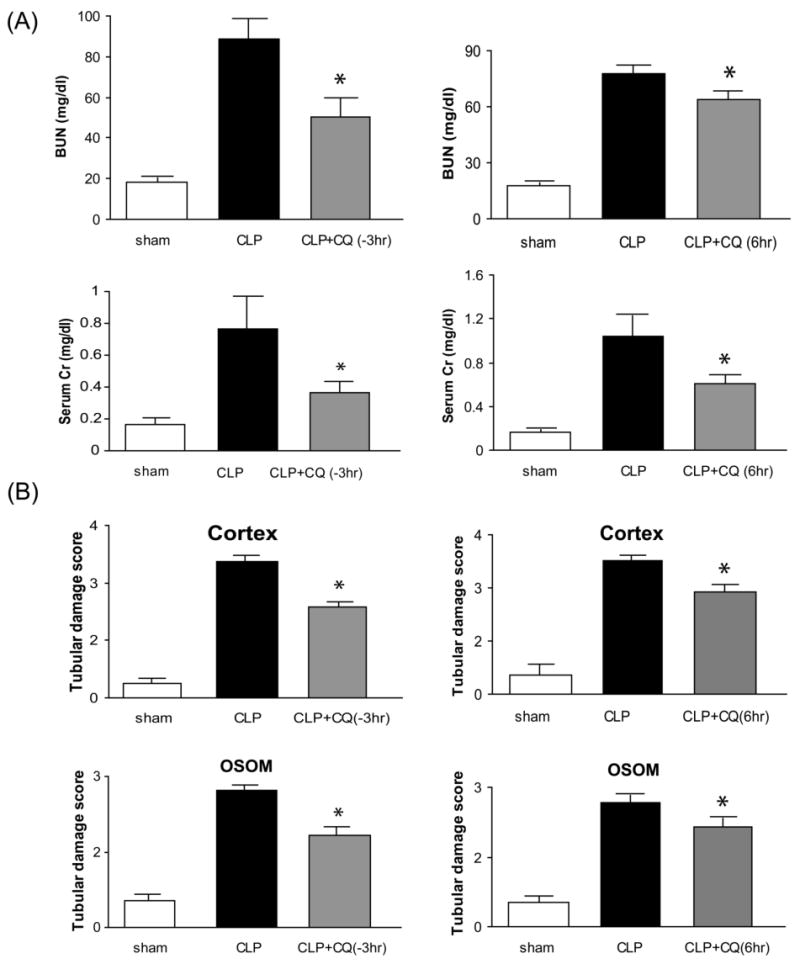
(A) Blood urea nitrogen and serum creatinine as an index of kidney function at 24 hr after sham surgery (white bar, N = 6), CLP surgery plus vehicle (black bar, N = 8-11), chloroquine 3 hr before CLP (gray bar, N = 11, left panel), or chloroquine 6 hr after CLP (gray bar, N = 8, right panel). (B); original magnification, 400X. (C) Kidney histology (periodic acid-Schiff stain) at 24 hr after surgery was scored (see Methods) for tubules in the renal cortex and outer stripe of the outer medulla (OSOM) at 24 hr after surgery (N = 5-6 per group). Values are mean ± SE. *, P < 0.05 vs. CLP plus vehicle.
Figure 3. Chloroquine inhibits multiple organ damage after polymicrobial sepsis.
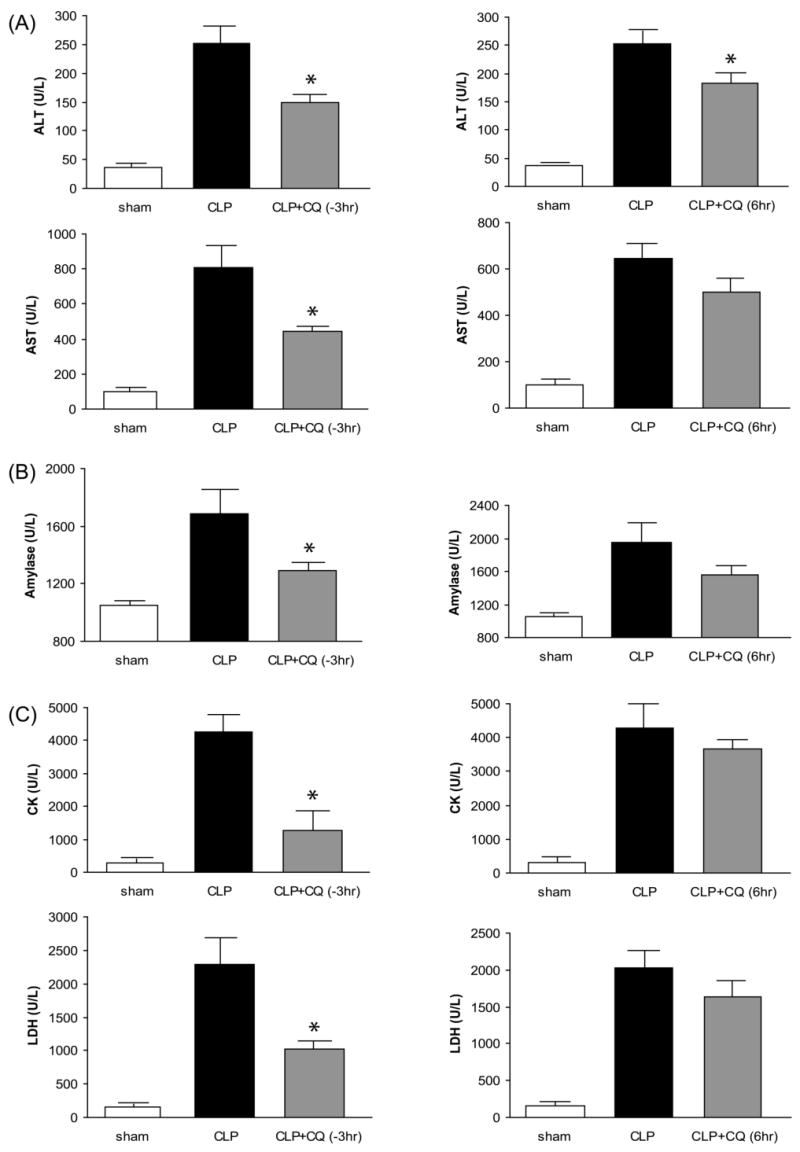
Serum chemistry 24 hr after sham surgery (white bars, N = 5), CLP (black bars, N = 6-9), or CLP after chloroquine treatment (gray bars, N = 6-9). Chloroquine treatment 3 hr before CLP is shown on the left and chloroquine treatment 6 hr after CLP is shown on the right. (A) alanine aminotransferase (ALT) and aspartate aminotransferase (AST) (B) amylase (C) creatine kinase (CK) and lactate dehydrogenase (LDH). Values are mean ± SE. *, P < 0.05 vs. CLP given vehicle.
Chloroquine administration reduces splenic apoptosis after polymicrobial sepsis
Apoptosis of splenocytes has been shown to worsen the outcome of sepsis and contribute to immune depression (19, 38, 46). Therefore we evaluated the effects of chloroquine administration on splenic apoptosis. Splenic apoptosis, as detected by active caspase3, occurred mostly in the white pulp. CLP-induced sepsis profoundly increased the number of active caspase3 positive cells in the white pulp of the spleen at 24 hr after CLP (Fig. 4A, Supplement Fig. 2), as previously described (11). Chloroquine, administered either 3 hr before or 6 hr after CLP, significantly decreased the number of active caspase3 positive cells in the spleen at 24 hr after CLP (Fig. 4A, Supplement Fig. 2). Chloroquine also significantly reduced bacterial counts in blood, but had no effect on bacterial counts in peritoneal fluid (Supplement Fig. 3).
Figure 4. Chloroquine inhibits spleen apoptosis and serum cytokines after polymicrobial sepsis.
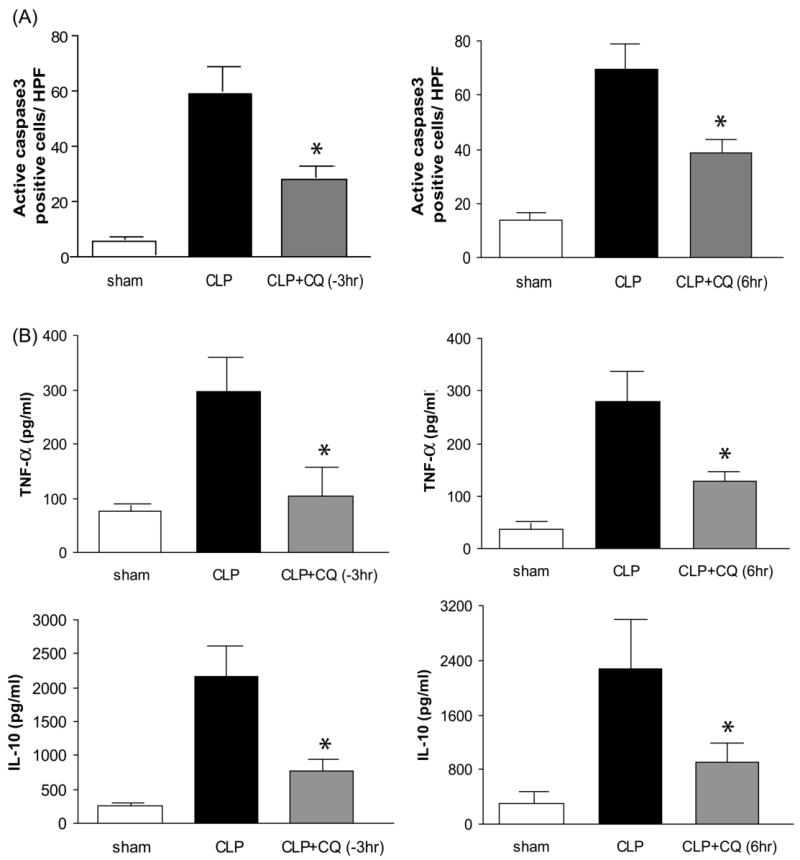
(A) Number of active caspase 3 positive cells in the spleen at 24 hr after CLP. (N = 5 per group). (B) Serum TNF-alpha and IL-10 at 24 hr after CLP (N = 6-12 per group). Values are mean ± SE. *, P < 0.05 vs. CLP given vehicle.
Effect of TLR9 deficiency and inhibition
Because of its sensitivity to chloroquine and its predominant expression in the spleen, TLR9 is a good candidate for mediating the beneficial effects of chloroquine. We used TLR9-deficient mice to determine whether the absence of TLR9 could mimic the effects of chloroquine. Survival at 96 hr after CLP was significantly improved from 23% in wild-type mice to 70% in TLR9-deficient mice (Fig. 5A). TLR9-deficient mice also had significantly reduced BUN and serum creatinine levels 24 hr after CLP compared to wild-type mice (Fig. 5C). Splenic apoptosis, as measured by active caspase3 staining was also significantly decreased in TLR9-deficient mice compared to wild-type mice (Fig. 5B, Supplement Fig. 4). Increases in proinflammatory and anti-inflammatory cytokines TNF-alpha and IL-10, respectively, were blunted in TLR9-deficient mice compared to wild-type mice (Fig. 5E).
Figure 5. TLR9 deficiency improves survival, acute kidney injury, splenic apoptosis, and circulating cytokines after polymicrobial sepsis.
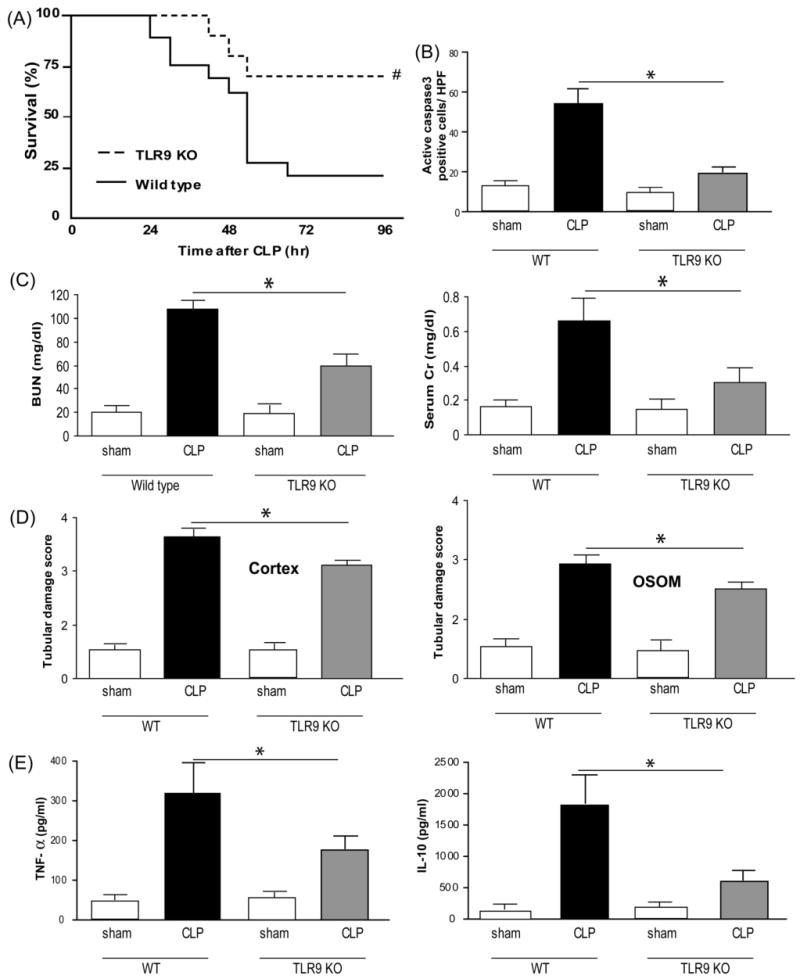
(A) Survival curves after CLP for wild-type (dashed line, N = 19) and TLR9-deficient (solid line, N = 16) mice. #, P < 0.05 vs wild-type. Mice were subjected to sham surgery (white bars) or CLP in wild-type (black bars) or TLR9-deficient (gray bars) mice. (B) Number of active caspase3 positive cells in the spleen 24 hr after sham surgery (wild-type, N = 4; TLR9-deficient, N = 4) or CLP (wild-type, N = 7; TLR9-deficient, N = 5). (C) Kidney function 24 hr after sham surgery (wild-type, N = 4; TLR9-deficient, N = 4) or CLP in wild-type (N = 10) and TLR9-deficient (N = 11) mice. (D) Kidney histology scores at 24 hr after surgery (see Methods) for tubules in the renal cortex and outer stripe of the outer medulla (OSOM) at 24 hr after surgery (N = 4-6 per group). (E) Serum TNF-alpha and IL-10 at 24 hr after CLP (N = 6-11 per group). Values are mean ± SE. *, P < 0.05 vs. wild-type.
To complement the studies on TLR9-deficient mice, we used a selective TLR9 oligodeoxynucleotide inhibitor H154 (28) and a non-inhibitory oligodeoxynucleotide A151. H154, but not A151, significantly inhibited the increase of BUN and serum creatinine after CLP (Fig. 6A), significantly inhibited the increase in serum AST, ALT, amylase (not significant), CK, and LDH (Fig. 6B,C), significantly inhibited the increase in splenic active caspase3 staining (Fig. 6D, Supplement Fig. 5), and significantly inhibited the increase in TNF-alpha and IL-10 (Fig. 6E), confirming the results from TLR9-deficient mice. By all of these parameters, TLR9 deficiency mirrored chloroquine treatment, implicating TLR9 as a substantial target that may account for much of the protective effects of chloroquine. However, H154 did not significantly reduce bacterial counts in blood (Supplement Fig. 3), which may reflect a TLR9-independent component of chloroquine action on immune function.
Figure 6. TLR9 inhibition by phosphorothioate oligodeoxynucleotides improves acute kidney injury, multiple organ damage, and serum cytokines.
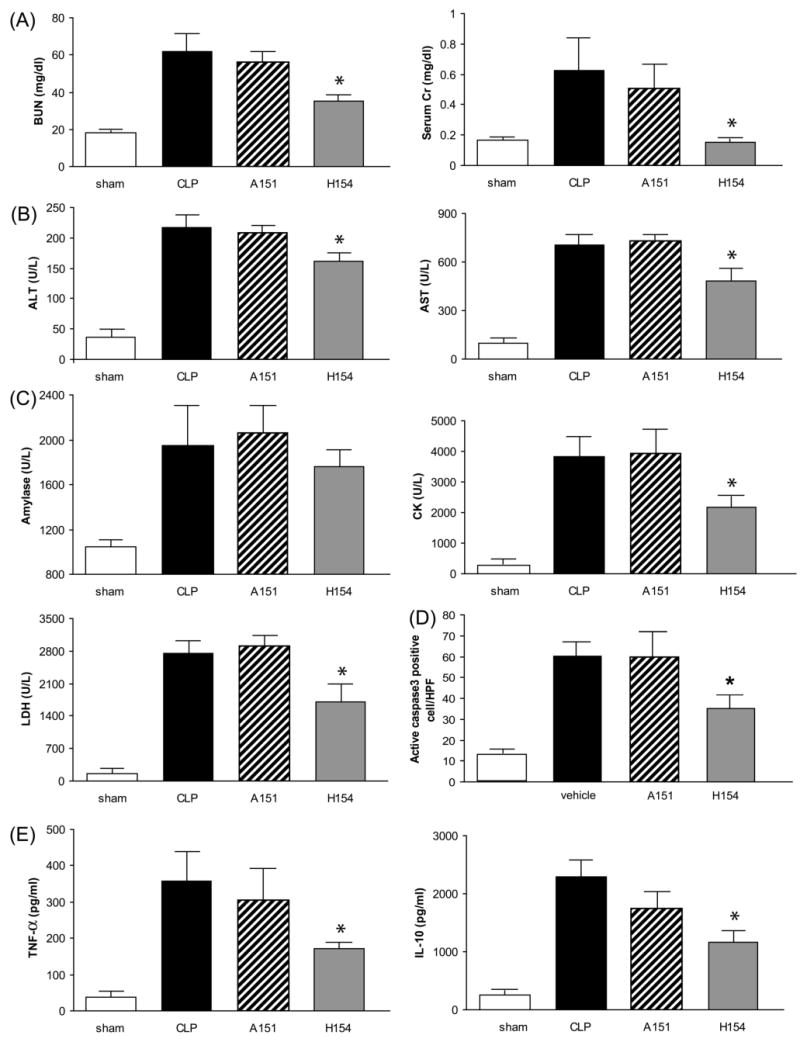
Mice were subjected to sham surgery (white bars, N = 5) or CLP (black bars, N = 8), and CLP was treated with control (A151, hatched bars, N = 8) or TLR9-selective (H154, gray bars, N = 8) phosphorothioate oligodeoxynucleotides, and evaluated at 24 hr for (A) kidney function (B) ALT, AST (C) amylase, CK, LDH (D) Number of active caspase3 positive cells in the spleen (E) TNF-alpha and IL-10. Values are mean ± SE. *, P < 0.05 vs. wild-type.
Finally, we examined the effect of chloroquine and TLR9 inhibition on TLR9 protein abundance. In SLE patients and a SLE animal model, TLR9 expression in renal tubules was upregulated (5), but we were unable to detect TLR9 in kidney after CLP (data not shown). By western blot both oligonucleotides induced moderate increases in splenic TLR9. Chloroquine, administered either 3 hr before or 6 hr after CLP, decreased splenic TLR9 to undetectable levels (Supplement Fig. 6). This provides a mechanism by which chloroquine could interfere with TLR9 signaling.
Discussion
In a clinically relevant model of polymicrobial sepsis, we found that (a) chloroquine improves mortality and reduces renal injury, even when delayed for 6 hr; (b) chloroquine reduces systemic inflammation and multiple organ damage, but perhaps more critically, improves splenic apoptosis, suggestive of a mechanism that includes reducing immune paralysis, (c) TLR9 deficiency or TLR9 inhibition has the same effect as chloroquine, making TLR9 a likely target for the actions of chloroquine. These findings are discussed below.
Chloroquine has preventative and therapeutic actions
Chloroquine improved survival, renal injury, and renal function, whether administered 3 hr prior to surgery or 6 hr after surgery, when the animals first become symptomatic. The degree of protection is similar to that seen with ethyl pyruvate (34, 41) or simvastatin (49). Chloroquine has also been shown to improve survival after CpG/LPS administration(18), in a 2 hit model of hemorrhage followed by CLP (13), and in a mouse cryptococcosis infection model(31). Hence, chloroquine might be useful for treating newly diagnosed sepsis, rather than just as a preventative agent.
Effect of chloroquine on kidney function
Chloroquine treatment either 3 hours prior to or 6 hours after CLP had a significant benefit on serum creatinine, BUN, and both cortical and OSOM histology scores. As chloroquine can have diuretic and natriuretic effects under normal conditions (3, 36), it is not known whether these phenomena contribute to the protective effect of chloroquine on kidney function during sepsis; we also cannot rule out an effect of chloroquine on sepsis-associated hypotension. We used serum enzyme markers as surrogates for multiple organ damage, as well as examined splenic apoptosis and reduction of circulating bacteria as surrogates for immune paralysis to assess whether chloroquine simply decreased the overall severity of sepsis, or whether there was any selectivity toward the kidney. Chloroquine treatment 3 hours prior to CLP showed a broad protective effect over every marker, including renal and splenic damage. However, chloroquine administration 6 hours after CLP, a more clinically relevant treatment, resulted in significant decreases in kidney and spleen injury markers, and ALT— but not AST, amylase, CK, or LDH. The differences between preventative (-3 hr) vs therapeutic (6 hr) chloroquine on organ function are not consistent with prevention causing a uniform but more intense dampening of the overall extent of sepsis vs. delayed chloroquine. While not conclusive, the tighter association between mortality and both kidney and splenic injury (compared to other organs) suggests a close mechanistic link between kidney and splenic injury that may significantly contribute to mortality. Alternatively, chloroquine may act through a different mechanism during earlier vs. later stages of sepsis.
We conclude that chloroquine does not act exclusively through the kidney, and any connection between splenic injury and kidney injury, while consistent with our data, is only speculative at this point. Disruption of one or more mediators of this putative organ-selective injury sequence would be needed to establish such a mechanism.
Similarities between chloroquine treatment and inhibition of TLR9
We had previously demonstrated that MyD88-deficient mice were resistant to sepsis and sepsis-induced AKI (11), consistent with a central role of TLR signaling. As TLR4-deficient mice had an intact sepsis-induced AKI response (11), and chloroquine inhibited sepsis-induced AKI, our data supports the hypothesis that one or more of the endosomal class of TLR: TLR3, TLR7/8, TLR9, was primarily involved in the development of sepsis-induced AKI. To test this more directly, we initially focused on TLR9 by using a) TLR9-deficient mice and b) a selective inhibitor of TLR9, the phosphorothioate oligodeoxynucleotide H154 and its corresponding control A151. TLR9 deficiency improved CLP sepsis-induced mortality and acute kidney injury, with improvements in renal function and histology, downstream systemic pro- and anti-inflammatory cytokines, and splenic apoptosis. The TLR9 inhibitor H154 improved renal function, reduced multiple organ damage, including splenic apoptosis, and dampened systemic pro- and anti-inflammatory cytokine responses. Chloroquine also decreased TLR9 protein levels in spleen to undetectable levels, analogous to TLR9-deficiency, further supporting a TLR9-centric mechanism for chloroquine. Previous studies have shown that survival after sepsis was improved in mice lacking the common TLR adapter protein MyD88 in a colon ascendens stent peritonitis (CASP) model (44). However, survival was not improved in mice lacking TLR2 or TLR4 in a CASP model (44), and mice lacking TLR4 were either not protected (11), or only marginally protected (12) against CLP sepsis-induced acute kidney injury. Our findings further support TLR9 as a key component of the host response to polymicrobial sepsis. We cannot eliminate significant roles of other TLRs, but if they are also important in polymicrobial sepsis, they at least require functional TLR9 signaling.
For each of the parameters tested chloroquine administration qualitatively had the same effect as TLR9 deficiency. However, chloroquine may have effects in addition to inhibiting TLR9. Other Toll-like receptors (TLR3, 7, and 8) are internalized and function through an endosomal pathway (10, 14-16, 30) and could be inhibited by chloroquine (13, 18). In support of this view, a) chloroquine was somewhat more effective than TLR9-deficiency to inhibit AKI, and b) chloroquine significantly decreased blood bacterial counts, whereas reduction by the TRL9 inhibitor H154 was not significant. Chloroquine has also been reported to interfere with ERK-mediated TNF-alpha upregulation in vitro (43), but the relative contribution of this pathway to sepsis in vivo is not known. Chloroquine also may have direct renal effects, including increases in urine flow, sodium excretion, and glomerular filtration rate (2, 3), that might contribute to protection of tubular damage induced by CLP; whether these are related to TLRs or other actions is unknown. Nevertheless, the concordance between TLR9 deficiency and chloroquine administration on almost all measured outcomes (survival, renal injury, cytokine levels), suggests that chloroquine is functioning in this model in large part via inhibition of splenic TLR9.
Chloroquine as a therapeutic agent
The spleen has become an attractive target for new therapeutic strategies for sepsis, particularly with regard to splenic apoptosis (22). Chloroquine administration compares favorably with previously studied methods to improve spleen function: caspase inhibitors (19, 24), Akt overexpression (6), TAT-BH4 or TAT-Bcl-XL peptides (21), anti-CD-40 antibody (39), caspase 8 or fas siRNA administration (46), or administration of exosomes from immature dendritic cells (33). Because of its low toxicity and acceptance in the clinic chloroquine may have fewer barriers in its development pipeline. Even though chloroquine retains its efficacy toward the kidney, spleen, and cytokines with delayed treatment, the window of opportunity has apparently passed to reduce multiple organ damage. Therefore, chloroquine treatment alone is unlikely to affect the outcome in the general septic patient population. The most promising outlook for chloroquine would be as a preventative agent or as a component of a combination therapeutic cocktail.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of NIH, NIDDK, NCI, and NHLBI. The authors thank Ms. Debra Currie (FDA) for breeding TLR9-deficient mice, and Drs. Anthony Suffredini (CC, NIH) and Daniel Doeuk (NIAID, NIH) for many helpful discussions.
References
- 1.Ahmad-Nejad P, Hacker H, Rutz M, Bauer S, Vabulas RM, Wagner H. Bacterial CpG-DNA and lipopolysaccharides activate Toll-like receptors at distinct cellular compartments. European J Immunol. 2002;32:1958–1968. doi: 10.1002/1521-4141(200207)32:7<1958::AID-IMMU1958>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed MH, Ashton N, Balment RJ. Renal function in a rat model of analgesic nephropathy: effect of chloroquine. J Pharmacol Exp Ther. 2003;305:123–130. doi: 10.1124/jpet.102.047233. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed MH, Ashton N, Balment RJ. The effect of chloroquine on renal function and vasopressin secretion: a nitric oxide-dependent effect. J Pharmacol Exp Ther. 2003;304:156–161. doi: 10.1124/jpet.102.042523. [DOI] [PubMed] [Google Scholar]
- 4.Akira S, Takeda K. Toll-like receptor signalling. Nature Reviews. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 5.Benigni A, Caroli C, Longaretti L, Gagliardini E, Zoja C, Galbusera M, Moioli D, Romagnani P, Tincani A, Andreoli L, Remuzzi G. Involvement of renal tubular Toll-like receptor 9 in the development of tubulointerstitial injury in systemic lupus. Arthritis and Rheumatism. 2007;56:1569–1578. doi: 10.1002/art.22524. [DOI] [PubMed] [Google Scholar]
- 6.Bommhardt U, Chang KC, Swanson PE, Wagner TH, Tinsley KW, Karl IE, Hotchkiss RS. Akt decreases lymphocyte apoptosis and improves survival in sepsis. J Immunol. 2004;172:7583–7591. doi: 10.4049/jimmunol.172.12.7583. [DOI] [PubMed] [Google Scholar]
- 7.Chung CS, Xu YX, Wang W, Chaudry IH, Ayala A. Is Fas ligand or endotoxin responsible for mucosal lymphocyte apoptosis in sepsis? Arch Surg. 1998;133:1213–1220. doi: 10.1001/archsurg.133.11.1213. [DOI] [PubMed] [Google Scholar]
- 8.Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420:885–891. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- 9.Coopersmith CM, Stromberg PE, Dunne WM, Davis CG, Amiot DM, 2nd, Buchman TG, Karl IE, Hotchkiss RS. Inhibition of intestinal epithelial apoptosis and survival in a murine model of pneumonia-induced sepsis. JAMA. 2002;287:1716–1721. doi: 10.1001/jama.287.13.1716. [DOI] [PubMed] [Google Scholar]
- 10.de Bouteiller O, Merck E, Hasan UA, Hubac S, Benguigui B, Trinchieri G, Bates EE, Caux C. Recognition of double-stranded RNA by human toll-like receptor 3 and downstream receptor signaling requires multimerization and an acidic pH. J Biol Chem. 2005;280:38133–38145. doi: 10.1074/jbc.M507163200. [DOI] [PubMed] [Google Scholar]
- 11.Dear JW, Yasuda H, Hu X, Hieny S, Yuen PS, Hewitt SM, Sher A, Star RA. Sepsis-induced organ failure is mediated by different pathways in the kidney and liver: Acute renal failure is dependent on MyD88 but not renal cell apoptosis. Kidney Int. 2006;69:832–836. doi: 10.1038/sj.ki.5000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El-Achkar TM, Huang X, Plotkin Z, Sandoval RM, Rhodes GJ, Dagher PC. Sepsis induces changes in the expression and distribution of Toll-like receptor 4 in the rat kidney. Am J Physiol Renal Physiol. 2006;290:F1034–1043. doi: 10.1152/ajprenal.00414.2005. [DOI] [PubMed] [Google Scholar]
- 13.Ertel W, Morrison MH, Ayala A, Chaudry IH. Chloroquine attenuates hemorrhagic shock-induced immunosuppression and decreases susceptibility to sepsis. Arch Surg. 1992;127:70–75. doi: 10.1001/archsurg.1992.01420010084012. discussion 75-76. [DOI] [PubMed] [Google Scholar]
- 14.Hacker H, Mischak H, Miethke T, Liptay S, Schmid R, Sparwasser T, Heeg K, Lipford GB, Wagner H. CpG-DNA-specific activation of antigen-presenting cells requires stress kinase activity and is preceded by non-specific endocytosis and endosomal maturation. EMBO J. 1998;17:6230–6240. doi: 10.1093/emboj/17.21.6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hart OM, Athie-Morales V, O'Connor GM, Gardiner CM. TLR7/8-mediated activation of human NK cells results in accessory cell-dependent IFN-gamma production. J Immunol. 2005;175:1636–1642. doi: 10.4049/jimmunol.175.3.1636. [DOI] [PubMed] [Google Scholar]
- 16.Heil F, Ahmad-Nejad P, Hemmi H, Hochrein H, Ampenberger F, Gellert T, Dietrich H, Lipford G, Takeda K, Akira S, Wagner H, Bauer S. The Toll-like receptor 7 (TLR7)-specific stimulus loxoribine uncovers a strong relationship within the TLR7, 8 and 9 subfamily. Eur J Immunol. 2003;33:2987–2997. doi: 10.1002/eji.200324238. [DOI] [PubMed] [Google Scholar]
- 17.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 18.Hong Z, Jiang Z, Liangxi W, Guofu D, Ping L, Yongling L, Wendong P, Minghai W. Chloroquine protects mice from challenge with CpG ODN and LPS by decreasing proinflammatory cytokine release. Int Immunopharmacol. 2004;4:223–234. doi: 10.1016/j.intimp.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 19.Hotchkiss RS, Chang KC, Swanson PE, Tinsley KW, Hui JJ, Klender P, Xanthoudakis S, Roy S, Black C, Grimm E, Aspiotis R, Han Y, Nicholson DW, Karl IE. Caspase inhibitors improve survival in sepsis: a critical role of the lymphocyte. Nature Immunol. 2000;1:496–501. doi: 10.1038/82741. [DOI] [PubMed] [Google Scholar]
- 20.Hotchkiss RS, Coopersmith CM, Karl IE. Prevention of lymphocyte apoptosis--a potential treatment of sepsis? Clin Infect Dis. 2005;41 7:S465–469. doi: 10.1086/431998. [DOI] [PubMed] [Google Scholar]
- 21.Hotchkiss RS, McConnell KW, Bullok K, Davis CG, Chang KC, Schwulst SJ, Dunne JC, Dietz GP, Bahr M, McDunn JE, Karl IE, Wagner TH, Cobb JP, Coopersmith CM, Piwnica-Worms D. TAT-BH4 and TAT-Bcl-xL peptides protect against sepsis-induced lymphocyte apoptosis in vivo. J Immunol. 2006;176:5471–5477. doi: 10.4049/jimmunol.176.9.5471. [DOI] [PubMed] [Google Scholar]
- 22.Hotchkiss RS, Nicholson DW. Apoptosis and caspases regulate death and inflammation in sepsis. Nature Reviews. 2006;6:813–822. doi: 10.1038/nri1943. [DOI] [PubMed] [Google Scholar]
- 23.Hotchkiss RS, Swanson PE, Freeman BD, Tinsley KW, Cobb JP, Matuschak GM, Buchman TG, Karl IE. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med. 1999;27:1230–1251. doi: 10.1097/00003246-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Hotchkiss RS, Tinsley KW, Swanson PE, Chang KC, Cobb JP, Buchman TG, Korsmeyer SJ, Karl IE. Prevention of lymphocyte cell death in sepsis improves survival in mice. Proc Natl Acad Sci U S A. 1999;96:14541–14546. doi: 10.1073/pnas.96.25.14541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hotchkiss RS, Tinsley KW, Swanson PE, Schmieg RE, Jr, Hui JJ, Chang KC, Osborne DF, Freeman BD, Cobb JP, Buchman TG, Karl IE. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J Immunol. 2001;166:6952–6963. doi: 10.4049/jimmunol.166.11.6952. [DOI] [PubMed] [Google Scholar]
- 26.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nature Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 27.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 28.Klinman DM. Immunotherapeutic uses of CpG oligodeoxynucleotides. Nature Rev. 2004;4:249–258. doi: 10.1038/nri1329. [DOI] [PubMed] [Google Scholar]
- 29.Kox WJ, Volk T, Kox SN, Volk HD. Immunomodulatory therapies in sepsis. Intensive Care Med. 2000;26 1:S124–128. doi: 10.1007/s001340051129. [DOI] [PubMed] [Google Scholar]
- 30.Lee J, Chuang TH, Redecke V, She L, Pitha PM, Carson DA, Raz E, Cottam HB. Molecular basis for the immunostimulatory activity of guanine nucleoside analogs: activation of Toll-like receptor 7. Proc Natl Acad Sci U S A. 2003;100:6646–6651. doi: 10.1073/pnas.0631696100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levitz SM, Harrison TS, Tabuni A, Liu X. Chloroquine induces human mononuclear phagocytes to inhibit and kill Cryptococcus neoformans by a mechanism independent of iron deprivation. J Clin Invest. 1997;100:1640–1646. doi: 10.1172/JCI119688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsumoto M, Funami K, Tanabe M, Oshiumi H, Shingai M, Seto Y, Yamamoto A, Seya T. Subcellular localization of Toll-like receptor 3 in human dendritic cells. J Immunol. 2003;171:3154–3162. doi: 10.4049/jimmunol.171.6.3154. [DOI] [PubMed] [Google Scholar]
- 33.Miksa M, Wu R, Dong W, Das P, Yang D, Wang P. Dendritic cell-derived exosomes containing milk fat globule epidermal growth factor-factor VIII attenuate proinflammatory responses in sepsis. Shock. 2006;25:586–593. doi: 10.1097/01.shk.0000209533.22941.d0. [DOI] [PubMed] [Google Scholar]
- 34.Miyaji T, Hu X, Yuen PS, Muramatsu Y, Iyer S, Hewitt SM, Star RA. Ethyl pyruvate decreases sepsis-induced acute renal failure and multiple organ damage in aged mice. Kidney Int. 2003;64:1620–1631. doi: 10.1046/j.1523-1755.2003.00268.x. [DOI] [PubMed] [Google Scholar]
- 35.Munford RS, Pugin J. Normal responses to injury prevent systemic inflammation and can be immunosuppressive. Am J Respir Crit Care Med. 2001;163:316–321. doi: 10.1164/ajrccm.163.2.2007102. [DOI] [PubMed] [Google Scholar]
- 36.Musabayane CT, Windle RJ, Forsling ML, Balment RJ. Arginine vasopressin mediates the chloroquine induced increase in renal sodium excretion. Trop Med Int Health. 1996;1:542–550. doi: 10.1046/j.1365-3156.1996.d01-81.x. [DOI] [PubMed] [Google Scholar]
- 37.Rutz M, Metzger J, Gellert T, Luppa P, Lipford GB, Wagner H, Bauer S. Toll-like receptor 9 binds single-stranded CpG-DNA in a sequence- and pH-dependent manner. European J Immunol. 2004;34:2541–2550. doi: 10.1002/eji.200425218. [DOI] [PubMed] [Google Scholar]
- 38.Saleh M, Mathison JC, Wolinski MK, Bensinger SJ, Fitzgerald P, Droin N, Ulevitch RJ, Green DR, Nicholson DW. Enhanced bacterial clearance and sepsis resistance in caspase-12-deficient mice. Nature. 2006;440:1064–1068. doi: 10.1038/nature04656. [DOI] [PubMed] [Google Scholar]
- 39.Schwulst SJ, Grayson MH, DiPasco PJ, Davis CG, Brahmbhatt TS, Ferguson TA, Hotchkiss RS. Agonistic monoclonal antibody against CD40 receptor decreases lymphocyte apoptosis and improves survival in sepsis. J Immunol. 2006;177:557–565. doi: 10.4049/jimmunol.177.1.557. [DOI] [PubMed] [Google Scholar]
- 40.Shirota H, Gursel I, Gursel M, Klinman DM. Suppressive oligodeoxynucleotides protect mice from lethal endotoxic shock. J Immunol. 2005;174:4579–4583. doi: 10.4049/jimmunol.174.8.4579. [DOI] [PubMed] [Google Scholar]
- 41.Ulloa L, Ochani M, Yang H, Tanovic M, Halperin D, Yang R, Czura CJ, Fink MP, Tracey KJ. Ethyl pyruvate prevents lethality in mice with established lethal sepsis and systemic inflammation. Proc Natl Acad Sci U S A. 2002;99:12351–12356. doi: 10.1073/pnas.192222999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 43.Weber SM, Chen JM, Levitz SM. Inhibition of mitogen-activated protein kinase signaling by chloroquine. J Immunol. 2002;168:5303–5309. doi: 10.4049/jimmunol.168.10.5303. [DOI] [PubMed] [Google Scholar]
- 44.Weighardt H, Kaiser-Moore S, Vabulas RM, Kirschning CJ, Wagner H, Holzmann B. Cutting edge: myeloid differentiation factor 88 deficiency improves resistance against sepsis caused by polymicrobial infection. J Immunol. 2002;169:2823–2827. doi: 10.4049/jimmunol.169.6.2823. [DOI] [PubMed] [Google Scholar]
- 45.Wellmer A, von Mering M, Spreer A, Diem R, Eiffert H, Noeske C, Bunkowski S, Gold R, Nau R. Experimental pneumococcal meningitis: impaired clearance of bacteria from the blood due to increased apoptosis in the spleen in Bcl-2-deficient mice. Infect Immun. 2004;72:3113–3119. doi: 10.1128/IAI.72.6.3113-3119.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wesche-Soldato DE, Chung CS, Lomas-Neira J, Doughty LA, Gregory SH, Ayala A. In vivo delivery of caspase-8 or Fas siRNA improves the survival of septic mice. Blood. 2005;106:2295–2301. doi: 10.1182/blood-2004-10-4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wheeler AP, Bernard GR. Treating patients with severe sepsis. N Engl J Med. 1999;340:207–214. doi: 10.1056/NEJM199901213400307. [DOI] [PubMed] [Google Scholar]
- 48.Yamada H, Gursel I, Takeshita F, Conover J, Ishii KJ, Gursel M, Takeshita S, Klinman DM. Effect of suppressive DNA on CpG-induced immune activation. J Immunol. 2002;169:5590–5594. doi: 10.4049/jimmunol.169.10.5590. [DOI] [PubMed] [Google Scholar]
- 49.Yasuda H, Yuen PS, Hu X, Zhou H, Star RA. Simvastatin improves sepsis-induced mortality and acute kidney injury via renal vascular effects. Kidney Int. 2006;69:1535–1542. doi: 10.1038/sj.ki.5000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yuen PS, Dunn SR, Miyaji T, Yasuda H, Sharma K, Star RA. A simplified method for HPLC determination of creatinine in mouse serum. Am J Physiol Renal Physiol. 2004;286:F1116–1119. doi: 10.1152/ajprenal.00366.2003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


