Abstract
Objective: We explored the relevance and significance of connective tissue growth factor (CTGF) as a determinant of renal and vascular complications among type 1 diabetic patients.
Methods and Results: We measured the circulating and urinary levels of CTGF and CTGF N fragment in 1050 subjects with type 1 diabetes from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study cohort. We found that hypertensive diabetic subjects have significantly higher levels of plasma log CTGF N fragment relative to normotensive subjects (P = 0.0005). Multiple regression analysis showed a positive and independent association between CTGF N fragment levels and log albumin excretion rate (P < 0.0001). In categorical analysis, patients with macroalbuminuria had higher levels of CTGF N fragment than diabetic subjects with or without microalbuminuria (P < 0.0001). Univariate and multiple regression analyses demonstrated an independent and significant association of log CTGF N fragment with the common and internal carotid intima-media thickness. The relative risk for increased carotid intima-media thickness was higher in patients with concomitantly elevated plasma CTGF N fragment and macroalbuminuria relative to patients with normal plasma CTGF N fragment and normal albuminuria (relative risk = 4.76; 95% confidence interval, 2.21–10.25; P < 0.0001).
Conclusion: These findings demonstrate that plasma CTGF is a risk marker of diabetic renal and vascular disease.
In patients with type 1 diabetes, circulating levels of connective tissue growth factor (CTGF) and CTGF N fragment are risk factors for vascular and renal disease, in that increasing plasma CTGF N fragments are associated with higher arterial pressure, albuminuria, and greater carotid intima-media thickness.
Diabetes mellitus is associated with increased morbidity and mortality derived mainly from cardiovascular complications. The progression of microvascular and macrovascular disease associated with diabetes is exacerbated by hypertension, but the mechanisms by which diabetes and hypertension accelerate vascular damage are as yet undefined (1,2).
The development of micro- and macroalbuminuria in diabetic and nondiabetic individuals also augments risk for the development of macrovascular disease. Type 1 diabetic patients with proteinuria have a 10-fold or greater risk of macrovascular disease relative to type 1 patients without proteinuria. The relation of microalbuminuria to vascular disease as measured by carotid intima-medial thickness (IMT) was recently illustrated in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) cohort of type 1 diabetic patients (3). Diabetic renal disease is associated with elevations of blood pressure and dyslipidemia, conditions that typically precede and accelerate the progression of vascular disease in diabetic patients (4).
The mechanisms that link hyperglycemia, hypertension, renal disease, and dyslipidemia with vascular disease are not fully defined. Emerging evidence indicates that connective tissue growth factor (CTGF) may act along a causal pathway for vascular and renal disease. Relatively high levels of expression of CTGF mRNA and protein concentrations occur in vascular smooth muscle cells and endothelial cells of advanced human atherosclerotic lesions (5). In addition, vascular smooth muscle cells expressing CTGF are localized predominantly in areas with extracellular matrix production and especially in areas around the fibrous cap, indicating that CTGF regulates the production of matrix proteins in these cells (5). Renal expression of CTGF is up-regulated in diabetes and in other progressive renal diseases (6). In addition, CTGF has been shown to increase collagen I expression in human mesangial cells (7).
The relation between plasma levels of CTGF and CTGF N fragment, the predominant form of CTGF present in blood and urine, and the development of vascular disease in diabetic patients has not been explored. The present study was conducted to determine whether circulating levels of CTGF and CTGF N fragment identify type 1 diabetic subjects at risk for vascular and renal disease.
Subjects and Methods
Study population
The study population was a subgroup of the DCCT/EDIC cohort. The DCCT/EDIC cohort is comprised of 1325 (94%) of the original 1441 type 1 diabetic DCCT subjects. The original DCCT cohort, enrolled between 1983 and 1989, consisted of men and women from the ages of 13–40 yr with 1–15 yr of diabetes at study entry (8). Half of the patient population was randomly assigned to conventional diabetes treatment, and the other half was assigned to intensive diabetes treatment. In 1993, the DCCT study was stopped after an average follow-up time of 6.5 yr, when intensive treatment was shown to reduce the development and progression of retinopathy, nephropathy, and neuropathy by as much as 76% (8). The subjects were then invited to enroll in EDIC, a multicenter longitudinal observational study of the development of macrovascular complications and development and further progression of microvascular complications (9). At EDIC baseline in 1994, the average age of the DCCT/EDIC cohort was 35 yr (range 19–50 yr). Fifty-four percent of the cohort were male, and the mean duration of diabetes was 12 ± 5 yr. Fasting plasma samples, collected from 1997–1999 (EDIC yr 3–5) from 1025 DCCT/EDIC subjects for CTGF measurements, were shipped directly from participating EDIC clinics to Medical University of South Carolina and stored at −80 C. The Institutional Review Boards of Medical University of South Carolina and all participating DCCT/EDIC clinics approved the study, and written informed consent was obtained from each participant.
EDIC procedures
Each EDIC subject had a standardized annual history and physical examination, including a detailed evaluation of overall health, diabetes management, occurrence of diabetic complications, development of new disease, and medications used. Annual evaluations also included glycosylated hemoglobin (HbA1c) measurements, resting electrocardiograms, and arm blood pressure (BP) measurements. All measurements were standardized and carried out according to the EDIC protocol and methods of operations. Renal function was assessed every other year and included measurements of the urinary albumin excretion rate (AER) in a standardized 4-h collection (8). Microalbuminuria has been defined by the DCCT as an AER of 40–299 mg/24 h and macroalbuminuria as an AER of at least 300 mg/24 h. Normal albumin excretion was defined as an AER of less than or equal to 40 mg/24 h. About 98% of all AER measurements were carried out within 1 yr of blood sampling for CTGF for this study.
Ultrasonography and image analysis
Carotid ultrasonography was performed in the EDIC cohort from 1999–2001 as previously described (10).
CTGF measurement
Plasma and urine samples for CTGF, obtained after an overnight fast, were measured with a sandwich ELISA using monoclonal antibodies generated against recombinant human CTGF (rhCTGF) (11). Pairs of CTGF-specific monoclonal antibodies were used to construct two different ELISA designed to capture and detect whole CTGF (CTGF W) or N-terminal CTGF fragments plus whole CTGF (CTGF N+W) (FibroGen Inc., South San Francisco, CA). CTGF N fragment levels are derived by subtracting CTGF W from CTGF N+W. Microtiter plates (Maxisorb; Nunc, Rochester, NY) were coated overnight at 4 C with an anti-CTGF monoclonal antibody (10 μg/ml) used to capture CTGF and then were washed and blocked with PBS containing 1% BSA for at least 2 h. A non-cross-blocking anti-CTGF monoclonal antiboyd different from that used as capture monoclonal antibody, conjugated directly to alkaline phosphatase, was used for detection. Para-nitrophenol phosphate was used as the substrate for the colorimetric reaction. The plate was read at 405 nm (Vmax Plate Reader; Molecular Devices, Sunnyvale, CA). Standard curves were generated using rhCTGF standards run in triplicate with each set of samples. Samples were diluted 1:10 in assay buffer containing 50 μg/ml heparin, 0.1% Triton X-100, 0.1% BSA before being assayed in duplicate, and the coefficient of variation on duplicates was within 15%. A quadratic fit to the standard curve was used for calibration. Spike-recovery experiments using rhCTGF demonstrated quantitative detection in patient samples. Assay sensitivities (lower limits of quantitation) were 0.6 ng/ml for urine CTGF samples in the N+W assay and 5 ng/ml CTGF for serum samples in the W assay or N+W assay. The antibodies used in the ELISA are specific for CTGF and do not cross-react with CCN family members cyr61 and nov. CTGF content reported in urine is the sum of full-length CTGF and CTGF N-terminal half-fragments. The form of CTGF present in urine as measured by these assays was essentially solely CTGF N fragment. Within-run and between-run coefficients of variations were 5 and 15%, respectively.
Statistical analysis
The levels of CTGF in plasma and urine followed a skewed distribution, and we applied the Box-Cox transformation to CTGF levels. Log transformation converted raw CTGF data to normality, and transformed values were used in the analyses. In addition, a logarithmic transformation of AER was used to provide normality of residuals. To analyze continuous outcomes vs. each covariate separately, t tests were used, and χ2 tests were used to analyze discrete outcomes vs. each covariate. Pearson's correlation coefficients and Spearman nonparametric correlations were computed to assess the association between plasma log CTGF N fragment and BP, AER, and IMT.
Log of AER and carotid IMT (common and internal) were used as outcomes in regression analyses. In particular, when plasma log CTGF N fragment was the outcome, ANOVA and analysis of covariance were used to determine differences between mean levels of log plasma CTGF N fragment in plasma and urine among nephropathy subgroups. With outcomes log AER and carotid IMT, results from the univariate analyses were used to develop initial models for multiple linear regressions, and backward model selection procedures were applied to eliminate variables that were collinear or had nonsignificant partial tests. Log plasma CTGF N fragment levels were included as a covariate in the regression models for both outcomes log AER and IMT. Log AER was considered as a covariate in the model for carotid IMT. In these regression models, we adjusted for other covariates such as age, HbA1c levels, duration of diabetes, gender, and DCCT treatment group. Multivariate analysis using generalized linear models were performed to determine whether log CTGF N fragment will associate with both the common and internal carotid IMT in the same model. The square of the multiple partial correlation coefficient was calculated to estimate the increase in the percentage of the variance of the dependent variable explained by introducing that variable into a model that included all the other covariates. All statistical analyses were performed using SAS (version 9.1). Bonferroni adjustment was performed for multiple comparisons.
Results
CTGF N fragment levels in DCCT/EDIC cohort of type 1 diabetic patients
The clinical characteristics of the DCCT/EDIC subpopulation on whom the CTGF W and CTGF N fragment measurements were performed (n = 1052) are shown in Table 1. The demographic characteristics did not differ from those in the entire cohort (n = 1325, data not shown).
Table 1.
Clinical characteristics of the DCCT/EDIC cohort by gender
| Parameter | Male
|
Female
|
P | ||
|---|---|---|---|---|---|
| n | Mean ± sd | n | Mean ± sd | ||
| Age (yr) | 562 | 40 ± 7 | 452 | 39 ± 7 | 0.0632 |
| Weight (kg) | 561 | 86.8 ± 14.8 | 451 | 72.5 ± 14.9 | 0.0001 |
| BMI (kg/m2) | 551 | 27.1 ± 3.9 | 439 | 26.3 ± 4.3 | 0.0055 |
| Waist-hip ratio | 550 | 0.9 ± 0.1 | 438 | 0.8 ± 0.1 | 0.0001 |
| Duration of diabetes (yr) | 562 | 17.2 ± 4.7 | 452 | 17.7 ± 4.9 | 0.0743 |
| HbA1c (%) | 555 | 8.2 ± 1.3 | 447 | 8.2 ± 1.4 | 0.8643 |
| DCCT group | 562 | 1.5 ± 0.5 | 452 | 1.5 ± 0.5 | 0.287 |
| SBP (mm Hg) | 551 | 122.0 ± 13.3 | 439 | 116.0 ± 13.8 | 0.0001 |
| DBP (mm Hg) | 551 | 77.1 ± 9.3 | 439 | 72.6 ± 9.0 | 0.0001 |
| MBP (mm Hg) | 551 | 92.3 ± 9.6 | 439 | 87.1 ± 9.6 | 0.0001 |
| LDL (mg/dl) | 529 | 118.5 ± 31.2 | 423 | 110.0 ± 29.8 | 0.0001 |
| Total cholesterol (mg/dl) | 533 | 189.7 ± 36.2 | 424 | 188.2 ± 33.8 | 0.5162 |
| Triglycerides (mg/dl) | 533 | 98.0 ± 70.9 | 424 | 77.2 ± 49.3 | 0.0001 |
| HDL (mg/dl) | 533 | 51.6 ± 12.9 | 424 | 62.7 ± 14.8 | 0.0001 |
| Log AER (mg/24 h) | 553 | 2.8 ± 1.5 | 446 | 2.5 ± 1.3 | 0.0011 |
| Common IMT (mm) | 520 | 0.60 ± 0.09 | 415 | 0.56 ± 0.08 | 0.0001 |
| Internal IMT (mm) | 511 | 0.67 ± 0.24 | 401 | 0.62 ± 0.18 | 0.0002 |
| % Hypertensive | 554 | 44 ± 49 | 442 | 26 ± 44 | 0.0001 |
Variables were evaluated between groups using t and χ2 test for continuous and categorical variables, respectively. HDL, High-density lipoprotein.
Univariate analyses showed a strong association between log plasma CTGF N fragment levels and age, duration of diabetes, DCCT intensive group, systolic BP (SBP), log AER, low density lipoprotein (LDL), total cholesterol, and internal and common carotid IMT. There was no association between log plasma CTGF N fragment levels and HbA1c, gender, weight, body mass index (BMI), or waist to hip ratio (Table 2).
Table 2.
Associations between log CTGF N fragment levels and clinical parameters
| Variables | Effect | 95% Confidence interval
|
P | |
|---|---|---|---|---|
| Lower | Upper | |||
| Plasma | ||||
| Age (yr) | 0.0129 | 0.007 | 0.0188 | 0.0001 |
| Weight (kg) | 0.001 | −0.0015 | 0.0035 | 0.4533 |
| BMI (kg/m2) | 0.0034 | −0.0068 | 0.0136 | 0.5145 |
| Waist-hip ratio | 0.3557 | −0.1325 | 0.8439 | 0.1513 |
| Duration of diabetes (yr) | 0.0124 | −0.0038 | 0.021 | 0.0047 |
| HbA1c (%) | −0.017 | −0.048 | 0.014 | 0.2826 |
| DCCT group | 0.1031 | 0.0208 | 0.1853 | 0.0141 |
| SBP (mm Hg) | 0.0039 | 0.001 | 0.0069 | 0.0091 |
| DBP (mm Hg) | 0.0005 | −0.004 | 0.0049 | 0.8393 |
| Log AER (mg/24 h) | 0.0779 | 0.0481 | 0.1077 | 0 |
| LDL | 0.0016 | 0.0002 | 0.0029 | 0.027 |
| Total Cholesterol | 0.0016 | 0.0004 | 0.0028 | 0.0079 |
| Triglycerides | 0.0006 | 0 | 0.0013 | 0.0672 |
| HDL | 0 | −0.0028 | 0.0029 | 0.9851 |
| Internal carotid IMT | 0.2767 | 0.0811 | 0.4723 | 0.0056 |
| Common carotid IMT | 1.1659 | 0.6744 | 1.6573 | 0 |
| Gender % (male) | −0.0374 | −0.1203 | 0.2704 | 0.3765 |
| Urine | ||||
| Age | −0.0044 | −0.0113 | 0.0026 | 0.2166 |
| Weight (kg) | 0.0037 | 0.0008 | 0.0067 | 0.0129 |
| BMI (kg/m2) | 0.005 | −0.0066 | 0.0167 | 0.3968 |
| Waist-hip ratio | 1.0875 | 0.5222 | 1.6527 | 0.0002 |
| Duration of diabetes (yr) | −0.0075 | −0.0175 | 0.0024 | 0.1392 |
| HbA1c (%) | 0.0061 | −0.0296 | 0.0417 | 0.7378 |
| DCCT group | 0.042 | −0.0538 | 0.1379 | 0.3899 |
| SBP (mm Hg) | 0.0065 | 0.003 | 0.0099 | 0.0002 |
| DBP (mm Hg) | 0.0107 | 0.0056 | 0.0158 | 0.0001 |
| Log AER (mg/24 h) | 0.0033 | −0.0316 | 0.0382 | 0.8527 |
| LDL | −0.0004 | −0.0019 | 0.0012 | 0.6586 |
| Total cholesterol | −0.0004 | −0.0018 | 0.001 | 0.5923 |
| Triglycerides | 0.0003 | −0.0005 | 0.0011 | 0.4188 |
| HDL | −0.0015 | −0.0048 | 0.0018 | 0.3714 |
| Internal carotid IMT | 0.1427 | −0.0836 | 0.369 | 0.4146 |
| Common carotid IMT | 0.2333 | −0.3395 | 0.806 | 0.4243 |
| Gender (male) | −0.2091 | −0.3045 | −0.1137 | 0.0001 |
HDL, High-density lipoprotein.
Univariate analyses were also performed between plasma whole CTGF (CTGF W), the precursor, and clinical parameters shown in Table 1. The results demonstrated an association between plasma CTGF W and duration of diabetes (P < 0.0033), LDL (P < 0.0150), total cholesterol (P < 0.0534), and common carotid IMT (P < 0.0160). No associations were observed between plasma CTGF W and SBP, log AER, internal carotid IMT, DCCT intensive group, age, HbA1c, angiotensin-converting enzyme inhibitor (ACEI), gender, weight, BMI, or waist to hip ratio.
A strong association between log urine CTGF N fragment levels and weight, waist to hip ratio, SBP, diastolic BP (DBP), and gender was observed. The excretion rate of log CTGF N fragment was not influenced by age, duration of diabetes, HbA1c, log AER, total cholesterol, LDL, and ACEI.
Association between plasma CTGF N fragment and blood pressure
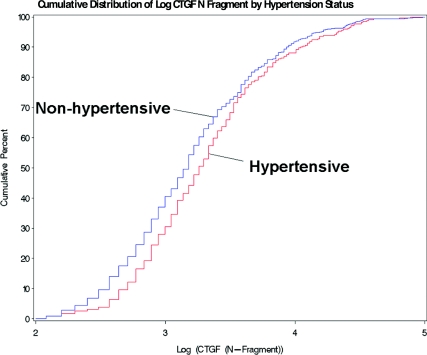
The results in Fig. 1 show the estimated cumulative distribution of log plasma CTGF N fragment plotted for nonhypertensive (n = 668) and hypertensive (n = 382) subjects (all patients diagnosed with hypertension with SBP > 140 or DBP > 90 or documented antihypertensive medication). This figure demonstrates that hypertensive subjects tend to have higher values of plasma log CTGF N fragment than do normotensive subjects.
Figure 1.
Log CTGF N fragment vs. cumulative distribution of hypertension status in the DCCT/EDIC cohort. The plasma CTGF N fragment levels in patients with hypertension are significantly higher than patients with normal blood pressure (P = 0.0005; n = 1050).
The mean values of plasma log CTGF N fragment for subjects with documented hypertension was significantly greater than subjects who did not develop hypertension (3.36 ± 0.04 vs. 3.21 ± 0.03 ng/ml, P = 0.0005). The actual mean plasma CTGF level measured in plasma of hypertensive patients was 41.57 ± 3.47 ng/ml compared with 32.28 ± 1.81 ng/ml in normotensive patients (P = 0.0109). A strong association was also observed between the urinary excretion rate of log CTGF N fragment and SBP, DBP, and mean blood pressure (all P < 0.0001).
Relationship between CTGF N fragment levels and albumin excretion
Plasma and urine CTGF N fragment levels of subjects with macroalbuminuria (65.79 ± 11.89 ng/ml) were significantly higher than those of subjects with microalbuminuria (32.39 ± 2.73 ng/ml) and normal albumin excretion rate (32.39 ± 2.73 ng/ml; P = 0.0001 for each comparison; Table 3). The urinary excretion rate of CTGF N fragment in subjects with macroalbuminuria was 18.53 ± 4.33 vs. 9.25 ± 0.70 μg/24 h in subjects with microalbuminuria and 10.61 ± 0.34 μg/24 h in subjects with normal AER (P = 0.0001 for each comparison).
Table 3.
Plasma and urine CTGF N fragment levels by albuminuria status
| Variable | AER < 40 mg/24 h (n = 896)
|
AER 40–299 mg/24 h (n = 105)
|
AER > 300 mg/24 h (n = 51)
|
|||||
|---|---|---|---|---|---|---|---|---|
| Mean | sd | Mean | sd | P | Mean | sd | P | |
| Plasma (ng/ml) | ||||||||
| Log CTGF N | 3.227 | 0.657 | 3.297 | 0.542 | 0.2487 | 3.75 | 0.921 | <0.0001 |
| CTGF N | 34.191 | 55.116 | 32.386 | 27.958 | 0.8250 | 65.792 | 84.842 | 0.0001 |
| Urine (μg/24 h) | ||||||||
| Log CTGF N | 2.098 | 0.719 | 1.943 | 0.821 | 0.0529 | 2.285 | 1.083 | 0.1096 |
| CTGF N | 10.611 | 10.142 | 9.248 | 7.189 | 0.2806 | 18.534 | 30.918 | <0.0001 |
P values are compared with baseline group (AER < 40 mg/24 h) and are adjusted for age.
Linear regression analyses were performed to further assess the relationship between plasma CTGF N fragment and albuminuria. A significant association was observed between CTGF N fragment and diabetic subjects with AER of at least 40 mg/24 h (estimate = 1.6326; P < 0.0011), demonstrating a link between CTGF N fragment and development of micro- and macroalbuminuria. In addition, the relationship between plasma CTGF N fragment and progression of microalbuminuria was assessed on patients who had normal AER (<40 mg/24 h) at closeout of DCCT (1993) and progressed to develop microalbuminuria (AER ≥ 40 mg) in EDIC, 5 yr later. A significant association was observed between CTGF N fragment and diabetic patients who progressed to develop microalbuminuria (estimate = 1.537; P < 0.0169), demonstrating that increases in plasma CTGF N fragment may predict development of early nephropathy.
Univariate regression analysis demonstrated that log plasma CTGF N fragment levels were positively associated with log AER (P = 0.0001). A multiple linear regression model was developed having log AER as the outcome. A number of variables that may influence log AER were included into the model. Nonsignificant variables and collinear variables were eliminated by backward regression analysis to develop a model that best explained the outcome as a function of the risk factors. The results in Table 4 with adjustment for age, weight, BMI, duration of diabetes, HbA1c levels, DCCT intensive group, SBP, and total cholesterol showed a significant association between log plasma CTGF and log AER (P < 0.0001). These results were interpreted such that a 2-fold greater plasma CTGF N fragment was associated with a 20% greater increase AER. Furthermore, the results in Table 4 also demonstrate that both CTGF N fragment and HbA1c are both highly associated with log AER. The estimate (log) for CTGF N fragment is 0.3183 and for HbA1c is 0.2414 (P < 0.0001 for both).
Table 4.
Multiple linear regression models for log AER
| Variable | Effect | 95% Confidence interval
|
P | |
|---|---|---|---|---|
| Lower | Upper | |||
| Intercept | -4.3388 | −5.5492 | −3.1824 | 0.0001 |
| Plasma log CTGF N fragment | 0.3183 | 0.1959 | 0.4407 | 0.0001 |
| Age (yr) | −0.0272 | −0.0393 | −0.015 | 0.0001 |
| Weight (kg) | 0.0008 | 0.0002 | 0.0161 | 0.0445 |
| BMI (kg/m2) | −0.0348 | −0.0645 | −0.005 | 0.0221 |
| Waist-hip ratio | 0.2832 | −0.8149 | 1.3859 | 0.6143 |
| Duration of diabetes (yr) | 0.0388 | 0.0219 | 0.0557 | 0.0001 |
| HbA1c (%) | 0.2414 | 0.1797 | 0.3026 | 0.0001 |
| DCCT intensive group | 0.3792 | 0.2179 | 0.5405 | 0.0001 |
| SBP (mm Hg) | 0.024 | 0.0179 | 0.0301 | 0.0001 |
| Total cholesterol (mg/dl) | 0.0052 | 0.0028 | 0.0076 | 0.0001 |
CTGF and glomerular filteration rate (GFR)
To assess the relationship between CTGF and renal function, we evaluated whether levels of plasma and urine CTGF were associated with renal function assessed by estimates of GFR, using the equation derived from the Modification of Diet in Renal Disease (MDRD) study (12,13): GFR (milliliters per minute per 1.73m2) = 186 × (Sc)−1.154 × (age)−0.203 × (0.742 if female) × (1.210 if black), where Sc is serum creatinine (milligrams per deciliter), age is given in years and weight in kilograms.
We elected to use the MDRD equation because it was shown to have better accuracy in diagnosing and stratifying chronic renal failure in diabetic subjects (14). The results adjusted for HbA1c levels, DCCT intensive group, and SBP showed a significant association between log plasma CTGF N fragment and MDRD estimated GFR (P < 0.0172). No association was observed between MDRD estimated GFR and plasma whole CTGF levels and/or urine log CTGF N fragment.
CTGF and carotid arterial wall thickness
We evaluated the relationship between CTGF level and common and/or internal carotid IMT. Univariate analysis demonstrated a significant association between log plasma CTGF N fragment levels and the common and internal carotid IMT both with P < 0.001 (Table 5).
Table 5.
Univariate regression analyses to predict carotid IMT
| Independent variables | Effect | 95% Confidence interval
|
P | |
|---|---|---|---|---|
| Lower | Upper | |||
| Internal carotid IMT | ||||
| Age (yr) | 0.0139 | 0.0125 | 0.0153 | 0.0001 |
| BMI (kg/m2) | 0.0051 | 0.0025 | 0.0077 | 0.0509 |
| Duration of diabetes (yr) | 0.0042 | 0.0021 | 0.0063 | 0.0206 |
| HbA1c (%) | 0.0209 | 0.0132 | 0.0286 | 0.0074 |
| SBP (mm Hg) | 0.0056 | 0.0049 | 0.0063 | 0.0001 |
| Log AER (mg/24 h) | 0.0421 | 0.0347 | 0.0495 | 0.0001 |
| Gender % (male) | 0.1218 | 0.1016 | 0.1419 | 0.0001 |
| Plasma log CTGF N fragment | 0.0419 | 0.0258 | 0.0582 | 0.0096 |
| Plasma log CTGF-W | 0.0158 | 0.0035 | 0.0281 | 0.1973 |
| Common carotid IMT | ||||
| Age (yr) | 0.0065 | 0.006 | 0.007 | 0.0001 |
| BMI (kg/m2) | 0.0036 | 0.0028 | 0.0044 | 0.0001 |
| Duration of Diabetes (yr) | 0.0022 | 0.0015 | 0.0029 | 0.0018 |
| HbA1c (%) | 0.003 | 0.0003 | 0.0057 | 0.254 |
| SBP (mm Hg) | 0.0023 | 0.0021 | 0.0025 | 0.0001 |
| Log AER (mg/24 h) | 0.0146 | 0.0121 | 0.0171 | 0.0001 |
| Gender % (male) | 0.0493 | 0.0425 | 0.0562 | 0.0001 |
| Plasma Log CTGF N fragment | 0.0257 | 0.0202 | 0.0312 | 0.0001 |
| Plasma log CTGF-W | 0.0094 | 0.0052 | 0.0136 | 0.026 |
Multiple linear regression analyses were performed to assess CTGF N fragment and other risk factors that predict progression of common carotid IMT. Association of CTGF N fragment and other covariates with common IMT at yr 6 (EDIC follow-up) was assessed with adjustment for measurement at yr 1 (EDIC baseline). The model containing log plasma CTGF N fragment, log AER, age, gender, BMI, duration of diabetes, total cholesterol, HbA1c, SBP, and ACEI use demonstrated that log CTGF N fragment levels were independently and significantly associated with the common carotid IMT (Table 6). The results of the univariate and multiple regression analysis indicated that the log N fragment strongly associates with both the common and internal IMT independently. We next performed multivariate analysis using generalized linear models to determine whether log CTGF N fragment will predict both the common and internal carotid IMT in the same model. The outcomes of interest are IMT common and internal, and the covariate is log CTGF N fragment. The results demonstrated that log CTGF N fragment significantly associated (Wilks' λ P value <.05) with both outcomes (common and carotid IMT) after adjusting for the following covariates (age, gender, and log AER).
Table 6.
Multiple linear regression models for common carotid IMT
| Variable | Effect | 95% Confidence interval
|
P | |
|---|---|---|---|---|
| Lower | Upper | |||
| Plasma log CTGF N fragment | 1.008 | 1.0044 | 1.0353 | 0.0273 |
| Age (yr) | 1.0044 | 1.004 | 1.0048 | <0.0001 |
| Duration of diabetes (yr) | 1.0013 | 1.0008 | 1.0018 | 0.0081 |
| Systolic blood pressure (mm Hg) | 1.0004 | 1.0002 | 1.0006 | 0.0487 |
| Body mass index (kg/m2) | 1.0021 | 1.0015 | 1.0026 | 0.0006 |
| Log AER (mg/24 h) | 1.0081 | 1.0061 | 1.0101 | <0.0001 |
| Total cholesterol (mg/dl) | 1.0001 | 1 | 1 | 0.3062 |
| Gender | 1.0313 | 1.0262 | 1.0364 | <0.0001 |
| HbA1c (%) | 1.0004 | 0.9985 | 1.0023 | 0.8436 |
| ACEI | 1.0051 | 0.9972 | 1.0129 | 0.5186 |
We next dichotomized IMT into high and low categories based on the 75th percentile and fitted a logistic regression model with this as the outcome. Age-adjusted logistic models confirmed the association of CTGF with increased carotid IMT. Subjects with high CTGF levels and macroalbuminuria experienced a significantly greater risk for increased carotid IMT (relative risk = 4.76; 95% confidence interval = 2.21–10.25; P < 0.0001) than subjects with low CTGF levels and normal AER (Table 7).
Table 7.
Relative risk for increased carotid IMT according to plasma CTGF N fragment and AER
| CTGF N | AER < 40 mg/d | AER > 40 < 299 mg/d | AER > 300 mg/d |
|---|---|---|---|
| High | RR = 1.86 (1.30–2.67) | RR = 2.54 (1.21–5.23) | RR = 4.76 (2.21–10.25) |
| n = 376 (P = 0.0007) | n = 41 (P = 0.0135) | n = 36 (P < 0.0001) | |
| Low | RR = 1.00 | RR = 2.42 (1.13–5.18) | RR = 2.69 (0.59–12.92) |
| n = 415 | n = 43 (P = 0.0224) | n = 9 (P = 0.1989) | |
| Total | n = 791 | n = 84 | n = 45 |
Increased IMT is defined as the top quartile of distribution for men and women combined. Estimates are age adjusted. RR, Relative risk (95% confidence interval).
Discussion
The findings of the present study indicate that vascular disease in type 1 diabetes is linked to increased CTGF levels. To our knowledge, these findings provide the first evidence of an independent and positive association between CTGF N fragment levels and common and internal carotid IMT, a well established biomarker of macrovascular disease. In addition, the findings demonstrate an independent association between CTGF N fragment levels and both hypertension and microalbuminuria, which are important risk factors for macrovascular disease. The relationship between CTGF levels and carotid IMT persisted even after adjusting for hypertension, microalbuminuria, and other cardiovascular risk factors.
Our data demonstrated that the strength of the association with risk factors or subclinical disease was generally stronger for CTGF N than for CTGF precursor. CTGF is characterized by 38 conserved cysteine residues that form the canonical feature of the CCN gene family (15). The two cysteine-free regions within CTGF are known as hinges and separate domains 2 and 3 (32 amino acids) and domains 3 and 4 (eight amino acids). CTGF is readily susceptible to cleavage by serine proteases and matrix metalloproteases within the so-called hinge region resulting in the amino-terminal fragment of CTGF (CTGF N fragment), the predominant form of CTGF present in blood and urine (16). The selective accumulation of CTGF N fragment may occur because the NH2-terminal region of CTGF is more stable than the whole CTGF molecule to degradation and may represent a marker of CTGF production and turnover rate (17). Although there is a growing body of information on generation and activity of CTGF fragments, little is known about specific production and biological significance of CTGF fragments. It was recently shown that CTGF N fragment mediates myofibroblast differentiation and collagen synthesis, whereas the C-terminal half mediates fibroblast proliferation (18). In addition, module 3 of CTGF was shown to exhibit integrin binding, signaling, and fibrogenic properties (19).
CTGF is an emerging determinant of progressive fibrotic diseases, and expression of this factor is up-regulated in atherosclerotic vascular and glomerular disease. However, the factors that regulate the expression of CTGF are not fully defined. Studies have shown that the glomerular expression of CTGF is induced by experimental diabetes and by high glucose, suggesting that hyperglycemia may be one of the factors that regulate the expression of CTGF (20,21). Our data did not show any relationship between CTGF and HbA1c, but we did observe a positive and significant association between plasma CTGF levels and LDL, demonstrating that LDL may modulate levels of CTGF in diabetic patients. This novel observation in diabetic subjects led us to investigate the mechanistic basis of this finding. We demonstrated at a cellular level that LDL can directly stimulate the expression of CTGF in mesangial and human aortic endothelial cells, and this effect was mediated via autocrine activation of TGF-β and c-Jun NH2-terminal kinase (22,23). In addition, we recently demonstrated that the expression of CTGF in the aorta of LDL receptor knockout (LDLR−/−) mice fed a high-fat diet was significantly higher than LDLR−/− mice fed a normal-fat diet (24). These findings demonstrate a novel mechanism of CTGF expression by lipoproteins and suggest that CTGF may provide a mechanistic pathway through which lipoproteins promote vascular sclerosis.
Microalbuminuria, an early marker of diabetic nephropathy, signifies high risk for progressive renal failure and cardiovascular disease. Microalbuminuria has also been associated with increased cardiovascular mortality in diabetic and nondiabetic populations and with generalized and glomerular endothelial dysfunction (25). Identifying biomarkers that contribute to the development of microalbuminuria may provide insights into the mechanisms of diabetic vascular injury. In this regard, our findings demonstrate that CTGF N fragment levels in plasma and urine of patients with macroalbuminuria were 2-fold higher than levels in patients with microalbuminuria or a normal albumin excretion rate. These findings suggest that CTGF is a marker for progressive nephropathy and atherosclerosis. Furthermore, univariate and multiple linear regression analyses supported an independent and positive association between CTGF N fragment levels and AER. This finding is in agreement with previous reports in the literature conducted in smaller populations of type 1 diabetic patients (11,26).
The findings of the present study also point to a relationship between plasma CTGF N fragment and renal function. Increased levels of plasma CTGF N fragment were associated with decreased GFR. The increased urinary excretion of CTGF N fragment we observed in our study could also result from increased fractional clearance of CTGF secondary to impaired permselectivity to the filtered load of CTGF. It is also important to point out that the increase in plasma CTGF levels we observed in diabetic patients with overt albuminuria is not due to a decrease in renal clearance of CTGF. If CTGF is merely a nonspecific marker of impaired renal function, then either the rise in plasma CTGF N fragment is due to decreased renal clearance (in which case it would be elevated in the plasma and not elevated in the urine) or the increase in urine is due to altered permselectivity (in which case it should not be elevated in the plasma). Thus, the only way CTGF N fragment can be elevated in both plasma and urine is if there is increased synthesis followed by increased proteolysis of CTGF W to CTGF N fragment.
The present data provide the first evidence of an association between CTGF N fragment and elevated blood pressure. Whether the increase in plasma CTGF levels contributes to the development of hypertension or results from increased blood pressure is yet to be determined. In this regard, a recent study demonstrated the increased production of extracellular matrix proteins in response to high static pressure was mediated via increased expression of CTGF (27). This finding is of significance because risk for progressive renal injury and cardiovascular disease in diabetes is accentuated by hypertension.
The current findings demonstrate an independent and positive association between plasma CTGF N fragment levels and common and internal carotid IMT, recognized markers for coronary as well as cerebral vascular disease in patients with type 1 diabetes (3). The enhanced atherosclerosis in diabetic subjects may result from increased matrix accumulation within the vessel wall that occurred as a result of increased CTGF activity. Given the influence of hypertension, lipoproteins, and hyperglycemia on CTGF regulation and their contribution to vascular and renal dysfunction, one can speculate that CTGF may be a mechanistic pathway through which high arterial pressure, lipoproteins, and hyperglycemia may mediate their deleterious effects on promoting renal and vascular injury in diabetic patients.
In summary, the findings in the present study demonstrate that plasma CTGF N fragment levels in type 1 diabetes are associated with higher arterial pressure, albuminuria, and greater carotid IMT. The level of association with log AER was comparable between HbA1c and CTGF N fragment.
Footnotes
This work was supported by National Institutes of Health Grants DK-46543, HL077192, and HL-55782. DCCT/EDIC is supported by contracts with the Division of Diabetes, Endocrinology, and Metabolic Diseases of the National Institute of Diabetes and Digestive and Kidney Diseases and the General Clinical Research Centers Program, National Center for Research Resources.
First Published Online March 11, 2008
Abbreviations: ACEI, Angiotensin-converting enzyme inhibitor; AER, albumin excretion rate; BMI, body mass index; BP, blood pressure; CTGF, connective tissue growth factor; DBP, diastolic BP; DCCT/EDIC, Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications; GFR, glomerular filtration rate; HAEC, human aortic endothelial cells; HbA1c, glycosylated hemoglobin; IMT, intima-medial thickness; LDL, low-density lipoprotein; MDRD, Modification of Diet in Renal Disease; rhCTGF, recombinant human CTGF; SBP, systolic BP.
References
- Christlieb AR, Warram JH, Krolewsky AS, Busick EG, Ganda OP, Asmal AC, Soeldnar JS, Bradley AF 1981 Hypertension: the major risk factor in juvenile-onset insulin dependent diabetics. Diabetes 30(Suppl 2):90–96 [DOI] [PubMed] [Google Scholar]
- Krolewski AS, Canessa JH, Laffel LMB, Christlieb R, Knowler WC, Lawrence PH, Li LR 1988 Predisposition to hypertension and susceptibility to renal disease in insulin-dependent diabetes mellitus. N Engl J Med 318:140–145 [DOI] [PubMed] [Google Scholar]
- Nathan DM, Lachin J, Cleary P, Orchard T, Brillon DJ, Backlund JY, O'Leary DH, Genuth S; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group 2003 Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitus. N Engl J Med 348:2294–2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakris GL, Sowers JR 2002 Microalbuminuria in diabetes: focus on cardiovascular and renal risk reduction. Curr Diab Rep 2:258–262 [DOI] [PubMed] [Google Scholar]
- Oemar BS, Werner A, Garnier JM, Do DD, Godoy N, Nauck M, Marz W, Rupp J, Pech M, Luscher T 1997 Human connective tissue growth factor is expressed in advanced atherosclerotic lesions. Circulation 4:831–839 [DOI] [PubMed] [Google Scholar]
- Ito Y, Aten J, Bende RJ, Oemar BS, Rabelink TJ, Weening JJ, and Goldschmeding R 1998 Expression of connective tissue growth factor in renal fibrosis. Kidney Int 53:853–861 [DOI] [PubMed] [Google Scholar]
- Gore-Hyer E, Shegogue D, Markiewicz M, Lo S, Hazen-Martin D, Greene EL, Grorendorst G, and Trojanowska M 2002 TGF-β and CTGF have overlapping and distinct fibrogenic effects on human renal cells. Am J Physiol 283:F707–F716 [DOI] [PubMed] [Google Scholar]
- 1993 The effect of intensive treatment of diabetes on the development and progression of long term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 329:977–986 [DOI] [PubMed] [Google Scholar]
- 1999 Epidemiology of Diabetes Interventions and Complications (EDIC). Design, implementation and preliminary results of long-term follow up of Diabetes Control and Complications Trial cohort. Diabetes Care 22:99–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1999 Effect of intensive diabetes treatment on carotid artery wall thickness in the epidemiology of diabetes interventions and complications. Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group. Diabetes 48:383–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert RE, Ardeniz A, Weitz S, Usinger WR, Molineaux C, Jones SE, Langham RG, Jerums G 2003 Urinary connective tissue growth factor excretion in patients with type 1 diabetes and nephropathy. Diabetes Care 26:2632–2636 [DOI] [PubMed] [Google Scholar]
- Levy AS, Bosch JP, Lewis JB, Greene T, Rojers N, Roth D 1999 A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation: Modification of Diet in Renal Disease Study Group. Ann Intern Med 130:461–470 [DOI] [PubMed] [Google Scholar]
- Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Paffrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW 2003 Kidney disease as a risk factor for development of cardiovascular disease. Circulation 108:2154–2169 [DOI] [PubMed] [Google Scholar]
- Rigalleau V, Lasseur C, Perlemoine C, Barthe N, Raffaitin C, Liu C, Chauveau P, Baillet-Blanco L, Beauvieux MC, Combe C, Gin H 2005 Estimation of glomerular filtration rate in diabetic subjects. Diabetes Care 28:838–843 [DOI] [PubMed] [Google Scholar]
- Bork P 1993 The modular architecture of a new family of growth regulators related to connective tissue growth factor. FEBS Lett 327:125–130 [DOI] [PubMed] [Google Scholar]
- Hinton DR, Spee C, He S, Weitz S, Usinger W, LaBree L, Oliver N, Lim JI 2004 Accumulation of NH2-yerminal fragment of connective tissue growth factor in the vitreous of patients with proliferative diabetic nephropathy. Diabetes Care 27:758–764 [DOI] [PubMed] [Google Scholar]
- Hashimoto G, Inoki I, Fujii Y, Aoki T, Ikeda E, Okada Y 2002 Matrix metalloproteinases cleave connective tissue growth factor and reactivate angiogenic activity of vascular endothelial growth factor 165. J Biol Chem 277:36288–36295 [DOI] [PubMed] [Google Scholar]
- Grotendorst GA, Duncan MR 2005 Individual domains of connective tissue growth factor regulate fibroblast proliferation and myofibroblast differentiation. FASEB J 19:729–738 [DOI] [PubMed] [Google Scholar]
- Tong ZY, Brigstock DR 2006 Intrinsic biological activity of the thrombospondin structural homology repeat in connective tissue growth factor. J Endocrinology 188:R1–R8 [DOI] [PubMed] [Google Scholar]
- Murphy M, Godson C, Cannon S, Kato S, Machenzie HS, Martin F, and Brady HR 1999 Suppression subtractive hybridization identifies high glucose levels as a stimulus for expression of connective tissue growth factor and other genes in human mesangial cells. J Biol Chem 274:5830–5834 [DOI] [PubMed] [Google Scholar]
- Riser BL, Denichilo M, Cortes P, Baker C, Grondin JM, Yee J, Narins RG 2000 Regulation of connective tissue growth factor activity in cultured rat mesangial cells and its expression in experimental diabetic glomerulosclerosis. J Am Soc Nephrol 11:25–38 [DOI] [PubMed] [Google Scholar]
- Sohn M, Tan Y, Wang B, Klein R, Trojanowska M, Jaffa AA 2006 Mechanism of low-density lipoprotein-induced expression of connective tissue growth factor in human aortic endothelial cells. Am J Physiol Heart Circ Physiol 290:1624–1634 [DOI] [PubMed] [Google Scholar]
- Sohn M, Tan Y, Klein R, Jaffa AA 2005 Evidence for low-density lipoprotein-induced expression of connective tissue growth factor in mesangial cells. Kidney Int 67:1286–1296 [DOI] [PubMed] [Google Scholar]
- Game BA, He L, Jarido V, Nareika A, Jaffa AA, Lopes-Virella M, Huang Y 2007 Pioglitazone inhibits connective tissue growth factor expression in advanced atherosclerotic plaques in low-density lipoprotein receptor deficient mice. Atherosclerosis 192:85–91 [DOI] [PubMed] [Google Scholar]
- Stehouwer C, Nauta J, Zeldenrust G, Hackeng W, Donker A, Ottolander G 1992 Urinary albumin excretion, cardiovascular disease and endothelial dysfunction in non insulin dependent diabetes. Lancet 340:319–323 [DOI] [PubMed] [Google Scholar]
- Roestenberg P, Nieuwenhoven FA, Wieten L, Boer P, Diekman T, Tiller AM, Wiersinga WM, Oliver N, Usinger WR, Weitz S, Schlingemann RO, Goldschmeding R 2004 Connective tissue growth factor is increased in plasma of type 1 diabetic patients with nephropathy. Diabetes Care 27:1164–1170 [DOI] [PubMed] [Google Scholar]
- Hishikawa K, Oemar BS, Nakaki T 2001 Static pressure regulates connective tissue growth factor expression in human mesangial cells. J Biol Chem 276:16797–16803 [DOI] [PubMed] [Google Scholar]