Abstract
Context: Genetically determined heightened androgen sensitivity may influence the phenotype of polycystic ovary syndrome (PCOS). To date, studies of the androgen receptor exon 1 polymorphic CAG repeat have produced conflicting results in PCOS.
Objective: We tested the hypothesis that a lower number of CAG repeats is associated with increased odds of PCOS. We also compared X-chromosome inactivation between cases and controls.
Design: Women with and without PCOS were genotyped for the CAG repeat and assessed for X-chromosome methylation. Association analyses were performed.
Setting: Subjects were recruited from the reproductive endocrinology clinic at the University of Alabama at Birmingham; controls were recruited from the surrounding community. Genotyping took place at Cedars-Sinai Medical Center in Los Angeles.
Participants: Participants included 330 women with PCOS and 289 controls (77% white, 23% black).
Main Measurements: Androgen receptor genotype, X-chromosome methylation, and phenotyping for PCOS were measured.
Results: A smaller biallelic mean of CAG repeats was associated with increased odds of PCOS. X-chromosome inactivation was not different comparing cases with controls; however, in the subset with nonrandom inactivation, the chromosome bearing the shorter CAG allele was preferentially active in PCOS women.
Conclusions: Association of shorter CAG repeats with PCOS is consistent with in vitro functional studies demonstrating higher activity of androgen receptors expressed from alleles with fewer CAG repeats, suggesting inherited alteration in androgen sensitivity may contribute to PCOS. In some women, such heightened sensitivity may also result from preferential expression of androgen receptors with shorter alleles. Thus, genetic and epigenetic changes may be involved in the pathogenesis of PCOS.
This study suggests that in addition to excess androgen production, the risk of polycystic ovary syndrome is associated with the inheritance of androgen receptors with shorter polyglutamine tracts, suggesting that genetic differences in androgen sensitivity may contribute to the development of the syndrome.
The androgen receptor (AR) is a member of the family of ligand-activated transcription factors that regulate many biological processes and is encoded by a gene located at Xq11-q12 (1,2). Exon 1 of the AR gene encodes the N-terminal transactivation domain and contains a variable length CAG repeat polymorphism that encodes a polyglutamine tract; CAG repeat number normally ranges between 8 and 35 and demonstrates a stable inheritance (3). In vitro studies have indicated that ARs with shorter polyglutamine tracts have greater ability to activate reporter genes with androgen response elements (4,5,6). Consistent with these functional studies, various metaanalyses indicated that the presence of longer CAG repeats increases the risk of male infertility (7), and shorter repeats slightly increase the risk of prostate cancer (8). Similarly, a smaller number of CAG repeats has been associated with hirsutism (9), premature pubarche, and ovarian hyperandrogenism in women (10) as well as androgen-dependent skin disorders in both men and women (11).
A decreased number of AR gene CAG repeats (and hence, increased androgen sensitivity) could explain the normal serum androgen levels found in up to 25% women with polycystic ovary syndrome (PCOS), despite obvious evidence of hyperandrogenism (e.g. hirsutism) (12). However, investigations determining the role of CAG repeats in PCOS have yielded conflicting results (13,14,15,16,17,18,19,20,21). We undertook the present study to assess the influence of the CAG repeats in a large cohort consisting of women with PCOS and healthy controls. We hypothesized that decreased CAG repeat numbers [i.e. (CAG)n] would be associated with an increased risk of PCOS and hirsutism. We also assessed the potential role of skewed X inactivation, with the hypothesis that shorter alleles would be preferentially active in PCOS.
Patients and Methods
Subjects and phenotyping
The study included 619 subjects, consisting of 330 with PCOS (aged 13–47 yr) and 289 controls (aged 14–60 yr). The majority of the patient population (474 subjects, 287 PCOS, and 187 controls) was white; 145 subjects (43 PCOS and 102 controls) were black. These women were recruited from the Birmingham, AL, area. All subjects were unrelated. Clinical characteristics of these subjects are presented in Table 1.
Table 1.
Clinical characteristics
| Control (n = 289) | PCOS (n = 330) | |
|---|---|---|
| Race (% black) | 35.3% | 13.0%a |
| Age (yr) | 34.0 (19.0) | 27.4 (11.0)a |
| BMI (kg/m2) | 25.5 (7.8) | 34.7 (13.3)a |
| WHR | 0.80 (0.10) | 0.83 (0.10)a |
| mFG score | 0 (0) | 8 (6)a |
| Hirsute (%) | 0 | 74.5%a |
| Total testosterone (ng/dl) | 39.0 (26.0) | 80.0 (31.0)a |
| Free testosterone (pg/ml) | 0.32 (0.25) | 0.84 (0.45)a |
| DHEAS (ng/ml) | 900.0 (814.8) | 2041.0 (1669.5)a |
| SHBG (nmol/liter)b | 220.0 (120.0) | 150.0 (60.0)a |
| Insulin (μIU/ml) | 8.2 (7.4) | 18.0 (18.6)a |
| Glucose (mg/dl) | 88.0 (10.0) | 88.0 (13.0) |
| HOMA-IR | 1.10 (0.98) | 2.29 (2.01)a |
| HOMA-%B | 115.9 (64.7) | 175.0 (93.9)a |
Data are median (interquartile range). To convert total testosterone from nanograms per deciliter to nanomole per liter, multiply by 0.03467; to convert free testosterone from picograms per milliliter to picomoles per liter, multiply by 34.67; to convert DHEAS from nanograms per milliliter to micromoles per liter, multiply by 0.002714; to convert insulin from microinternational units per milliliter to picomoles per liter, multiply by 7.175; to convert glucose from milligrams per deciliter to millimoles per liter, multiply by 0.05551. WHR, Waist to hip ratio; DHEAS, dehydroepiandrosterone sulfate; HOMA-%B, β-cell function estimated by the homeostatic model assessment
P < 0.001, compared with control group.
SHBG activity was measured by competitive binding analysis, using Sephadex G-25 and [3H]T as the ligand; this assay gives values of approximately 100–300 nmol/liter in normal adult women.
PCOS subjects were recruited consecutively from the reproductive endocrine practice of one of the investigators (R.A.) at the University of Alabama at Birmingham. Participation in research studies was offered to patients meeting inclusion criteria (premenopausal, nonpregnant, on no hormonal therapy, including oral contraceptives, for at least 3 months, and meeting diagnostic criteria for PCOS). To ensure the inclusion of women with the classic disorder, the presence of PCOS was defined by the 1990 National Institutes of Health (NIH) consensus criteria (22), including: 1) clinical hyperandrogenism and/or hyperandrogenemia, 2) oligoovulation, and 3) the exclusion of related disorders, including androgen-producing tumors, nonclassic 21-hydroxylase-deficient adrenal hyperplasia, hyperprolactinemia, active thyroid disease, or Cushing's syndrome. The specific parameters for defining hirsutism, hyperandrogenemia, ovulatory dysfunction, and exclusion of related disorders have been previously reported (23).
Controls were healthy women, with regular menstrual cycles or a history of regular menstrual cycles before menopause and without family history of hirsutism. These women had no evidence of hirsutism, acne, or alopecia or endocrine dysfunction. Controls were recruited by word of mouth and advertisements in the Birmingham area through a call for healthy women without detailing further the nature of the studies.
The comprehensive physical examination and hormonal evaluation protocol used in our research has been previously described in detail (23). Hormone levels were obtained between d 3 and 8 (follicular phase) after a spontaneous menstrual cycle or progesterone-induced withdrawal bleed. The same laboratory assays were used for all subjects. Completeness of data was 93%. For association analyses involving androgen levels, only data from premenopausal women were used.
All subjects gave written informed consent, and the study was performed according to the guidelines of the Institutional Review Boards of University of Alabama at Birmingham and Cedars-Sinai Medical Center.
Genotyping and X-chromosome inactivation analysis
The CAG repeat was genotyped using a PCR-based assay. Genomic DNA was amplified by PCR using fluorescently labeled primers that flank the CAG repeat. The forward primer (5′-ACCGAGGAGCTTTCCAGAAT-3′) was labeled at the 5′ end with the laser-activated fluorescent dye 6FAM. The reverse primer (5′-gtttcttTGGGGAGAACCATCCTCAC-3′) contained a pigtail sequence (lower-case letters) at the 5′ end to reduce addition of nontemplated nucleotides (24). The amplified products were separated on a denaturing polyacrylamide gel using an ABI 3100 genetic analyzer (Applied Biosystems, Foster City, CA). The genotyping success rate was 90%. The size of fragments was estimated by comparison with the internal standard TAMRA 500, and a subset was sequenced to confirm exact repeat size. The primers used for sequencing were forward 5′-CGCGAAGTGATCCAGAACC-3′ and reverse 5′-CTCATCCAGGACCAGGTAGC-3′.
X-chromosome inactivation analysis is possible because of the presence of two methylation-sensitive HpaII restriction sites less than 70 bp from the CAG repeat; these sites are amplified with the CAG repeat during PCR. When this site is methylated, it is not cut by the restriction enzyme; when the site is not methylated, it is cut by the enzyme. Thus, when HpaII digestion is carried out before the PCR, only methylated sequences are intact for PCR amplification. Comparison between the digested and undigested samples allows determination of the relative methylation of each allele in subjects heterozygous at (CAG)n (in homozygotes the two alleles are indistinguishable). Nonrandom X inactivation is defined as more than 60% inactivation of either allele; skewed X inactivation is defined as more than 80% inactivation of either allele.
X inactivation analysis was carried out using a modification of the protocol by Hickey et al. (14). For the 484 heterozygous subjects, 200 ng of DNA were either digested with 10 U of HpaII or incubated in digestion buffer alone for 5 h at 37 C, followed by incubation at 80 C for 20 min to denature the enzyme. Next, 1 μl of digested and mock-digested products was amplified using the first set of PCR primers above and peak areas for both alleles determined using an ABI 3730 genetic analyzer running data collection software and Genemapper version 3.5. X inactivation (relative methylation of each allele) was quantified as previously described (25), by comparing the ratio to which each allele contributed to the total peak area between digested and undigested samples. To determine the degree of variance in the methylation assay, we initially ran 33 digested and undigested samples in duplicate. Whereas there was variability in total peak area, the peak ratios and the X-inactivation data based on these were very consistent, with a mean difference of only 2.0% (sd 2.9%). We then proceeded to assay the entire population, and the overall X-inactivation analysis was based on one pair of digested and undigested samples for each subject, with the exception of any samples with extreme departure (>90%) from random X inactivation. The latter samples were repeated to validate their results. The X-inactivation analysis entailed repeat genotyping of all heterozygotes; the allele calls were 100% concordant with the original genotyping runs.
After completing X-inactivation analysis, we then generated the X-weighted biallelic mean using the method of Hickey et al. (14), whereby each allele in a genotypic pair is multiplied by its percent activation, and then the two adjusted repeat values are added together. This value integrates CAG repeat number with expression of constituent alleles. Homozygous subjects were included in the set of X-weighted biallelic means because variation of allele expression would not alter the mean value of alleles of equivalent repeat number. X-weighted biallelic mean was considered quantitatively and qualitatively as performed for the simple biallelic mean (described below).
Statistical analysis
Unpaired t tests and χ2 tests were used to compare clinical characteristics between women with and without PCOS. Quantitative trait values were log or square root transformed as appropriate to reduce nonnormality.
The primary genotypic unit used in the association analyses was the biallelic mean (the mean of the CAG repeat number from the two alleles in each subject). Biallelic mean was considered in two ways. First, it was analyzed as a continuous quantitative variable. Second, it was treated as a binary qualitative variable, with the two states being either less than or greater than or equal to the median repeat number observed in the control group. To allow the two racial groups to be combined in the latter analysis, we used the race-specific median derived from the control subjects of each group.
The primary phenotype of interest was presence/absence of PCOS. Association analyses of biallelic mean (quantitative or qualitative) with PCOS were carried out using logistic regression, adjusting for age and body mass index (BMI) by including both of them as independent variables in every analysis. When whites and blacks were analyzed together, race was also taken as a covariate. Association with quantitative traits in women with PCOS was evaluated using analysis of covariance, handling covariates of age, BMI, and race in the same manner. Significance was taken as P < 0.05. Analyses were carried out using Statview 5.0 (SAS Institute, Cary, NC).
Results
Association of CAG repeats with PCOS
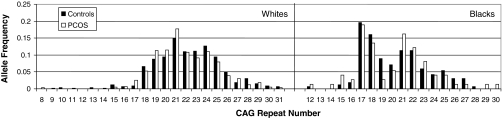
In the white women with PCOS, the range of CAG repeats was 8–31, with a median of 21; in control women CAG repeat numbers ranged from 10 to 31, with a median of 22 (Fig. 1). In the black women with PCOS, the CAG number ranged from 12 to 28, with a median of 20. In control black women, repeat numbers ranged from 10 to 31, with a median of 20 (Fig. 1). The difference in median repeat number was highly significant comparing whites with blacks, in both total and subdivided by PCOS diagnosis (P < 0.0001). In the combined analyses, race-specific median was defined as 22 for whites and 20 for blacks (the control medians).
Figure 1.
Distribution of CAG alleles. Distribution of CAG alleles is shown for white and black subjects. Each subject contributed two alleles (paternal and maternal) to the histogram.
In the entire cohort, biallelic mean of repeats was associated with PCOS. Each additional unit increment in the biallelic mean reduced the odds of PCOS by 13% (as determined by logistic regression, P = 0.0057). In the total population, a biallelic mean repeat number less than the race-specific control median conferred an increased odds of PCOS [odds ratio (OR) 2.08, P = 0.0018] (Table 2).
Table 2.
Associations of CAG biallelic mean with PCOS
| PCOS (%) | Controls (%) | OR (95% CI) | P valuea | |
|---|---|---|---|---|
| Combined cohort (n = 556) | ||||
| Biallelic mean less than median | 50.2 | 41.4 | 2.08 (1.32–3.30) | 0.0018 |
| X-weighted biallelic mean less than median | 54.3 | 48.4 | 1.74 (1.10–2.74) | 0.017 |
| Whites (n = 435) | ||||
| Biallelic mean less than 22 | 48.9 | 40.0 | 1.73 (1.02–2.92) | 0.041 |
| X-weighted biallelic mean less than 22 | 55.1 | 48.2 | 1.76 (1.04–2.96) | 0.034 |
| Blacks (n = 121) | ||||
| Biallelic mean less than 20 | 59.5 | 44.0 | 3.73 (1.35–10.32) | 0.011 |
| X-weighted biallelic mean less than 20 | 48.6 | 48.8 | 1.50 (0.58–3.88) | 0.40 |
CI, Confidence interval.
P values from logistic regression. In all analyses, covariates included age and BMI. In the combined group, race was also taken as a covariate.
We assessed the effects of the CAG repeat in each ethnic group separately (Table 2). Biallelic mean CAG repeat number was associated with frequency of PCOS in the white population, with each additional unit increment in biallelic mean reducing the odds by 11% (P = 0.040). A mean repeat number less than 22 was associated with increased odds of PCOS (OR 1.73, P = 0.041).
Among black women, the biallelic mean was associated with a trend to PCOS risk, with each unit increment in biallelic mean reducing odds of PCOS by 15% (P = 0.14). Having a biallelic mean less than 20 was associated with increased odds of PCOS (OR 3.73, P = 0.011).
X-chromosome inactivation analysis
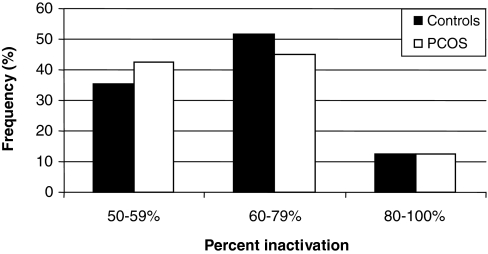
In the combined cohort, there was a nonsignificant trend toward a higher frequency of nonrandom X-inactivation in the control women than those with PCOS (P = 0.068, Table 3 and Fig. 2). There was no difference in the frequency of women with skewed X inactivation between women with and without PCOS. There were no significant differences in nonrandom or skewed X-inactivation among whites or blacks separately.
Table 3.
Relationship of X-inactivation to PCOS
| PCOS (%) | Controls (%) | OR (95% CI) | P valuea | |
|---|---|---|---|---|
| Combined cohort | ||||
| Nonrandom X inactivation | 57.6 | 64.4 | 0.64 (0.39–1.03) | 0.068 |
| Skewed X inactivation | 12.6 | 12.6 | 1.02 (0.49–2.15) | 0.95 |
| Among subjects with nonrandom X inactivation, active alleles less than median | 54.3 | 46.2 | 2.00 (1.05–3.83) | 0.036 |
| Whites | ||||
| Norandom X inactivation | 58.7 | 61.7 | 0.69 (0.39–1.20) | 0.19 |
| Skewed X inactivation | 12.6 | 10.1 | 1.32 (0.53–3.28) | 0.55 |
| Among subjects with nonrandom X inactivation, active alleles less than 22 | 54.8 | 46.7 | 1.95 (0.89–4.29) | 0.095 |
| Blacks | ||||
| Nonrandom X inactivation | 50.0 | 69.9 | 0.47 (0.17–1.28) | 0.14 |
| Skewed X inactivation | 12.5 | 17.8 | 0.68 (0.16–2.81) | 0.59 |
| Among subjects with nonrandom X inactivation, active alleles less than 20 | 50.0 | 45.1 | 1.55 (0.43–5.50) | 0.50 |
CI, Confidence interval.
P values from logistic regression. In all analyses, covariates included age and BMI. In the combined group, race was also taken as a covariate.
Figure 2.
Frequency of X inactivation in PCOS vs. controls. The overall distribution of X inactivation was not different between women with PCOS (n = 262) and controls (n = 222). Nonrandom X inactivation is defined as more than 60%, and skewed X inactivation is defined as more than 80% preferential inactivation of either allele.
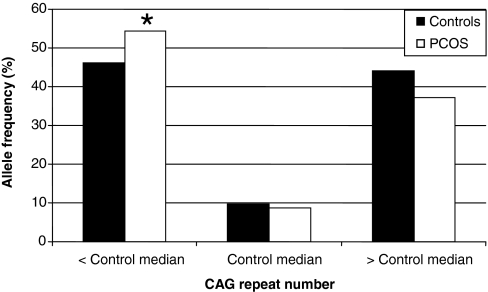
We then examined the women with nonrandom X inactivation to determine whether shorter (less than the median) alleles were preferentially active in PCOS vs. controls. In PCOS women with nonrandom X inactivation, 54.3% had preferential activation of their shorter allele, whereas 46.2% of such control women had preferential activation of their shorter allele (Table 3 and Fig. 3). Among women with nonrandom X inactivation, preferential activation of the allele shorter than the median was associated with a 2-fold increased odds ratio of PCOS (P = 0.036). A similar trend and magnitude of effect was observed within the white women (OR 1.95, P = 0.095). This was less significant in the black women (OR 1.55, P = 0.50) due to smaller sample size.
Figure 3.
Distribution of CAG alleles with 60–100% activity. In the subgroup of women (151 PCOS, 143 controls) with nonrandom X inactivation, shorter (less than the race specific control median), CAG alleles were preferentially active in PCOS. Women with PCOS had 54.3% of active alleles less than the median, whereas controls had 46.2% of active alleles less than the median. *, P = 0.036.
We then reevaluated the association of CAG repeat number with PCOS status using the X-weighted biallelic means. In analyses considering X-weighted biallelic mean as a continuous variable, associations with PCOS were identical with those observed with simple biallelic mean; each additional unit increment in the X-weighted biallelic mean reduced the odds of PCOS by 13% (P = 0.0053). Results in each race group separately were also unchanged. X-weighted mean considered qualitatively displayed no consistent effect on the associations that we had observed with the simple biallelic mean (Table 2).
CAG repeats and androgen-related traits in PCOS
There was no apparent influence of (CAG)n on the severity of hirsutism. The mean modified Ferriman-Gallwey (mFG) score of the patients with biallelic mean less than the median was 8.7, compared with 8.2 in those whose biallelic mean was greater than or equal to the median number of repeats (P = 0.46). There was also no association of biallelic mean (considered quantitatively or qualitatively) with total or free testosterone levels.
The control group included 26 white and 20 black menopausal women. We repeated the CAG repeat association analyses and X-inactivation analyses excluding these subjects. The results remained essentially the same (supplementary Tables 1 and 2, published as supplemental data on The Endocrine Society's Journals Online Web site at http://jcem.endojournals.org).
Discussion
This is the largest study (in terms of number of case subjects) that has examined the AR exon 1 CAG repeat variant in PCOS. We found a significant association between shorter repeat length and the presence of PCOS. A smaller biallelic mean (considered quantitatively or qualitatively) was associated with increased frequency of PCOS. Whereas overall X inactivation was not different between cases and controls, in the subgroup of women with nonrandom X inactivation, the shorter allele was preferentially active in PCOS. The large number of subjects in the cohort gives our study an advantage over previous studies to assess the influence of CAG repeat number on PCOS. Our results are consistent with previous functional studies that found higher activity in ARs with shorter repeats (4,5,6). Replication studies are critical to the confirmation (or repudiation) of genetic findings in complex disorders, particularly replication studies that are larger in size than previous reports, such as the present study.
Unlike most other androgenic conditions (7,8,9,10,11), association studies of PCOS with (CAG)n have been inconsistent and at times at variance with the in vitro functional studies. In one study (16), no overall difference was shown in the length of CAG repeats between the PCOS and control women. In the same study, all subjects with CAG repeat length 15 or less had PCOS, suggesting some influence of the shorter CAG repeat in the pathogenesis of PCOS. In a recent report, a trend to shorter CAG repeats (P = 0.1) in PCOS vs. controls was observed (21). In another study, no difference was seen in CAG repeat length between PCOS subjects and controls; however, in a subgroup with lower testosterone levels, there was a trend for lower CAG biallelic mean in women with PCOS, consistent with a role for androgen hypersensitivity in the development of the syndrome (17). Similarly, another study found no overall association of CAG repeats with PCOS but observed higher mean CAG repeats in a subgroup with higher testosterone levels (20). Alternatively, in the study by Hickey et al. (14), paradoxically a longer CAG repeat biallelic mean was associated with an increased risk of PCOS, a finding that appears to be contrary to functional data. Finally, a large study of several candidate genes found nonsignificant evidence of linkage of AR variation with PCOS (19).
DNA methylation is an example of an epigenetic mechanism that results in the modification of the DNA and altered gene expression without changing the primary DNA sequence. It is an important mechanism in X-chromosome inactivation (26). X inactivation, which occurs in early embryogenesis, is thought to be a random event. Nonrandom X inactivation, either due to environmental exposures or allelic differences, may contribute to the expression of PCOS because the AR gene resides on the X chromosome. Escobar-Morreale and colleagues (13) found no significant difference in pattern of X inactivation between women with hyperandrogenic hirsutism, idiopathic hirsutism, or controls. These results contrasted with a study that found preferential inactivation of the longer CAG repeat alleles in women with idiopathic hirsutism, compared with controls, resulting in expression of the smaller alleles predicted to increase androgen sensitivity (27). More recently a study of 122 infertile women with PCOS and 83 controls produced the unexpected result that preferential expression of longer CAG repeat alleles occurred in PCOS (14). Subsequently the same investigators conducted a family-based study of X inactivation consisting of 40 families that yielded 88 sister pairs. Sister pairs with the same AR CAG genotype and the same pattern of X inactivation were almost 30 times more likely to have the same phenotype (both affected or both unaffected) as sister pairs with the same genotype but different pattern of X inactivation (15). The latter study implicated nonrandom X inactivation in PCOS pathogenesis, although without comment on which allele was being inactivated. Our result of preferential activation of X chromosomes with shorter alleles was as hypothesized, i.e. that ARs with longer CAG repeats, which are presumably less active, are preferentially inactivated in PCOS; however, this finding was observed only when X inactivation was nonrandom.
Some, but not all, prior studies (including both non-PCOS and PCOS subjects) found an association between CAG repeats and testosterone levels (14,17,20,28,29). Other investigators found that the CAG repeat length of the AR influenced the relationship between free testosterone and insulin resistance [measured by homeostatic model assessment (HOMA-IR)], such that at lower CAG lengths, free testosterone and HOMA-IR were positively correlated (18). Another study found that shorter CAG repeats were associated with increased androgen levels only in the presence of longer alleles of the SHBG (TAAAA)n variant, suggesting that simultaneous lesions in both androgen availability and sensitivity could result in hyperandrogenism (21). Presumably alterations in androgen sensitivity could lead to altered androgen secretion, similar to altered insulin secretion when insulin resistance is present. However, this hypothesis was not borne out in our study because we found no relationship between testosterone level and CAG repeats.
This the first large study of PCOS wherein the association of CAG repeats with PCOS as well as X-inactivation patterns agree with the in vitro evidence that shorter AR alleles produce more active receptors. Our study differs from previous investigations in not only size but also how PCOS was defined. Most other studies examining the CAG repeat in PCOS did not use the 1990 NIH criteria but instead used various broader or more restrictive definitions of the disorder (13,14,16,17,19,20). Another difference may reside in the fact that we adjusted for age and BMI when assessing association with PCOS. Age-related changes in X inactivation have been previously observed (30,31), and we observed a weak but statistically significant correlation between age and biallelic mean (r = −0.093, P = 0.032), suggesting the need to adjust association analyses for this parameter. Whereas BMI was not correlated with CAG repeats in PCOS women in our study or that of others (16), in non-PCOS studies, the number of CAG repeats influenced obesity and body composition in women (32) and men (33,34,35), providing a rationale for adjusting for BMI.
Our cohort was composed of both white and black women. Combining them was possible because we were studying a single functional coding variant. This contrasts with association studies that attempt to find association by linkage disequilibrium mapping, in which case combining different ethnic groups is inadvisable because patterns of linkage disequilibrium often differ between the groups. Consistent with prior reports (36,37), we found slightly lower repeat number in blacks, compared with whites. We accounted for this by using race-specific medians as cutoffs as well as adjusting for race in combined analyses. Combined analyses were reasonable because there was no a priori reason to believe that CAG repeats would affect AR function differently in whites and blacks, which was confirmed by the similarity of the association results we observed in each group separately.
Our study had some limitations. There were proportionately more white than black women with PCOS; given that whites had higher repeat numbers, this might have weakened the observed association of lower (CAG)n with PCOS in the combined analysis, despite adjustment for race. Another limitation of our study is that we could not separate the effects of (CAG)n on PCOS vs. hirsutism because the majority of PCOS cases had hirsutism. We were also unable to detect an effect of (CAG)n on androgen levels or mFG score, given that such analyses could be conducted only within PCOS cases, which reduced the sample size. Study of (CAG)n in a much larger, racially homogeneous cohort will be needed to overcome these limitations and confirm our results.
A large number of AR coactivators and corepressors have been identified, which are critical to AR function and may mediate tissue-specific androgen effects (38). Variation in expression or function of these coregulators could also contribute to development of PCOS. Our study examined the AR in isolation. Future genetic and physiologic work is warranted to identify functional interactions between CAG repeats and AR coregulators.
In conclusion, our data suggest that in addition to excess androgen production, the risk of PCOS is associated with the inheritance of ARs with shorter polyglutamine tracts, suggesting that genetic differences in androgen sensitivity may contribute to development of the syndrome. Association of PCOS with other genes (39) that influence target organ response to androgens further supports this hypothesis, which may explain the often observed disparity between the clinical severity of hyperandrogenism and the degree of hyperandrogenemia.
Supplementary Material
Acknowledgments
We thank Theresa Hickey for valuable technical advice.
Footnotes
This study was supported in part by NIH Grants R01-HD29364, K24-HD01346 (to R.A.), and M01-RR00425 (General Clinical Research Center Grant from the National Center for Research Resources) and an endowment from the Helping Hand of Los Angeles, Inc.
Disclosure Statement: N.A.S., H.J.A., M.P., K.D.T., and M.O.G. have nothing to declare. R.A. has received consulting fees from Procter & Gamble, Merck & Co., and Organon.
First Published Online February 26, 2008
Abbreviations: AR, Androgen receptor; BMI, body mass index; HOMA-IR, homeostatic model assessment for insulin resistance; mFG, modified Ferriman-Gallwey; OR, odds ratio; PCOS, polycystic ovary syndrome.
References
- Lubahn DB, Joseph DR, Sullivan PM, Willard HF, French FS, Wilson EM 1988 Cloning of human androgen receptor complementary DNA and localization to the X chromosome. Science 240:327–330 [DOI] [PubMed] [Google Scholar]
- Brown CJ, Goss SJ, Lubahn DB, Joseph DR, Wilson EM, French FS, Willard HF 1989 Androgen receptor locus on the human X chromosome: regional localization to Xq11–12 and description of a DNA polymorphism. Am J Hum Genet 44:264–269 [PMC free article] [PubMed] [Google Scholar]
- Rajender S, Singh L, Thangaraj K 2007 Phenotypic heterogeneity of mutations in androgen receptor gene. Asian J Androl 9:147–179 [DOI] [PubMed] [Google Scholar]
- Chamberlain NL, Driver ED, Miesfeld RL 1994 The length and location of CAG trinucleotide repeats in the androgen receptor N-terminal domain affect transactivation function. Nucleic Acids Res 22:3181–3186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tut TG, Ghadessy FJ, Trifiro MA, Pinsky L, Yong EL 1997 Long polyglutamine tracts in the androgen receptor are associated with reduced trans-activation, impaired sperm production, and male infertility. J Clin Endocrinol Metab 82:3777–3782 [DOI] [PubMed] [Google Scholar]
- Beilin J, Ball EM, Favaloro JM, Zajac JD 2000 Effect of the androgen receptor CAG repeat polymorphism on transcriptional activity: specificity in prostate and non-prostate cell lines. J Mol Endocrinol 25:85–96 [DOI] [PubMed] [Google Scholar]
- Davis-Dao CA, Tuazon ED, Sokol RZ, Cortessis VK 2007 Male infertility and variation in CAG repeat length in the androgen receptor gene: a meta-analysis. J Clin Endocrinol Metab 92:4319–4326 [DOI] [PubMed] [Google Scholar]
- Zeegers MP, Kiemeney LA, Nieder AM, Ostrer H 2004 How strong is the association between CAG and GGN repeat length polymorphisms in the androgen receptor gene and prostate cancer risk? Cancer Epidemiol Biomarkers Prev 13:1765–1771 [PubMed] [Google Scholar]
- Legro RS, Shahbahrami B, Lobo RA, Kovacs BW 1994 Size polymorphisms of the androgen receptor among female Hispanics and correlation with androgenic characteristics. Obstet Gynecol 83:701–706 [PubMed] [Google Scholar]
- Ibanez L, Ong KK, Mongan N, Jaaskelainen J, Marcos MV, Hughes IA, De Zegher F, Dunger DB 2003 Androgen receptor gene CAG repeat polymorphism in the development of ovarian hyperandrogenism. J Clin Endocrinol Metab 88:3333–3338 [DOI] [PubMed] [Google Scholar]
- Sawaya ME, Shalita AR 1998 Androgen receptor polymorphisms (CAG repeat lengths) in androgenetic alopecia, hirsutism, and acne. J Cutan Med Surg 3:9–15 [DOI] [PubMed] [Google Scholar]
- Chang WY, Knochenhauer ES, Bartolucci AA, Azziz R 2005 Phenotypic spectrum of polycystic ovary syndrome: clinical and biochemical characterization of the three major clinical subgroups. Fertil Steril 83:1717–1723 [DOI] [PubMed] [Google Scholar]
- Calvo RM, Asuncion M, Sancho J, San Millan JL, Escobar-Morreale HF 2000 The role of the CAG repeat polymorphism in the androgen receptor gene and of skewed X-chromosome inactivation, in the pathogenesis of hirsutism. J Clin Endocrinol Metab 85:1735–1740 [DOI] [PubMed] [Google Scholar]
- Hickey T, Chandy A, Norman RJ 2002 The androgen receptor CAG repeat polymorphism and X-chromosome inactivation in Australian Caucasian women with infertility related to polycystic ovary syndrome. J Clin Endocrinol Metab 87:161–165 [DOI] [PubMed] [Google Scholar]
- Hickey TE, Legro RS, Norman RJ 2006 Epigenetic modification of the X chromosome influences susceptibility to polycystic ovary syndrome. J Clin Endocrinol Metab 91:2789–2791 [DOI] [PubMed] [Google Scholar]
- Jaaskelainen J, Korhonen S, Voutilainen R, Hippelainen M, Heinonen S 2005 Androgen receptor gene CAG length polymorphism in women with polycystic ovary syndrome. Fertil Steril 83:1724–1728 [DOI] [PubMed] [Google Scholar]
- Mifsud A, Ramirez S, Yong EL 2000 Androgen receptor gene CAG trinucleotide repeats in anovulatory infertility and polycystic ovaries. J Clin Endocrinol Metab 85:3484–3488 [DOI] [PubMed] [Google Scholar]
- Mohlig M, Jurgens A, Spranger J, Hoffmann K, Weickert MO, Schlosser HW, Schill T, Brabant G, Schuring A, Pfeiffer AF, Gromoll J, Schofl C 2006 The androgen receptor CAG repeat modifies the impact of testosterone on insulin resistance in women with polycystic ovary syndrome. Eur J Endocrinol 155:127–130 [DOI] [PubMed] [Google Scholar]
- Urbanek M, Legro RS, Driscoll DA, Azziz R, Ehrmann DA, Norman RJ, Strauss JF, Spielman RS, Dunaif A 1999 Thirty-seven candidate genes for polycystic ovary syndrome: strongest evidence for linkage is with follistatin. Proc Natl Acad Sci USA 96:8573–8578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Choung SH, Choi YM, Yoon SH, Kim SH, Moon SY 2008 Androgen receptor gene CAG repeat polymorphism in women with polycystic ovary syndrome. Fertil Steril, in press [DOI] [PubMed] [Google Scholar]
- Xita N, Georgiou I, Lazaros L, Psofaki V, Kolios G, Tsatsoulis A 2008 The role of sex hormone-binding globulin and androgen receptor gene variants in the development of polycystic ovary syndrome. Hum Reprod 23:693–698 [DOI] [PubMed] [Google Scholar]
- Zawadzki JK, Dunaif A 1992 Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Dunaif A, Givens JR, Haseltine F, Merriam GR, eds. Polycystic ovary syndrome. Cambridge, UK: Blackwell Scientific Publications; 377–384 [Google Scholar]
- Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO 2004 The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab 89:2745–2749 [DOI] [PubMed] [Google Scholar]
- Brownstein MJ, Carpten JD, Smith JR 1996 Modulation of non-templated nucleotide addition by Taq DNA polymerase: primer modifications that facilitate genotyping. Biotechniques 20:1004–1006, 1008–1010 [DOI] [PubMed] [Google Scholar]
- Naumova AK, Plenge RM, Bird LM, Leppert M, Morgan K, Willard HF, Sapienza C 1996 Heritability of X chromosome—inactivation phenotype in a large family. Am J Hum Genet 58:1111–1119 [PMC free article] [PubMed] [Google Scholar]
- Heard E, Clerc P, Avner P 1997 X-chromosome inactivation in mammals. Annu Rev Genet 31:571–610 [DOI] [PubMed] [Google Scholar]
- Vottero A, Stratakis CA, Ghizzoni L, Longui CA, Karl M, Chrousos GP 1999 Androgen receptor-mediated hypersensitivity to androgens in women with nonhyperandrogenic hirsutism: skewing of X-chromosome inactivation. J Clin Endocrinol Metab 84:1091–1095 [DOI] [PubMed] [Google Scholar]
- Westberg L, Baghaei F, Rosmond R, Hellstrand M, Landen M, Jansson M, Holm G, Bjorntorp P, Eriksson E 2001 Polymorphisms of the androgen receptor gene and the estrogen receptor β gene are associated with androgen levels in women. J Clin Endocrinol Metab 86:2562–2568 [DOI] [PubMed] [Google Scholar]
- Brum IS, Spritzer PM, Paris F, Maturana MA, Audran F, Sultan C 2005 Association between androgen receptor gene CAG repeat polymorphism and plasma testosterone levels in postmenopausal women. J Soc Gynecol Investig 12:135–141 [DOI] [PubMed] [Google Scholar]
- Busque L, Mio R, Mattioli J, Brais E, Blais N, Lalonde Y, Maragh M, Gilliland DG 1996 Nonrandom X-inactivation patterns in normal females: lyonization ratios vary with age. Blood 88:59–65 [PubMed] [Google Scholar]
- Gale RE, Fielding AK, Harrison CN, Linch DC 1997 Acquired skewing of X-chromosome inactivation patterns in myeloid cells of the elderly suggests stochastic clonal loss with age. Br J Haematol 98:512–519 [DOI] [PubMed] [Google Scholar]
- Gustafson DR, Wen MJ, Koppanati BM 2003 Androgen receptor gene repeats and indices of obesity in older adults. Int J Obes Relat Metab Disord 27:75–81 [DOI] [PubMed] [Google Scholar]
- Lapauw B, Goemaere S, Crabbe P, Kaufman JM, Ruige JB 2007 Is the effect of testosterone on body composition modulated by the androgen receptor gene CAG repeat polymorphism in elderly men? Eur J Endocrinol 156:395–401 [DOI] [PubMed] [Google Scholar]
- Walsh S, Zmuda JM, Cauley JA, Shea PR, Metter EJ, Hurley BF, Ferrell RE, Roth SM 2005 Androgen receptor CAG repeat polymorphism is associated with fat-free mass in men. J Appl Physiol 98:132–137 [DOI] [PubMed] [Google Scholar]
- Zitzmann M, Gromoll J, von Eckardstein A, Nieschlag E 2003 The CAG repeat polymorphism in the androgen receptor gene modulates body fat mass and serum concentrations of leptin and insulin in men. Diabetologia 46:31–39 [DOI] [PubMed] [Google Scholar]
- Platz EA, Rimm EB, Willett WC, Kantoff PW, Giovannucci E 2000 Racial variation in prostate cancer incidence and in hormonal system markers among male health professionals. J Natl Cancer Inst 92:2009–2017 [DOI] [PubMed] [Google Scholar]
- Sartor O, Zheng Q, Eastham JA 1999 Androgen receptor gene CAG repeat length varies in a race-specific fashion in men without prostate cancer. Urology 53:378–380 [DOI] [PubMed] [Google Scholar]
- Heemers HV, Tindall DJ 2007 Androgen receptor (AR) coregulators: a diversity of functions converging on and regulating the AR transcriptional complex. Endocr Rev 28:778–808 [DOI] [PubMed] [Google Scholar]
- Goodarzi MO, Shah NA, Antoine HJ, Pall M, Guo X, Azziz R 2006 Variants in the 5α-reductase type 1 and type 2 genes are associated with polycystic ovary syndrome and the severity of hirsutism in affected women. J Clin Endocrinol Metab 91:4085–4091 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.