Abstract
Uncoupling protein-2 (UCP2) regulates insulin secretion by controlling ATP levels in β cells. While UCP2 deficiency improves glycemic control in mice, increased expression of UCP2 interferes with glucose-stimulated insulin secretion. These observations link UCP2 to β cell dysfunction in type 2 diabetes with a perplexing evolutionary role. We found higher residual serum insulin levels and blunted lipid metabolic responses in fasted ucp2−/− mice, supporting the concept that UCP2 evolved to suppress insulin effects and to accommodate the fuel switch to fatty acids during starvation. In the absence of UCP2, fasting initially promotes peripheral lipolysis and hepatic fat accumulation at less than expected rates, but culminates in protracted steatosis indicating diminished hepatic utilization and clearance of fatty acids. We conclude that UCP2-mediated control of insulin secretion is a physiologically relevant mechanism of the metabolic response to fasting.
Keywords: Prolonged fasting, lipolysis, steatosis, insulin secretion
Uncoupling protein-2 (UCP2), a member of the anion carrier protein superfamily, is a widely distributed constituent of the mitochondrial inner membrane (6, 35). Although UCP2 was identified over 10 years ago by molecular cloning, its biological role and evolutionary aspects remain debated (6, 29, 35, 40). Based on its significant homology with the brown adipose tissue-specific UCP1 (thermogenin), UCP2 was predicted to regulate energy balance. Subsequent observations did not confirm this prediction and UCP2 appears to have a complex function yet to be fully explored (6, 29, 35, 40). Similar to other uncoupling proteins, UCP2 lowers the mitochondrial membrane potential (Δψm) by mediating proton leak across the inner membrane (6). As a result, UCP2 has the ability to interfere with the function of F1F0 ATP synthase and to alter the matrix ATP/ADP ratio (6).
Soon after its discovery, the importance of UCP2 was recognized in pancreatic β cells, where UCP2 acts as a negative regulator of glucose-stimulated insulin secretion (GSIS) (30, 51). Pancreatic β cells sense blood glucose through its metabolism that results in increased levels of intracellular ATP and triggers GSIS to maintain glucose homeostasis (41). Due to more efficient oxidative phosphorylation and increased rates of mitochondrial ATP synthesis, UCP2 deficiency is associated with enhanced β cell insulin secretory capacity in ucp2−/− mice and allows the partial correction of diabetes in leptin-deficient ob/ob mice (51). In contrast, overexpression of UCP2 in β cells reduces intracellular ATP levels leading to diminished GSIS (21). Because increased UCP2 expression of β cells is associated with obesity, UCP2 has been linked to impaired insulin production in type 2 diabetes (44, 51). Consequently, however, the evolutionary meaning of UCP2 in β cells has been questioned and its effect on GSIS was even labeled a ‘surprising whim of nature’ (40). Nonetheless, the obesity epidemic is a recent (and essentially human-specific) development and one may reasonably assume that the ability of UCP2 to interfere with the regulation of insulin in β cells has a true physiologic function.
UCP2 expression is elevated in skeletal muscle during periods of fasting (4, 37) and this ‘starvation paradox’ served as a major argument against the role of UCP2 in thermogenesis or energetic inefficiency (13). Most recently, increased UCP2 expression was also described in the whole pancreas and in islets of starved mice, raising speculations that upregulation of UCP2 in β cells reflects a physiological mechanism for suppression of GSIS in the fasting state (3, 10). Under fed conditions, glucose is preferentially metabolized to generate ATP, while surplus carbohydrates are converted into triacylglycerols and stored as peripheral fat. Under fasting conditions, glucose is not sufficiently available and lipids become the predominant source of fuel (1, 15). Thus, hepatic glycogen stores are rapidly depleted while triacylglycerols are released from white adipose tissue and transported as non-esterified fatty acids to liver mitochondria for β-oxidation (1, 15). Acetyl-CoA, the end product of β-oxidation, is then used for ketogenesis and re-esterification into very low-density lipoproteins (VLDL) to supply the energy demand of extrahepatic tissues (1). With the exception of hepatocellular uptake of fatty acids (47), these metabolic pathways are under tight hormonal regulation in which insulin has a pivotal inhibitory role that needs to be suppressed in fasting (45).
Since fatty acids stimulate both the expression (30, 36) and the activity of UCP2 in β cells (28), higher levels of circulating fatty acids may signal the upregulation and activation of UCP2 in fasting and contribute to the suppression of insulin secretion, thus suggesting an amplification loop through which UCP2 plays a role in normal β cell physiology. Moreover, recent work found that the half-life of UCP2 is unusually short (~30 min), making UCP2 a suitable candidate for regulating rapid biological responses (43).
Here we investigated a specific aspect of lipid metabolism by testing the hypothesis that modulation of insulin secretion by UCP2 is necessary for the fasting-induced fuel switch to fatty acid oxidation. To this end, we analyzed fasting-induced changes in the lipid metabolism of ucp2−/− mice and conclude that the physiologic response to fasting requires the presence of UCP2.
Materials and Methods
Animals and study design
Founders of ucp2−/− mice were obtained from Dr. Bradford B. Lowell (Harvard Medical School, Boston, MA). Genotypes were determined as described (51). Male ucp2−/− mice and their wild type littermates (n = 4 to 10) at the age of 12 to 14 weeks were fasted by removing food at 8:00AM for either 24 hours or 72 hours. Control groups were fed ad libitum with regular chow diet. All animals had unlimited access to drinking water. At the time of sacrifice animals were weighed, their blood glucose was measured from the tail vein, and the liver and epididymal fat pads were removed, weighed, and snap-frozen or processed for histological studies. Blood was obtained by cardiac puncture for additional biochemical assays. All experiments were performed by the approval of the Lifespan Animal Welfare Committee of Rhode Island Hospital.
Biochemical measurements
We used commercially available kits to determine plasma levels of non-esterified fatty acids (Wako, Richmond, VA), triacylglycerols, and β-hydroxybutyrate (StanBio, Boerne, TX). Plasma insulin levels were measured by ELISA (Linco, St. Charles, MO). Total liver tissue lipid content was determined by the chloroform: methanol extraction method as originally described (2).
Histological studies and image analysis
Liver tissue pieces were embedded and frozen in Tissue-Tek medium (Sakura, Torrance, CA), and sectioned at 4 μm thickness. To estimate the extent of hepatic lipid accumulation, we prepared digitized images of liver slides stained with oil-red-O (MicroPublisher 3.3 RTV, Qimaging, Burnaby, British Columbia). We recorded the area of oil-red-O staining above a constant optical density threshold using Image Pro Plus 5.1 (MediaCybernetics, Silver Springs, MD) to calculate the percentage of area stained positive for oil-red-O. Constant optical conditions were maintained along the entire morphometric evaluation.
Western blot analysis
Liver tissue lysates were prepared as described (22). Equal amounts of protein were size-fractionated by 10% SDS-PAGE, transferred to a PVDF membrane (PerkinElmer Life Sciences, Waltham, MA), and immunoblots developed using polyclonal rabbit antibodies against phosphorylated HSL (Cell Signaling, Danvers, MA), PPAR-α (Sigma), PPAR-γ and SREBP1-c (Santa Cruz, Santa Cruz, CA). Secondary donkey antibody (Santa Cruz) was conjugated with horseradish peroxidase and immunoblots detected by ECL (PerkinElmer). Equal loading was confirmed using mouse anti-β-actin antibody (Sigma) and secondary goat antibody (Santa Cruz).
Real time quantitative polymerase chain reaction
Total RNA was extracted from snap-frozen liver tissue specimens with TRIzol reagent (Invitrogen, Carlsbad, CA) and contaminating genomic DNA was removed with DNase-I, RNase-free (Roche, Indianapolis, IN) prior to reverse transcription using first-strand cDNA synthesis kit (Roche). Polymerase chain reaction (PCR) was performed using an iCycler iQ Multi-Color Real Time PCR Detection System (Bio-Rad, Hercules, CA) and SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA). We utilized the TATA box-binding protein (TBP) as reference gene (49). The full-length mouse TBP gene (957 bp) was amplified and cloned into pCR2.1 vector to create standard curves by serial dilutions. Sample cDNAs (equivalent of 5 ng total RNA) were used as template with gene-specific primers (Table 1). Each sample was normalized using its TBP mRNA content and data are given as relative abundance over fed controls.
Table 1.
Primer sequences of genes used for mRNA quantification
| Protein | NCBI Accession # | Forward Primer (5′ to 3′) | Reverse Primer (5′ to 3′) |
|---|---|---|---|
| ACC-α | NM_133360 | CAGGGACTATGTCCTGAAGCA | GGTCATGTGGACGATGGAGT |
| AOX | NM_015729 | TTATGCGCAGACAGAGATGG | TATGTGGCAGTGGTTTCCAA |
| CPT1A | NM_013495 | CAGCAGCAGGTGGAACTGT | GGAAACACCATAGCCGTCAT |
| CYP2E1 | NM_021282 | CCAAAGAGAGGCACACTTCC | GCACAGCCAATCAGAAAGGT |
| FAS | NM_007988 | GGCATCATTGGGCACTCCTT | GCTGCAAGCACAGCCTCTCT |
| mtHMGS | NM_008256 | CCTACCGCAAGAAGATCCAG | GAAAGGCTGGTTGTTTCCAG |
| MCAD | NM_007382 | TGATCAACGCGCACATTC | GAACGTTCCCAGGCCAAG |
| MTTP | NM_008642 | GGATCCTCTTCTGCCTATACTGG | TGTCAAGGCTGTATGTGGAC |
| SCD-1 | NM_009127 | CCTCCGGAAATGAACGAGAG | CAGGACGGATGTCTTCTTCCA |
| TBP | NM_013684 | ACTTCGTGCAAGAAATGCTGAA | TGTCCGTGGCTCTCTTATTCTCA |
The abbreviations used are: ACC-α, acyl-CoA carboxylase-alpha; AOX, acyl-CoA oxidase; CPT1A, carnitine palmitoyl transferase 1A; CYP2E1, cytochrome P450 2E1; FAS, fatty acid synthase complex; mtHMGS, hydroxy-methyl-glutaryl CoA synthase (mitochondrial form); MCAD, medium chain acyl-CoA dehydrogenase; MTTP, microsomal triacylglycerol transfer protein; SCD-1, stearoyl CoA desaturase-1; TBP, TATA box-binding protein.
Statistical analysis
We presented the results as mean ± SE. The data were analyzed with unpaired Student t test or ANOVA when multiple comparisons were made. Association between categorical groups was evaluated by the Fisher’s exact probability, Mann-Whitney U test, and binomial exact calculations. Differences with calculated P values <0.05 were regarded as significant.
RESULTS
Biochemical indicators reveal blunted response to fasting in ucp2−/− mice
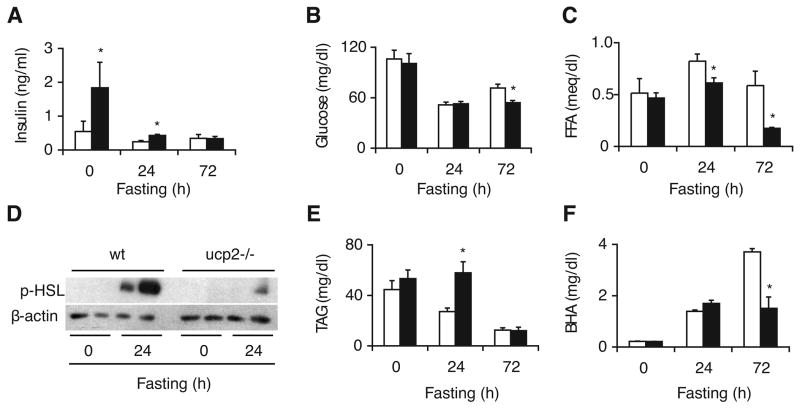
We fasted ucp2−/− mice and wild type littermates up to 72 hours to analyze the impact of UCP2 deficiency on the metabolic response to fasting. In previous studies, serum insulin levels were found to be elevated in ucp2−/− mice (51). Importantly, hyperinsulinemia in ucp2−/− mice is not the result of insulin resistance since it is associated with mild hypoglycemia and normal insulin tolerance test (51). We confirmed 3-fold higher serum insulin levels in ucp2−/− mice during the fed state when compared to wild type controls (Fig. 1A). These differences are known to persist at lower serum insulin levels following an overnight fast (51), but the effect of UCP2 deficiency in response to longer periods of fasting has not been reported before. Serum insulin levels in ucp2−/− mice remained higher even after 24-hour fast, while this difference disappeared when fasting continued for a total of 72 hours (Fig. 1A). Although ambient blood glucose levels and fasting-induced hypoglycemia in this cohort of ucp2−/− mice were not significantly different from wild type, blood glucose levels following 72-hour fast in ucp2−/− mice were lower compared to wild type controls, consistent with a continued imbalance of metabolic regulation in UCP2 deficiency (Fig. 1B).
Fig. 1.
Metabolic response to fasting is blunted in ucp2−/− mice. Biochemical parameters determined in the plasma of fed and fasted ucp2−/− mice and their wild type (wt) littermates (n = 4 to 10) include (A) insulin, (B) glucose, (C) free fatty acids (FFA), (E) total triacylglycerols (TAG), and (F) β-hydroxybutyrate (BHA). White bars, wild type mice; black bars, ucp2−/− mice. Error bars denote SE. *, Significantly different from wild type (P < 0.05). (D) Immunoblot analysis of phosphorylated hormone-sensitive lipase (p-HSL) levels in epididymal fat pad of fed and fasted (24h) ucp2−/− mice and their wild type littermates. Beta-actin served as loading control.
As expected, serum levels of fatty acids increased in wild type mice after 24-hour fast, while this response was diminished in ucp2−/− mice (Fig. 1C). This genotypic difference was even more apparent after 72-hour fast, when fatty acid levels dropped below pre-fasting values in ucp2−/− mice, suggesting insufficient availability (Fig. 1C). Peripheral lipolysis is one of the earliest responses of lipid metabolism to fasting, accounting for elevated serum levels of fatty acids (24). During lipolysis, triacylglycerols stored in white adipose tissue are hydrolyzed into fatty acids that subsequently help coordinate the complex fasting response. Hormone-sensitive lipase (HSL) is traditionally viewed as a major determinant of this process (39). HSL is activated by phosphorylation, but this reaction is very sensitive to the inhibitory effect of insulin (8). We therefore hypothesized that higher serum insulin levels persisting in fasted ucp2−/− mice may be associated with diminished activation of HSL. To assess the lipolytic activity in peripheral adipose tissue, we used immunoblot analysis to determine the abundance of phosphorylated HSL in the epididymal fat pad of fasted animals. There was no genotypic difference between the weight of epididymal fat pads before and after fasting (not shown). Following 24-hour fast, amounts of phosphorylated HSL dramatically increased in wild type mice indicating release from insulin-mediated inhibition, but this change was attenuated in ucp2−/− mice (Fig. 1D). Thus, fasting-induced lipolysis is diminished in ucp2−/− mice, coinciding with higher residual serum insulin levels. (Notably, 72-hour fast resulted in massive lipolysis regardless of the genotype, resulting in the disappearance of epididymal fat pads in most animals and preventing the systematic analysis of HSL activation at this extreme phase of fasting.)
Serum levels of triacylglycerols in wild type mice became gradually lower as fasting continued for up to 72 hours, consistent with dwindling peripheral fat stores due to increased hydrolysis and utilization (Fig. 1E). Interestingly, this trend was delayed in ucp2−/− mice, complementing changes in serum levels of fatty acids as described above. The genotypic difference disappeared after 72-hour fast when serum triacylglycerols levels were uniformly low, consistent with the overall depletion of peripheral fat stores. We then assessed fasting-induced ketogenesis by measuring serum levels of β-hydroxybutyrate (BHA). Importantly, while wild type mice responded with a sharp and continuous increase in BHA production, ucp2−/− mice were unable to maintain the same trend in response to 72-hour fast, indicating impaired fasting-induced ketogenesis in the absence of UCP2 (Fig. 1F).
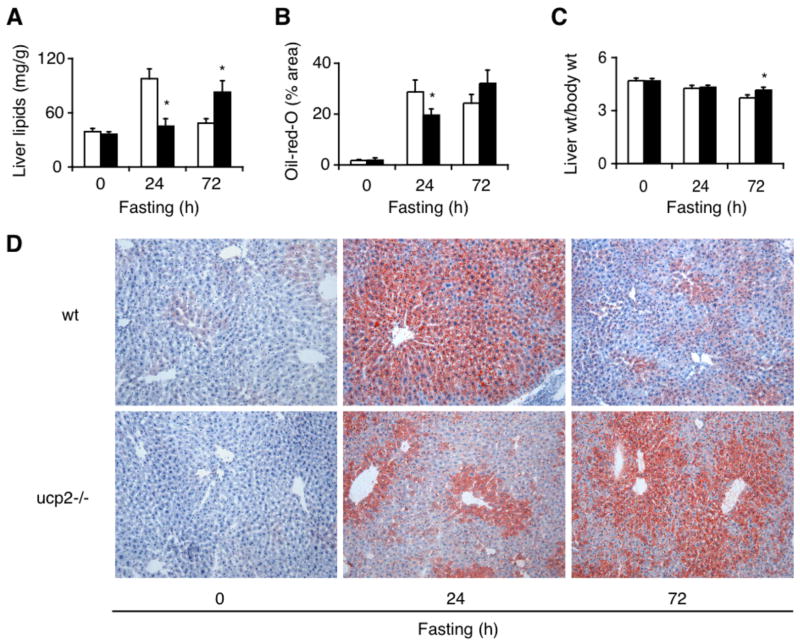
Fasting-induced hepatic steatosis is delayed in ucp2−/− mice
As a result of fasting-induced peripheral lipolysis, large amounts of fatty acids are transported to the liver where they provide an alternative energy source (24). Flux rates of fatty acids in starvation often exceed normal hepatocellular capacity for mitochondrial β-oxidation and result in transient accumulation of triacylglycerols, manifesting as steatosis (32, 47). Since fasting-induced hyperfattyacidemia is diminished in ucp2−/− mice, we predicted that steatosis would be less prominent in these animals. Indeed, reduced amounts of fat accumulated in livers of ucp2−/− mice after 24-hour fast as assessed by total tissue lipid extraction (Fig. 2A) and oil-red-O staining (Fig. 2 B, D). Surprisingly, however, steatosis became more severe in ucp2−/− mice after 72-hour fast when it already showed substantial resolution in wild type mice. Increased hepatic fat accumulation in ucp2−/− mice was also indicated by elevated liver weight/body weight ratios after 72-hour fast (Fig. 2C). These findings suggest that hepatocytes in ucp2−/− mice fasted for a prolonged period fail to channel fatty acids efficiently into lipid catabolic pathways such as mitochondrial β-oxidation and ketogenesis, resulting in delayed hepatocellular lipid clearance.
Fig. 2.
Fasting-induced steatosis is delayed in the liver of ucp2−/− mice. (A) Liver lipid content assessed in fed and fasted ucp2−/− mice and their wild type (wt) littermates (n = 4 to 10) by chloroform:methanol extraction and by (B) image analysis of oil-red-O staining with lipid content expressed as the percentage of stained over total tissue area. (C) Ratio of liver weight (wt) over body weight. White bars, wild type mice; black bars, ucp2−/− mice. Error bars denote SE. *, Significantly different from wild type mice (P < 0.05). (D) Representative images of liver tissue slides stained with oil-red-O. Original magnification, 200X.
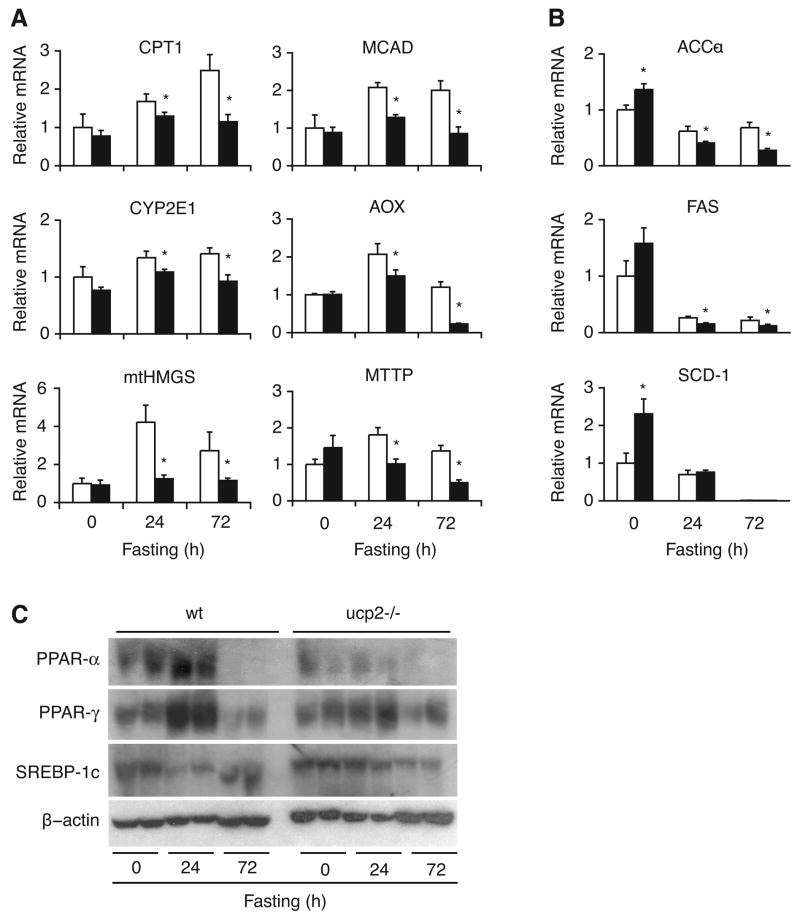
Hepatic expression of key lipid regulatory molecules in fasted ucp2−/− mice
Lipid and energy metabolism in the liver is primarily regulated by insulin at the transcriptional level with the mediation of various transcription factors (45). To better understand the mechanisms by which UCP2 affects the metabolic fate of fatty acids in fasting, we analyzed the gene expression pattern of key lipid regulatory enzymes in the liver. Gene expression for carnitine palmitoyltransferase 1 (CPT1), the rate-limiting enzyme for mitochondrial fatty acid uptake (33), gradually increased with fasting in wild type mice, but showed no change in ucp2−/− mice (Fig. 3A). The pattern of gene expression was similar for medium chain-specific acyl-CoA dehydrogenase (MCAD), a key enzyme for mitochondrial β-oxidation (14), indicating that mitochondrial capacity for fasting-induced lipid utilization is relatively diminished in hepatocytes of ucp2−/− mice (Fig. 3A). Fatty acid oxidation may occur through accessory pathways during starvation (42). Therefore, we also analyzed expression of acyl-CoA oxidase (AOX) and cytochrome p450 2E1 (CYP2E1) to estimate peroxisomal β-oxidation and microsomal ω-oxidation, respectively. Both genes had lower expression in fasted ucp2−/− mice, indicating lack of compensation at the transcriptional level for diminished mitochondrial β-oxidation by accessory fatty acid oxidation pathways (Fig. 3A).
Fig. 3.
Expression of key lipid regulatory molecules is altered in the liver of fasted ucp2−/− mice. Quantitative real time PCR analysis of genes encoding for enzymes involved in metabolic pathways of lipid breakdown (A) and synthesis (B) was performed on whole liver tissue of fed and fasted ucp2−/− mice and their wild type (wt) littermates (n = 4 to 10). Relative abundance of mRNA content over the mRNA of TATA box-binding protein expressed in arbitrary units referenced to fed control. CPT1, carnitine palmitoyl transferase-1 (mitochondrial fatty acid uptake); MCAD, medium-chain acyl-CoA dehydrogenase (mitochondrial β-oxidation); CYP2E1, cytochrome P450 2E1 (microsomal ω-oxidation); AOX, acyl-CoA oxidase (peroxisomal β-oxidation); mtHMGS, mitochondrial 3-hydroxy-3-methyl glutarate CoA synthase (ketogenesis); MTTP, microsomal triglyceride transfer protein (VLDL transport); ACC-α, acetyl CoA carboxylase-alpha; FAS, fatty acid synthase complex; SCD-1, stearoyl CoA desaturase-1 (lipogenesis). White bars, wild type mice; black bars, ucp2−/− mice. Error bars denote SE. *, Significantly different from wild type mice (P < 0.05). (C) Immunoblot analysis of peroxisome proliferator-activated receptor α (PPAR-α), PPAR-γ, and sterol regulatory element binding protein-1c (SREBP-1c) in livers of fed and fasted ucp2−/− mice and their wild type (wt) littermates. Beta-actin served as loading control.
Gene expression for mitochondrial β-hydroxy-β-methylglutaryl-CoA synthase (mtHMGS), a pivotal enzyme of ketogenesis (19), rose markedly upon fasting in wild type mice, but remained unchanged in ucp2−/− mice (Fig. 3A), in line with diminished BHA production. In addition, gene expression for microsomal triglyceride transfer protein (MTTP), the protein responsible for exporting VLDL from hepatocytes (48), became progressively diminished in ucp2−/− mice, suggesting that impaired clearance of de novo synthesized triacylglycerols may contribute to protracted fasting-induced steatosis in the absence of UCP2 (Fig. 3A). These findings support the notion that UCP2 is required for proper induction of ketogenesis and VLDL transport in response to fasting.
Since anabolic pathways of lipid metabolism contribute to steatosis, we also assessed the impact of UCP2 deficiency on fasting-induced changes in hepatic expression of key lipogenic enzymes. We measured mRNA levels of acetyl CoA carboxylase (ACC), fatty acid synthase (FAS), and stearoyl CoA desaturase (SCD), enzymes that are under positive transcriptional control by insulin and that mediate de novo production of fatty acids in the liver (7, 45). Consistent with higher insulin levels in UCP2 deficiency, mRNA levels for ACC-α, FAS, and SCD-1 in the fed state were higher in ucp2−/− mice than in wild type controls (Fig. 3B). Gene expression of all 3 lipogenic enzymes markedly diminished in both genotypes in response to fasting, although the decline in mRNA levels was more robust in ucp2−/− mice (Fig. 3B). This finding is at variance with higher residual insulin levels found in UCP2 deficiency, suggesting that non-insulin regulatory pathways may contribute to the genotypic difference. Nonetheless, the gene expression profile suggests that catabolic rather than anabolic lipid pathways correlate with fasting-induced steatosis and serum metabolic parameters in ucp2−/− mice. Thus, impairment of lipid breakdown appears to dominate the effect of UCP2 deficiency in our animal model.
To further analyze the metabolic response to fasting in ucp2−/− mice, we measured hepatic expression of key transcription factors that regulate lipid homeostasis. Peroxisome proliferator-activated receptors PPAR-α and PPAR-γ are nuclear hormone receptors naturally activated by fatty acids and their derivatives (25). PPAR-α regulates the genes of mitochondrial, peroxisomal, and microsomal fatty acid oxidation and ketogenesis (31), while PPAR-γ promotes adipocyte differentiation and lipid storage with hepatic expression mostly seen in fatty livers related to obesity and starvation (20, 34). In agreement with observations that both PPAR-α and PPAR-γ are upregulated in the liver in response to fasting (50), hepatic PPAR-α and PPAR-γ levels increased following a 24-hour fast in wild type mice, while this response was attenuated in ucp2−/− mice (Fig. 3C). Sterol regulatory element-binding protein-1c (SREBP-1c) controls de novo hepatic synthesis of fatty acids and triacylglycerols by inducing the expression of lipogenic genes such as ACC, FAS, and SCD-1 (7, 16). In contrast to PPAR-α and PPAR-γ, starvation normally results in down-regulation of hepatic SREBP-1c expression (23). As predicted, liver tissue SREBP-1c levels in wild type mice decreased in response to 24-hour fast, but remained unchanged in ucp2−/− mice (Fig. 3C). Thus, UCP2 deficiency attenuates fasting-induced changes in the levels of major lipid regulatory transcription factors examined in our study. Notably, 72-hour fast resulted in uniformly low levels of PPAR-α, PPAR-γ, and SREBP-1c regardless of the genotype, suggesting general dysfunction of metabolic regulation at this extreme phase of fasting.
DISCUSSION
There is considerable effort to elucidate the molecular mechanisms that contribute to the rapidly growing prevalence of obesity and type 2 diabetes in developed societies (38). It has been argued that this epidemic reflects the maladaptation of a ‘thrifty’ phenotype that originally evolved in our hunter-gatherer ancestors as a trait to promote efficient energy storage, but now it fails to meet rapid environmental changes such as continuous food availability and sedentary lifestyle (9, 17). Better understanding of how the metabolic response to fasting is regulated under physiologic conditions may therefore identify molecular targets that erroneously promote this alarming trend.
In this study we identified UCP2 as a modulator of the lipid metabolic response to fasting in mice since the complex biochemical response of the liver, which involves the efficient breakdown, conversion, and redistribution of fatty acids, is perturbed in UCP2 deficiency. Thus, fasting-induced physiologic changes in peripheral lipolysis and hepatic lipid utilization appear blunted in ucp2−/− mice as assessed by biochemical parameters and by expression of key lipid regulatory enzymes and transcription factors.
Peroxisomal β-oxidation and microsomal ω-oxidation are minor pathways of fatty acid oxidation (46), suggested to gain significance as a result of impaired mitochondrial β-oxidation or during increased delivery of fatty acids to the liver as it occurs in fasting (42). While insufficient β-oxidation capacity of mitochondria in UCP2 deficiency could theoretically repartition the catabolism of fatty acids into peroxisomes and microsomes, and this mechanism has recently been described in the liver of subjects with non-alcoholic fatty liver disease (27), we found no signs for such compensation. In fact, lower gene expression of AOX and CYP2E1 in fasted ucp2−/− mice suggests that non-mitochondrial routes for fatty acid breakdown remain suppressed in this animal model.
The effect of fasting on the expression of lipid regulatory transcription factors has been well characterized. As a result of peripheral lipolysis and rapid delivery of fatty acids to the liver, fasting stimulates hepatic expression of both PPAR-α (26) and PPAR-γ (20). The impact of PPAR-α and PPAR-γ on hepatic lipid metabolism, however, is different. While overexpression of PPAR-γ in the mouse liver causes lipid accumulation (50), PPAR-α promotes lipid utilization as demonstrated by marked hepatic steatosis and diminished fatty acid oxidation and ketogenesis in fasted PPAR-α knockout mice (18, 31). In our experiments, 24-hour fast resulted in increased expression of hepatic PPAR-α and PPAR-γ in wild type mice, indicating a dynamic response of lipid metabolism impaired in the livers of ucp2−/− mice (Fig. 3C). Notably, fasting-induced changes in hepatic SREBP-1c expression did not correlate with the degree of steatosis in our animal model. Thus, 24-hour fast resulted in decreased amounts of hepatic SREBP-1c in wild type mice, but remained unchanged in ucp2−/− mice (Fig. 3C), while genotype-specific changes in steatosis during starvation showed an opposing trend (Fig. 1). Together with the similarly discordant expression pattern of SREBP-1c target genes ACC-α, FAS, and SCD-1 (Fig. 3B), we conclude that SREBP-1c contributes little to the metabolic consequences of UCP2 deficiency in the fasting liver.
Our findings show that metabolic disparities between ucp2−/− and wild type mice become increasingly prominent with prolonged starvation. Thus, serum levels of fatty acids and BHA (Fig. 1) as well as the hepatic expression pattern of genes responsible for fatty acid oxidation, ketogenesis, and VLDL export (Fig. 3) are increasingly abnormal after 72-hour fast in the absence of UCP2. In addition, although initially delayed, fasting-induced hepatic lipid accumulation in ucp2−/− mice becomes more severe by 72 hours, by which time steatosis is already resolving in wild type mice (Fig. 2). It is likely that insufficient lipolysis in fasted ucp2−/− mice initially provides reduced amounts of fatty acids for hepatic uptake, accounting for less steatosis and for diminished impact of fatty acids on metabolic regulation. When fasting continues, steatosis rapidly resolves as hepatocytes process fatty acids in order to distribute lipid-derived energy in compensation for insufficiently available glucose (32, 47). In contrast, hepatic lipid utilization in response to fasting is apparently impaired in ucp2−/− mice, likely accounting for protracted steatosis.
Insulin plays a critical negative regulatory role both in fasting-induced peripheral lipolysis and in hepatic lipid breakdown (24, 45). It is known from previous work that GSIS is enhanced and hyperinsulinemia develops in ucp2−/− mice as a result of altered glucose sensing in pancreatic β cells (51). Here we show that residual serum insulin levels remain higher in ucp2−/− mice even after a 24-hour fast (Fig. 1A), indicating that UCP2 deficiency in β cells may impair the fasting response of hepatic lipid metabolism. These data support recent speculations on the evolutionary role of UCP2 in suppressing GSIS of β cells (3, 10) and suggest that insufficient suppression of insulin secretion may perturb the fasting response of lipid metabolism in ucp2−/− mice. However, plasma insulin levels in ucp2−/− mice after 24-hour fast are comparable to non-fasting baseline levels in wild type mice and some of the metabolic changes are most prominent after 72-hour fast when fasting insulin levels in ucp2−/− and wild type mice are no longer different. Although insulin tolerance test in our previous studies indicated no change in the peripheral insulin sensitivity of ucp2−/− mice (51), enhanced insulin signaling was recently described in the adipose tissue of mice treated with antisense oligonucleotides to UCP2 (11). Altered peripheral insulin action may therefore contribute to changes seen in the liver of fasted ucp2−/− mice, a possibility that warrants further studies.
In conclusion, fasting-induced changes in the lipid metabolism of mice gain from the presence of UCP2. Our findings lend experimental support to the concept that upregulation of UCP2 in β cells is a physiologically important response to fasting (3, 10). Increasing UCP2 levels diminish insulin release from β cells, thus facilitating peripheral lipolysis and hepatic lipid utilization in fasting. Accordingly, augmented presence of UCP2 seems beneficial under these conditions. UCP2 upregulation and activation in β cells may in fact involve an amplification loop driven by peripherally mobilized fatty acids during the initial phase of fasting. This regulatory mechanism, however, is misdirected in type 2 diabetes where steady abundance of UCP2 may result in β cell dysfunction, thus contributing to profound metabolic disturbances. Moreover, enduring hyperglycemia and hyperfattyacidemia in type 2 diabetes may induce oxidative stress in β cells, permanently activating the superoxide-UCP2 pathway (5, 29). There is growing evidence that inhibition of UCP2 by genetic ablation (51), antisense oligonucleotides (12), RNA interference (44), or the herbal derivative genipin (52) may restore the insulin-secreting ability of β cells and improve type 2 diabetes. Whether one needs to be concerned about the loss of physiologic effects of UCP2 under these conditions, however, remains to be seen.
Acknowledgments
This work has been supported by NIH grant DK-61890 (G.B.)
References
- 1.Bartlett K, Eaton S. Mitochondrial beta-oxidation. Eur J Biochem. 2004;271:462–469. doi: 10.1046/j.1432-1033.2003.03947.x. [DOI] [PubMed] [Google Scholar]
- 2.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 3.Bordone L, Guarente L. Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat Rev Mol Cell Biol. 2005;6:298–305. doi: 10.1038/nrm1616. [DOI] [PubMed] [Google Scholar]
- 4.Boss O, Samec S, Dulloo A, Seydoux J, Muzzin P, Giacobino JP. Tissue-dependent upregulation of rat uncoupling protein-2 expression in response to fasting or cold. FEBS Lett. 1997;412:111–114. doi: 10.1016/s0014-5793(97)00755-2. [DOI] [PubMed] [Google Scholar]
- 5.Brand MD, Affourtit C, Esteves TC, Green K, Lambert AJ, Miwa S, Pakay JL, Parker N. Mitochondrial superoxide: production, biological effects, and activation of uncoupling proteins. Free Radic Biol Med. 2004;37:755–767. doi: 10.1016/j.freeradbiomed.2004.05.034. [DOI] [PubMed] [Google Scholar]
- 6.Brand MD, Esteves TC. Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metab. 2005;2:85–93. doi: 10.1016/j.cmet.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest. 2004;114:147–152. doi: 10.1172/JCI22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carmen GY, Victor SM. Signalling mechanisms regulating lipolysis. Cell Signal. 2006;18:401–408. doi: 10.1016/j.cellsig.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Chakravarthy MV, Booth FW. Eating, exercise, and “thrifty” genotypes: connecting the dots toward an evolutionary understanding of modern chronic diseases. J Appl Physiol. 2004;96:3–10. doi: 10.1152/japplphysiol.00757.2003. [DOI] [PubMed] [Google Scholar]
- 10.Chan CB, Kashemsant N. Regulation of insulin secretion by uncoupling protein. Biochem Soc Trans. 2006;34:802–805. doi: 10.1042/BST0340802. [DOI] [PubMed] [Google Scholar]
- 11.De Souza CT, Araujo EP, Stoppiglia LF, Pauli JR, Ropelle E, Rocco SA, Marin RM, Franchini KG, Carvalheira JB, Saad MJ, Boschero AC, Carneiro EM, Velloso LA. Inhibition of UCP2 expression reverses diet-induced diabetes mellitus by effects on both insulin secretion and action. FASEB J. 2007;21:1153–1163. doi: 10.1096/fj.06-7148com. [DOI] [PubMed] [Google Scholar]
- 12.De Souza CT, Gasparetti AL, Pereira-da-Silva M, Araujo EP, Carvalheira JB, Saad MJ, Boschero AC, Carneiro EM, Velloso LA. Peroxisome proliferator-activated receptor gamma coactivator-1-dependent uncoupling protein-2 expression in pancreatic islets of rats: a novel pathway for neural control of insulin secretion. Diabetologia. 2003;46:1522–1531. doi: 10.1007/s00125-003-1222-5. [DOI] [PubMed] [Google Scholar]
- 13.Dulloo AG, Samec S. Uncoupling proteins: their roles in adaptive thermogenesis and substrate metabolism reconsidered. Br J Nutr. 2001;86:123–139. doi: 10.1079/bjn2001412. [DOI] [PubMed] [Google Scholar]
- 14.Eaton S, Bartlett K, Pourfarzam M. Mammalian mitochondrial beta-oxidation. Biochem J. 1996;320 (Pt 2):345–357. doi: 10.1042/bj3200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finn PF, Dice JF. Proteolytic and lipolytic responses to starvation. Nutrition. 2006;22:830–844. doi: 10.1016/j.nut.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Foufelle F, Ferre P. New perspectives in the regulation of hepatic glycolytic and lipogenic genes by insulin and glucose: a role for the transcription factor sterol regulatory element binding protein-1c. Biochem J. 2002;366:377–391. doi: 10.1042/BJ20020430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull. 2001;60:5–20. doi: 10.1093/bmb/60.1.5. [DOI] [PubMed] [Google Scholar]
- 18.Hashimoto T, Fujita T, Usuda N, Cook W, Qi C, Peters JM, Gonzalez FJ, Yeldandi AV, Rao MS, Reddy JK. Peroxisomal and mitochondrial fatty acid beta-oxidation in mice nullizygous for both peroxisome proliferator-activated receptor alpha and peroxisomal fatty acyl-CoA oxidase. Genotype correlation with fatty liver phenotype. J Biol Chem. 1999;274:19228–19236. doi: 10.1074/jbc.274.27.19228. [DOI] [PubMed] [Google Scholar]
- 19.Hegardt FG. Mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase: a control enzyme in ketogenesis. Biochem J. 1999;338 (Pt 3):569–582. [PMC free article] [PubMed] [Google Scholar]
- 20.Heijboer AC, Donga E, Voshol PJ, Dang ZC, Havekes LM, Romijn JA, Corssmit EP. Sixteen hours of fasting differentially affects hepatic and muscle insulin sensitivity in mice. J Lipid Res. 2005;46:582–588. doi: 10.1194/jlr.M400440-JLR200. [DOI] [PubMed] [Google Scholar]
- 21.Hong Y, Fink BD, Dillon JS, Sivitz WI. Effects of adenoviral overexpression of uncoupling protein-2 and -3 on mitochondrial respiration in insulinoma cells. Endocrinology. 2001;142:249–256. doi: 10.1210/endo.142.1.7889. [DOI] [PubMed] [Google Scholar]
- 22.Horimoto M, Fulop P, Derdak Z, Wands JR, Baffy G. Uncoupling protein-2 deficiency promotes oxidant stress and delays liver regeneration in mice. Hepatology. 2004;39:386–392. doi: 10.1002/hep.20047. [DOI] [PubMed] [Google Scholar]
- 23.Horton JD, Bashmakov Y, Shimomura I, Shimano H. Regulation of sterol regulatory element binding proteins in livers of fasted and refed mice. Proc Natl Acad Sci U S A. 1998;95:5987–5992. doi: 10.1073/pnas.95.11.5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jensen MD, Haymond MW, Gerich JE, Cryer PE, Miles JM. Lipolysis during fasting. Decreased suppression by insulin and increased stimulation by epinephrine. J Clin Invest. 1987;79:207–213. doi: 10.1172/JCI112785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kersten S, Desvergne B, Wahli W. Roles of PPARs in health and disease. Nature. 2000;405:421–424. doi: 10.1038/35013000. [DOI] [PubMed] [Google Scholar]
- 26.Kersten S, Seydoux J, Peters JM, Gonzalez FJ, Desvergne B, Wahli W. Peroxisome proliferator-activated receptor alpha mediates the adaptive response to fasting. JCI. 1999;103:1489–1498. doi: 10.1172/JCI6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kohjima M, Enjoji M, Higuchi N, Kato M, Kotoh K, Yoshimoto T, Fujino T, Yada M, Yada R, Harada N, Takayanagi R, Nakamuta M. Re-evaluation of fatty acid metabolism-related gene expression in nonalcoholic fatty liver disease. Int J Mol Med. 2007;20:351–358. [PubMed] [Google Scholar]
- 28.Koshkin V, Wang X, Scherer PE, Chan CB, Wheeler MB. Mitochondrial functional state in clonal pancreatic beta-cells exposed to free fatty acids. J Biol Chem. 2003;278:19709–19715. doi: 10.1074/jbc.M209709200. [DOI] [PubMed] [Google Scholar]
- 29.Krauss S, Zhang CY, Lowell BB. The mitochondrial uncoupling-protein homologues. Nat Rev Mol Cell Biol. 2005;6:248–261. doi: 10.1038/nrm1592. [DOI] [PubMed] [Google Scholar]
- 30.Lameloise N, Muzzin P, Prentki M, Assimacopoulos-Jeannet F. Uncoupling protein 2: a possible link between fatty acid excess and impaired glucose-induced insulin secretion? Diabetes. 2001;50:803–809. doi: 10.2337/diabetes.50.4.803. [DOI] [PubMed] [Google Scholar]
- 31.Leone TC, Weinheimer CJ, Kelly DP. A critical role for the peroxisome proliferator-activated receptor alpha (PPARalpha) in the cellular fasting response: the PPARalpha-null mouse as a model of fatty acid oxidation disorders. Proc Natl Acad Sci U S A. 1999;96:7473–7478. doi: 10.1073/pnas.96.13.7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewis GF, Carpentier A, Adeli K, Giacca A. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr Rev. 2002;23:201–229. doi: 10.1210/edrv.23.2.0461. [DOI] [PubMed] [Google Scholar]
- 33.Louet JF, Le May C, Pegorier JP, Decaux JF, Girard J. Regulation of liver carnitine palmitoyltransferase I gene expression by hormones and fatty acids. Biochem Soc Trans. 2001;29:310–316. doi: 10.1042/0300-5127:0290310. [DOI] [PubMed] [Google Scholar]
- 34.Matsusue K, Haluzik M, Lambert G, Yim SH, Gavrilova O, Ward JM, Brewer B, Jr, Reitman ML, Gonzalez FJ. Liver-specific disruption of PPARgamma in leptin-deficient mice improves fatty liver but aggravates diabetic phenotypes. J Clin Invest. 2003;111:737–747. doi: 10.1172/JCI17223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mattiasson G, Sullivan PG. The emerging functions of UCP2 in health, disease, and therapeutics. Antioxid Redox Signal. 2006;8:1–38. doi: 10.1089/ars.2006.8.1. [DOI] [PubMed] [Google Scholar]
- 36.Medvedev AV, Robidoux J, Bai X, Cao W, Floering LM, Daniel KW, Collins S. Regulation of the uncoupling protein-2 gene in INS-1 beta-cells by oleic acid. J Biol Chem. 2002;277:42639–42644. doi: 10.1074/jbc.M208645200. [DOI] [PubMed] [Google Scholar]
- 37.Millet L, Vidal H, Andreelli F, Larrouy D, Riou JP, Ricquier D, Laville M, Langin D. Increased uncoupling protein-2 and -3 mRNA expression during fasting in obese and lean humans. J Clin Invest. 1997;100:2665–2670. doi: 10.1172/JCI119811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moller DE, Kaufman KD. Metabolic syndrome: a clinical and molecular perspective. Annu Rev Med. 2005;56:45–62. doi: 10.1146/annurev.med.56.082103.104751. [DOI] [PubMed] [Google Scholar]
- 39.Nakamura MT, Cheon Y, Li Y, Nara TY. Mechanisms of regulation of gene expression by fatty acids. Lipids. 2004;39:1077–1083. doi: 10.1007/s11745-004-1333-0. [DOI] [PubMed] [Google Scholar]
- 40.Nedergaard J, Cannon B. The ‘novel’ uncoupling proteins UCP2 and UCP3: What do they really do? Pros and cons for suggested functions. Exp Physiol. 2003;88:65–84. doi: 10.1113/eph8802502. [DOI] [PubMed] [Google Scholar]
- 41.Newgard CB, McGarry JD. Metabolic coupling factors in pancreatic beta-cell signal transduction. Annu Rev Biochem. 1995;64:689–719. doi: 10.1146/annurev.bi.64.070195.003353. [DOI] [PubMed] [Google Scholar]
- 42.Orellana M, Fuentes O, Rosenbluth H, Lara M, Valdes E. Modulation of rat liver peroxisomal and microsomal fatty acid oxidation by starvation. FEBS Lett. 1992;310:193–196. doi: 10.1016/0014-5793(92)81327-i. [DOI] [PubMed] [Google Scholar]
- 43.Rousset S, Mozo J, Dujardin G, Emre Y, Masscheleyn S, Ricquier D, Cassard-Doulcier AM. UCP2 is a mitochondrial transporter with an unusual very short half-life. FEBS Lett. 2007;581:479–482. doi: 10.1016/j.febslet.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 44.Saleh MC, Wheeler MB, Chan CB. Endogenous islet uncoupling protein-2 expression and loss of glucose homeostasis in ob/ob mice. J Endocrinol. 2006;190:659–667. doi: 10.1677/joe.1.06715. [DOI] [PubMed] [Google Scholar]
- 45.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 46.Wada F, Usami M. Studies on fatty acid omega-oxidation. Antiketogenic effect and gluconeogenicity of dicarboxylic acids. Biochim Biophys Acta. 1977;487:361–368. [PubMed] [Google Scholar]
- 47.Wahren J, Sato Y, Ostman J, Hagenfeldt L, Felig P. Turnover and splanchnic metabolism of free fatty acids and ketones in insulin-dependent diabetics at rest and in response to exercise. J Clin Invest. 1984;73:1367–1376. doi: 10.1172/JCI111340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wetterau JR, Zilversmit DB. A triglyceride and cholesteryl ester transfer protein associated with liver microsomes. J Biol Chem. 1984;259:10863–10866. [PubMed] [Google Scholar]
- 49.Yu L, Liu C, Vandeusen J, Becknell B, Dai Z, Wu YZ, Raval A, Liu TH, Ding W, Mao C, Liu S, Smith LT, Lee S, Rassenti L, Marcucci G, Byrd J, Caligiuri MA, Plass C. Global assessment of promoter methylation in a mouse model of cancer identifies ID4 as a putative tumor-suppressor gene in human leukemia. Nat Genet. 2005;37:265–274. doi: 10.1038/ng1521. [DOI] [PubMed] [Google Scholar]
- 50.Yu S, Matsusue K, Kashireddy P, Cao WQ, Yeldandi V, Yeldandi AV, Rao MS, Gonzalez FJ, Reddy JK. Adipocyte-specific gene expression and adipogenic steatosis in the mouse liver due to peroxisome proliferator-activated receptor gamma1 (PPARgamma1) overexpression. J Biol Chem. 2003;278:498–505. doi: 10.1074/jbc.M210062200. [DOI] [PubMed] [Google Scholar]
- 51.Zhang C, Baffy G, Perret P, Krauss S, Peroni O, Grujic D, Hagen T, Vidal-Puig AJ, Boss O, Kim Y, Zheng XX, Wheeler MB, Shulman GI, Chan CB, Lowell BB. Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, beta cell dysfunction, and type 2 diabetes. Cell. 2001;105:745–755. doi: 10.1016/s0092-8674(01)00378-6. [DOI] [PubMed] [Google Scholar]
- 52.Zhang CY, Parton LE, Ye CP, Krauss S, Shen R, Lin CT, Porco JA, Jr, Lowell BB. Genipin inhibits UCP2-mediated proton leak and acutely reverses obesity- and high glucose-induced beta cell dysfunction in isolated pancreatic islets. Cell Metab. 2006;3:417–427. doi: 10.1016/j.cmet.2006.04.010. [DOI] [PubMed] [Google Scholar]