Abstract
We describe the construction and use of two sets of vectors for the over-expression and purification of protein from E. coli. The set of pTEV plasmids (pTEV3, 4, 5) directs the synthesis of a recombinant protein with a N-terminal hexahistidine (His6) tag that is removable by the tobacco etch virus (TEV) protease. The set of pKLD plasmids (pKLD66, 116) directs the synthesis of a recombinant protein that contains a N-terminal His6 and maltose-binding protein tags in tandem, which can also be removed with TEV protease. The usefulness of these plasmids is illustrated by the rapid, high-yield purification of the 2-methylcitrate dehydratase (PrpD) protein of Salmonella enterica, and the 2-methylaconitate isomerase (PrpF) protein of Shewanella oneidensis, two enzymes involved in the catabolism of propionate to pyruvate via the 2-methylcitric acid cycle.
Keywords: TEV protease-cleavable tags, rapid protein purification, propionate catabolism, 2-methylcitric acid cycle enzymes, 2-methylaconitate isomerase, 2-methylcitrate dehydratase
INTRODUCTION
The synthesis of the first biologically functional bacterial plasmid (Cohen et al., 1973) signaled the beginning of molecular cloning and recombinant DNA technology. With the advent of recombinant DNA technology, researchers were first able to selectively increase the expression of single genes, which in turn increased yields of protein purifications. However purification of proteins in their native form is still a difficult and time-consuming process.
Not until the advent of affinity tag purification did protein purification become a faster and more efficient process. Development of affinity tags such as the maltose-binding tag (di Guan et al., 1988; Maina et al., 1988), the polyhistidine tags of the pET vector series, (Rosenberg et al., 1987; Studier & Moffatt, 1986; Studier et al., 1990), and the IMPACT® intein chitin-binding tag (Chong et al., 1997; Chong et al., 1998) allowed for more efficient and rapid purification of diverse proteins at yields much higher than would have been possible through native purification. The development of instruments such as the Maxwell™ 16 Instrument and the Maxwell™ 16 Polyhistidine Protein Purification Kit (Promega), have taken protein purification a step further by now allowing for the automated purification of up to 16 polyhistidine tagged proteins in less than one hour.
While the addition of affinity tags allow for ease of purification, it does render the protein into a non-native state and the affinity tag can often hamper subsequent work with the protein. While the IMPACT® system requires cleavage of the protein from the tag as an elution step, other systems require a subsequent cleavage and purification to remove the affinity tag. One method of tag removal is through the use of the tobacco etch virus (TEV) protease. TEV protease has become one of the proteases of choice for cleaving fusion proteins due to its high degree of specificity, its resistance to many protease inhibitors used in protein purification, and the ease of separation of both the protease and affinity tag from the protein of interest (Parks et al., 1994). Additionally, improvement to the ability to purify large amounts of TEV protease (Blommel & Fox, 2007; Lucast et al., 2001; van den Berg et al., 2006) can make the TEV protease a relatively inexpensive option for cleavage of fusion proteins when compared to other commercially available options.
Previous work has established that various protein fusion vectors featuring rTEV protease cleavage sites are viable for the purification of high levels of recombinant protein through high-throughput approaches (Dummler et al., 2005; Korf et al., 2005) or via ligation independent cloning (Cabrita et al., 2006). Here we report on the construction of two new series of vectors to the growing family of rTEV-cleavable protein fusion vectors that allow for efficient purification of recombinant bacterial proteins. The pTEV series is based off the Novagen pET vector series and incorporates a TEV protease cleavage site as well as an improved variety of restriction endonucleases sites to increase cloning options. The pKLD series utilizes the pET vector backbone while incorporating both a polyhistidine tag as well as the maltose binding tag from the New England Biolabs pMAL vector series. The pKLD series also have a TEV protease cleavage site and improved multiple cloning sites.
RESULTS AND DISCUSSION
pTEV plasmids direct the synthesis of proteins fused to a hexahistidine (H6) N terminal tag
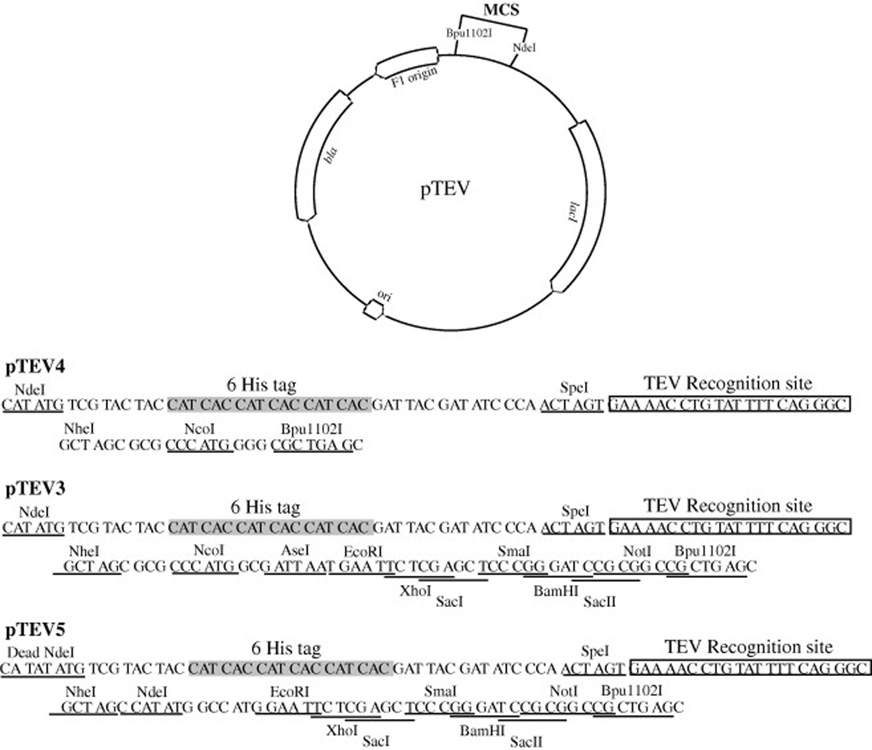
pTEV vectors used to overproduce proteins with TEV-cleavable N-terminal His6 tags were constructed by modifying the Novagen ketosteroid isomerase (KSI) fusion plasmid pET-31b(+). The KSI coding sequence was removed by digestion restriction enzymes NdeI and Bpu1102I (EspI). A hexahistidine (His6) tag coding sequence, a seven amino acid spacer sequence, and a SpeI site were introduced at the NdeI and Bpu1102I sites with the rTEVLink1 DNA fragment (Table 1, supplemental material). This temporary plasmid was designated pKLD35. A rTEV protease cleavage site was inserted into plasmid pKLD35 at the SpeI and Bpu1102I sites with the rTEVLink2 DNA fragment (Table 1, supplemental material). The resulting plasmid was named pTEV4 (Figure 1). If the NheI site is used for cloning the coding sequence of the gene of choice, after TEV protease cleavage the resulting protein only has three additional amino acids (Gly-Ala-Ser) attached to the N-terminus (Figure 1).
Table 1. Oligonucleotides used to construct pTEV plasmids.
The SpeI sites in rTEVLink1F and rTEVLink1R are in bold type. The sequence introduced into pMAL–c2x with the pMALHisMut mutagenic oligonucleotides is underlined; all other sequence is complementary.
| Name | Sequence 5’ → 3’ |
|---|---|
| rTEVLink1F | TATGTCGTACTACCATCACCATCACCATCACGATTACGATATCCCAACTAGTGGCGC |
| rTEVLink1R | TCAGCGCCACTAGTTGGGATATCGTAATCGTGATGGTGATGGTGATGGTAGTACGACA |
| rTEVLink2F | CTAGTGAAAACCTGTATTTTCAGGGCGCTAGCGCGCCCATGGGGCGC |
| rTEVLink2 | TCAGCGCCCCATGGGCGCGCTAGCGCCCTGAAAATACAGGTTTTCA |
| TEV MCS 1 | CATGGCGATTAATGAATTCTCGAGCTCCCGGGATCCGCGGCCGC |
| TEV MCS 2 | TGAGCGGCCGCGGATCCCGGGAGCTCGAGAATTCATTAATCGC |
| TEV Start MCS 1 | CTAGCCATATGGCCATGG |
| TEV Start MCS 2 | AATTCCATGGCCATATGG |
| pMALHisMutF | CCAACAAGGACCATAGCATATGGGCAGCCATCACCATCACCATCACTCCGGTAAAATCGAAGAAGGTAAACTGG |
| pMALHisMutR | CCAGTTTACCTTCTTCGATTTT ACCGGAGTGATGGTGATGGTGATGGCTGCC CATATGCTATGGTCCTTGTTGG |
| MCSInsertF | CGAGCGGAACCGCCTCGGGCGGTGCAACCACGTCAGAGAATCTCTACTTCCAAGGTACCTCGGACT |
| MCSInsertR | CTAGAGTCCGAGGTACCTTGGAAGTAGAGATTCTCTGACGTGGTTGCACCGCCCGAGGCGGTTCCGCTCGAGCT |
| NEW66f | CGAGCGGAACCGCCTCGGGCGGTGCAACCACTAGTGAGAATCTCTACTTCCAAGGCCTTAGCAGGT |
| NEW66r | GCATGTGGACGTCCCGGGCTAGCCATGGCCTGCA |
Figure 1. Map of pTEV plasmids.
Plasmid schematic is shown with relevant genetic elements. Plasmid sequence between the NdeI and Bpu1102I restrictions sites is shown below for each individual plasmid.
To increase the usefulness of plasmid pTEV4, the number of restriction sites available in the multiple cloning site (MCS) was increased. The TEV MCS DNA fragment (Table 1) was ligated into plasmid pTEV4 at the NheI and Bpu1102I (EspI) restriction sites. The resulting plasmid was named pTEV3 (Figure 1).
Moving genes from pET vectors into pTEV plasmids
The NdeI site of pTEV3 was inactivated thorough digestion and end filling. A new NdeI site was introduced with the TEVStartMCS DNA fragment (Table 1, supplemental material) at the NheI and EcoRI restriction sites. This final plasmid was given the name pTEV5 (Figure 1). Use of the NdeI site as a 5’ cloning site results in four additional residues (Gly-Ala-Ser-His) at the N-terminus of the protein after TEV protease cleavage (Figure 1). While the use of this site adds several additional residues, the NdeI site is a common 5’ cloning site in other pET vectors and addition of it to the pTEV vectors allows for the quick removal of an insert from pET vectors and insertion into the pTEV vector series.
Use of His6-TEV vectors to isolate the 2-methylaconitate isomerase (PrpF) enzyme of Shewanella oneidensis
To assess the usefulness of pTEV plasmids, we expressed the prpF gene from Shewanella oneidensis after cloning it into the NheI and NcoI sites of plasmid pTEV4. The resulting plasmid (pPRP196) was transformed into E. coli strain Bl21(λDE3) (New England Biolabs), a strain widely used to overproduce recombinant proteins.
His6-PrpF was purified from clarified cell-free extracts using the Novagen His-Bind® nickel affinity chromatography kit following manufacturer’s instructions and the results can be seen in Figure 2. Enzymatic assays were performed to determine that the protein was active and the average yield of protein from purification, after rTEV cleavage, was 15 mg per gram of wet cells. The purity of the protein was not determined as no contaminating bands could be observed in SDS-PAGE gels, so the protein was assumed to be greater than 95% pure.
Figure 2. Purification of rTEV-cleavable His-Tagged 2-methylaconitate isomerase (PrpF) enzyme.
A culture of the over expression strain was grown at 37°C in LB supplemented with ampicillin to an OD650 of ~0.6 and induced with IPTG (0.3 mM) for approximately 18 h. Cells were harvested and protein was purified using Novagen’s His-Bind® Kit and manufacturer’s procedures. A 12% SDS-PAGE gel was loaded with 20 μg of lysed cells (Lane 2), soluble cell free extract (Lane 3), column flow-through (Lane 4), and 10 μg of wash fraction (Lane 5) and eluted protein (Lane 6). Bio-Rad’s Precision Plus Protein™ Standard were loaded in lane 1. The gel was stained with Coomassie Brilliant Blue.
Along with pTEV4, both pTEV3 and pTEV5 have been used to purify large amounts of highly purified protein with an average protein yield after removal of the tag, varying from 10–20 mg protein per gram of wet cells, depending upon the individual protein purified. These proteins have been used in both enzymatic and crystallographic studies which helps demonstrate the functionality of these plasmids (Garvey et al., 2007; Gray & Escalante-Semerena, 2007; Lewis & Escalante-Semerena, 2007; St Maurice et al., 2007).
Construction of His-MBP-TEV vectors
In addition to the modified pET vectors, we engineered a maltose binding protein (MBP) fusion vector to include a rTEV protease cleavage site. First, a His6 tag was introduced 5’ from the malE gene in vector pMAL-c2x (NEB). The pMALHisMut DNA fragment (Table 1, supplemental material) was ligated to the plasmid 5’ to the MalE gene. This temporary plasmid was given the name pKLD54. To introduce a rTEV cleavage site and amino acid spacer downstream from the malE gene, plasmid pKLD54 was cut with restriction endonucleases SacI and XbaI and the DNA fragment MCSInsert (Table 1, supplemental material) was ligated into the plasmid. The resulting plasmid was named pKLD55. pKLD55 was cut with restriction endonucleases NdeI and HindIII to remove the His6-malE-TEV fragment, which was inserted into pET21a(+) at the same sites. The resulting plasmid was named pKLD66 (Figure 3). To increase the number of restriction sites in the multiple cloning site, the NEW66 DNA fragment (Table 1, supplemental material) was ligated into plasmid pKLD66 at the SacI and SbfI sites. The resulting plasmid was named pKLD116 (Figure 3). In plasmid pKLD66, use of the KpnI restriction site as a 5’ cloning site results in recombinant protein with two additional residues (Gly-Thr) at the N-terminus of the protein (Figure 3). The StuI site in plasmid pKLD116 permits blunt-end cloning at the 5’ end and can, depending on the restriction enzyme used to cut the insert, result in a single glycine added to the N-terminus of the protein.
Figure 3. Map of pKLD plasmids.
Plasmid schematic is shown with relevant genetic elements. Plasmid sequence between NdeI and NotI restriction sites is shown for each individual plasmid. The male gene is shown in a truncated form for space considerations.
Isolation of 2-methylcitrate dehydratase (PrpD) protein of Salmonella enterica using His- MBP-TEV vectors
To assess the usefulness of the MBP tag in the isolation of recombinant proteins, the 2-methylcitrate dehydratase gene (prpD) gene of Salmonella enterica was cloned into the KpnI and NotI sites of plasmid pKLD66 to yield plasmid pPRP222. The latter was introduced by transformation into E. coli strain Bl21(λDE3), the prpD gene was expressed, and the fusion protein was purified (Figure 4).
Figure 4. Purification of MBP-tagged 2-mehtylcitrate dehydratase (PrpD) protein.
Cells were grown in LB supplemented with ampicillin at 37°C to an OD650 of ~0.7 and induced with IPTG for 16 h. Cells were harvested by centrifugation and broken with Novagen's BugBuster® plus lysozyme (1mg/ml). Protein was purified using Bio-Rad Laboratories Amylose Resin High Flow as per manufacturer’s protocols. A 12% SDS PAGE gel was loaded with 20 μg of cell slurry (lane 2), cell-free extract (lane 3), and column flow-through (lane 4), column wash fraction (lane 5) and 3 μg of eluted protein (lane 6). Bio Rad’s Precision Plus Protein™ Standards were loaded in lane 1. The gel was stained with Coomassie Brilliant Blue.
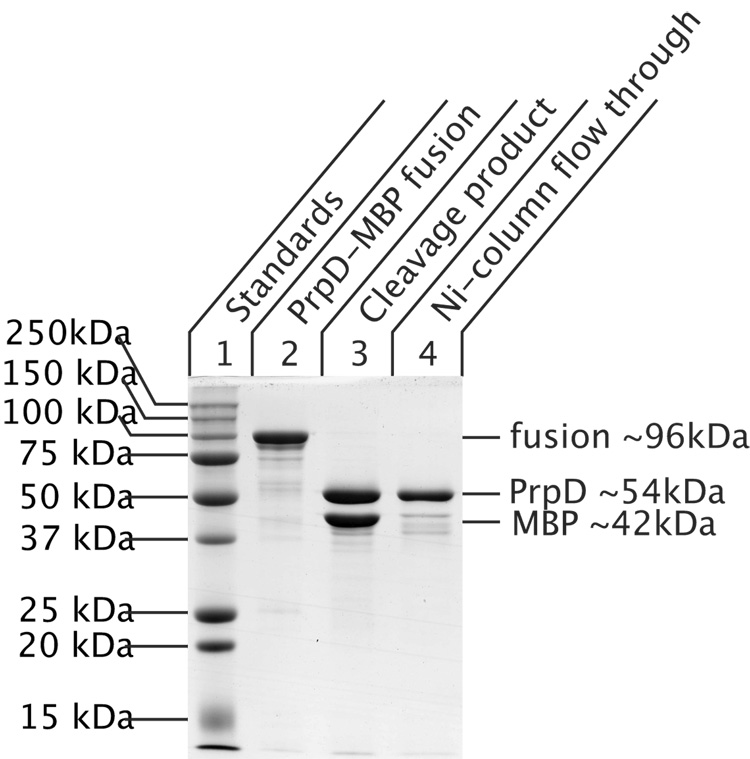
The yield of purified protein was high (~ 25 mg of recombinant protein per gram of wet cells) and could be increased based on the large amount of recombinant protein that was visible in the column flow through (Figure 4, Lane 4). Other experiments have shown that this protein was soluble and active, suggesting that in all probability the binding capacity of the column used in this experiment was exceeded. Recombinant protein was digested with rTEV protease, purified as described by Blommel and Fox (Blommel & Fox, 2007), and the resulting PrpD protein was purified from the His6-MBP tag and His6-rTEV (Figure 5). Approximately 90% of PrpD protein was recovered from the rTEV digestion reaction, and assays indicated that the enzyme was active. The high yield of recovery of cleaved PrpD protein indicated that the cleavage of the MBP tag and recovery was a very efficient process.
Figure 5. Cleavage and purification of recombinant His6-MBP-PrpD protein.
Lane 1: Bio-Rad Precision Plus Protein™ Standards; lane 2: recombinant His6-MBP-PrpD protein; lane 3: PrpD protein after rTEV cleavage; lane 4: purified PrpD protein. Cleavage of recombinant His6-MBP-PrpD with rTEV protease was performed at a 50:1 mg:mg ratio during overnight dialysis at 4°C against Tris•HCl buffer (20 mM, pH 7.9 at 25°C) containing NaCl (200 mM), DTT (5 mM). DTT was removed by dialysis Tris•HCl buffer (20 mM, pH 7.9 at 25°C) containing 400 mM NaCl and the cleaved protein was purified by running over a Novagen His-Bind® Resin column to remove both the His6-MBP tag and His6-rTEV protease.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by PHS grant GM62203 to J.C.E.-S, and by grant AR35186 to I.R.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Accesion #: pTEV4, bankit1044661; pTEV3, bankit 1044673; pTEV5, bankit1044675; pKLD66, bankit1044676; pKLD116, banlit1044677.
REFERENCES
- Bertani G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 1951;62:293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertani G. Lysogeny at mid-twentieth century: P1, P2, and other experimental systems. J Bacteriol. 2004;186:595–600. doi: 10.1128/JB.186.3.595-600.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blommel PG, Fox BG. A combined approach to improving large-scale production of tobacco etch virus protease. Protein Expr Purif. doi: 10.1016/j.pep.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–255. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cabrita LD, Dai W, Bottomley SP. A family of E. coli expression vectors for laboratory scale and high throughput soluble protein production. BMC Biotechnol. 2006;6:12. doi: 10.1186/1472-6750-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong S, Mersha FB, Comb DG, et al. Single-column purification of free recombinant proteins using a self-cleavable affinity tag derived from a protein splicing element. Gene. 1997;192:271–281. doi: 10.1016/s0378-1119(97)00105-4. [DOI] [PubMed] [Google Scholar]
- Chong S, Montello GE, Zhang A, Cantor EJ, Liao W, Xu MQ, Benner J. Utilizing the C-terminal cleavage activity of a protein splicing element to purify recombinant proteins in a single chromatographic step. Nucleic Acids Res. 1998;26:5109–5115. doi: 10.1093/nar/26.22.5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SN, Chang AC, Boyer HW, Helling RB. Construction of biologically functional bacterial plasmids in vitro. Proc Natl Acad Sci U S A. 1973;70:3240–3244. doi: 10.1073/pnas.70.11.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Guan C, Li P, Riggs PD, Inouye H. Vectors that facilitate the expression and purification of foreign peptides in Escherichia coli by fusion to maltose-binding protein. Gene. 1988;67:21–30. doi: 10.1016/0378-1119(88)90004-2. [DOI] [PubMed] [Google Scholar]
- Dummler A, Lawrence AM, de Marco A. Simplified screening for the detection of soluble fusion constructs expressed in E. coli using a modular set of vectors. Microb Cell Fact. 2005;4:34. doi: 10.1186/1475-2859-4-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey GS, Rocco CJ, Escalante-Semerena JC, Rayment I. The three-dimensional crystal structure of the PrpF protein of Shewanella oneidensis complexed with trans-aconitate: insights into its biological function. Protein Sci. 2007;16:1274–1284. doi: 10.1110/ps.072801907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray MJ, Escalante-Semerena JC. Single-enzyme conversion of FMNH2 to 5,6- dimethylbenzimidazole, the lower ligand of B12. Proc Natl Acad Sci U S A. 2007;104:2921–2926. doi: 10.1073/pnas.0609270104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H, Nojima H, Okayama H. High efficiency transformation of Escherichia coli with plasmids. Gene. 1990;96:23–28. doi: 10.1016/0378-1119(90)90336-p. [DOI] [PubMed] [Google Scholar]
- Korf U, Kohl T, van der Zandt H, et al. Large-scale protein expression for proteome research. Proteomics. 2005;5:3571–3580. doi: 10.1002/pmic.200401195. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage and structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lewis JA, Escalante-Semerena JC. Tricarballylate Catabolism in Salmonella enterica. The TcuB Protein Uses 4Fe-4S Clusters and Heme to Transfer Electrons from FADH(2) in the Tricarballylate Dehydrogenase (TcuA) Enzyme to Electron Acceptors in the Cell Membrane. Biochemistry. 2007;46:9107–9115. doi: 10.1021/bi7006564. [DOI] [PubMed] [Google Scholar]
- Lucast LJ, Batey RT, Doudna JA. Large-scale purification of a stable form of recombinant tobacco etch virus protease. Biotechniques. 2001;30:544–546. doi: 10.2144/01303st06. 548, 550 passim. [DOI] [PubMed] [Google Scholar]
- Maina CV, Riggs PD, Grandea AG, 3rd,, Slatko BE, Moran LS, Tagliamonte JA, McReynolds LA, Guan CD. An Escherichia coli vector to express and purify foreign proteins by fusion to and separation from maltose-binding protein. Gene. 1988;74:365–373. doi: 10.1016/0378-1119(88)90170-9. [DOI] [PubMed] [Google Scholar]
- Parks TD, Leuther KK, Howard ED, Johnston SA, Dougherty WG. Release of proteins and peptides from fusion proteins using a recombinant plant virus proteinase. Anal Biochem. 1994;216:413–417. doi: 10.1006/abio.1994.1060. [DOI] [PubMed] [Google Scholar]
- Rosenberg AH, Lade BN, Chui DS, Lin SW, Dunn JJ, Studier FW. Vectors for selective expression of cloned DNAs by T7 RNA polymerase. Gene. 1987;56:125–135. doi: 10.1016/0378-1119(87)90165-x. [DOI] [PubMed] [Google Scholar]
- Sasse J. Detection of proteins. In: Ausubel FA, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Current Protocols in Molecular Biology. New York: Wiley Interscience; 1991. pp. 10.16.11–10.16.18. [Google Scholar]
- St Maurice M, Mera PE, Taranto MP, Sesma F, Escalante-Semerena JC, Rayment I. Structural characterization of the active site of the PduO-type ATP:Co(I)rrinoid adenosyltransferase from Lactobacillus reuteri. J Biol Chem. 2007;282:2596–2605. doi: 10.1074/jbc.M609557200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier FW, Moffatt BA. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986;189:113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Studier FW, Rosenberg AH, Dunn JJ, Dubendorff JW. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- van den Berg S, Lofdahl PA, Hard T, Berglund H. Improved solubility of TEV protease by directed evolution. J Biotechnol. 2006;121:291–298. doi: 10.1016/j.jbiotec.2005.08.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.