Abstract
Objective: Osteoprotegerin (OPG) is an important regulator of bone turnover through its effects on osteoclastogenesis, yet findings from previous studies of circulating OPG and commonly measured bone indices in humans have been conflicting. We conducted a cross-sectional study to evaluate the association between plasma OPG and femoral neck (FN) bone density (BMD) and geometry in a large cohort of women and men.
Design: Participants included 1379 postmenopausal women and 1165 men, aged 50–89 yr (mean, 64 yr), in the Framingham Offspring Study. Dual x-ray absorptiometry was used to evaluate FN BMD and geometry (bone width, section modulus, and cross-sectional area at the narrow neck region). Plasma OPG concentrations were measured by ELISA. Sex-specific analysis of covariance was used to calculate means and assess linear trend in BMD and geometry values across OPG quartiles, adjusted for confounders.
Results: OPG concentrations were greater in women than men, increased with age, and were greater in smokers and those with diabetes and heart disease. Multivariable-adjusted mean FN BMD in women increased from the lowest to the highest OPG quartile (trend, P < 0.01). However, no linear trend between FN BMD and OPG was observed in men (trend, P = 0.34). Section modulus and bone width increased with OPG in men (trend, P < 0.01), whereas no association between hip geometry indices and OPG was observed in women.
Conclusion: Higher OPG concentration may indicate greater skeletal strength in women and men, possibly through reducing bone loss in women and increasing periosteal apposition in men.
A study in postmenopausal women and men, aged 50–89 years, examining the association between osteoprotegerin and femoral neck bone density and geometry finds that osteoprotegerin concentration increased with bone density in women and with bone width and bending strength in men.
The osteoprotegerin (OPG), receptor activator of nuclear factor-κβ (RANK), and RANK ligand (RANKL) system is an important regulator of osteoclastogenesis. Expressed by the osteoblast (and other cells), OPG acts as a decoy receptor for RANKL and prevents RANKL from binding to RANK on the surface of the osteoclast, thereby inhibiting differentiation of the osteoclast (1). In addition to reducing the number of differentiated osteoclasts, OPG decreases the activity of the remaining osteoclasts and increases mature osteoclast apoptosis. Thus, in vitro, OPG prevents bone loss by decreasing resorption. In animal models, OPG deficiency causes osteoporosis, and transgenic overexpression of OPG results in osteopetrosis (2,3,4,5).
Furthermore, treatment with recombinant OPG has been shown to improve structural and mechanical properties of bone in rodents and primates (6,7,8,9,10).
The clinical significance to the human skeleton of circulating OPG concentration is not known. Most studies report no association between OPG concentration and bone mineral density (11,12,13,14,15), and none have evaluated the relation of OPG to bone geometry of the femoral neck, the fracture site associated with the greatest morbidity, mortality, and public health costs (16,17,18).
We used bone densitometry to measure traditional bone density as well as structural geometry to evaluate the cross-sectional association between these indices of bone strength at the femoral neck and plasma OPG concentration in a large cohort of women and men.
Subjects and Methods
Subjects
Participants for this study were members of the Framingham Offspring Cohort. The Framingham Offspring Cohort was established in 1971 with enrollment of 5124 adult children and spouses of members of the original Framingham Heart Study cohort (19,20). Initiated in 1949, the original study was established as a population-based cohort selected from a two thirds sample of residents living in the town of Framingham, MA (21). Every 4 yr, members of the Offspring Cohort have been undergoing comprehensive clinical examinations conducted according to standardized protocols.
To be eligible for the current study, members of the Offspring Cohort were required to have undergone bone densitometry and phlebotomy testing between 1996 and 2001. Participants younger than age 50 yr (153 women, 121 men) and women who were 50 yr and older but still premenopausal (85) were excluded. There were no exclusions according to disease status. Thus, the current study included 2527 members of the Offspring Cohort, 1371 women and 1156 men.
Performed as part of an ancillary study to the Framingham Osteoporosis Study, (22), hip structure analysis was conducted for 2032 participants (84%) of 2527 individuals in the current analysis, including 1081 women and 951 men.
Participants provided written informed consent, and the Institutional Review Board for Human Research at Boston University and Hebrew Rehabilitation Center approved the study.
Bone density and geometry
Dual-energy x-ray absorptiometry (DXA) scans were used to evaluate femoral neck bone mineral density (Lunar DPX-L; Lunar Radiation Corp., Madison, WI). Coefficient of variation was 1.7% (23).
Based on a principle described by Martin and Burr (24), hip structure analysis uses two-dimensional images acquired by DXA densitometry to characterize structural dimensions of bone cross-sections. The algorithm averages geometric measurements over five parallel lines of pixels, each spaced 1 pixel apart, along the narrowest point of the femoral neck (25). Geometric parameters are measured directly from the mass profiles, with the narrow neck region of the femur modeled as a circular annulus with a 60:40 ratio of cortical to trabecular bone.
Bone width (centimeters), the outer diameter of the narrow neck of the femur, was determined as the blur-corrected width of the mass profile.
Cross-sectional area (square centimeters), a measure of compressive (axial) strength, was computed as the integral of the bone mass profile (grams) divided by a constant density of bone mineral (ρm= 1.05 g/cm2). Cross-sectional area measures the amount of bone surface area, excluding trabecular and soft tissue spaces, and can be interpreted as bone mineral content.
Used to calculate the section modulus, the cross-sectional moment of inertia (centimeters4) was computed as the integral of mass (grams) multiplied by the square of the distance (centimeters) from the profile center of mass, divided by the constant density of mineral (ρm= 1.05 g/cm2).
Section modulus (cubic centimeters), an estimate of bending strength (in the image plane), was calculated as the cross-sectional moment of inertia (cm4) divided by the maximum distance (centimeters) from the center of mass to the medial or lateral margin.
Whereas reliability of hip structure parameters was not assessed in the current study, Khoo et al. (26) evaluated precision for 119 pairs of Lunar DPX scans obtained from women with osteoporosis (aged 42–83 yr, mean age 69 yr) enrolled in clinical trials. Coefficients of variation for geometry indices of the narrow neck region measured by hip structure analysis using Lunar DPX scans were 6% for cross-sectional area, 3% for bone width, and 10% for section modulus (26).
OPG
Blood specimens were drawn in the morning after an overnight fast and stored at −80 C without freeze-thaw cycles. Total OPG concentrations (picomoles per liter) were determined by an ELISA according to the manufacturer's instructions (Biomedica, Vienna, Austria). Measurements were performed without knowledge of participant characteristics. Intraassay coefficient of variation was 4%.
Confounders
Clinical and lifestyle characteristics previously identified as risk factors for osteoporosis were considered as potential confounding variables. Assessments made at the time of bone densitometry were used for the analyses. Information on participants' age, smoking, use of osteoporosis medications (bisphophonate, selective estrogen receptor modulator, calcitonin), and estrogen replacement therapy (for women) was obtained by structured interview. Individuals who reported smoking at least one cigarette per day in the past year were classified as current smokers. Height, to the nearest one quarter inch and weight, to the nearest half pound, were measured using a stadiometer and balance beam scale, respectively. Body mass index was calculated as weight divided by the square of height. Diabetes was defined as a fasting plasma glucose level of more than 126 mg/dl or treatment with insulin or oral hypoglycemic agents. Coronary heart disease was defined as recognized or unrecognized myocardial infarction (identified by electrocardiogram or enzymes), angina pectoris, coronary insufficiency, or congestive heart failure (27).
Glomerular filtration rate was estimated using the Modification of Diet in Renal Disease equation (glomerular filtration rate = 186.3 × (serum creatinine)−1.154 × age−0.203 × (0.742 for women) (28). Serum creatinine was measured using the modified Jaffe method.
Statistical analysis
Characteristics of participants were compared according to sex-specific quartiles of OPG concentrations using analysis of covariance.
Sex-specific analysis of covariance was used to calculate adjusted least-squares means and assess linear trend in bone mineral density and geometry values across OPG quartiles. We present results first for age-adjusted analysis and second for multivariable-adjusted analysis in which models included age (years), height (inches), body mass index (pounds per square inch), current smoking (yes/no), osteoporosis medications (yes/no), and current estrogen use (yes/no) in women. Diabetes, heart disease, and kidney function (as measured by glomerular filtration rate) were not confounders and therefore not retained in multivariable models. However, we repeated the analysis excluding these individuals with diabetes or heart disease to examine whether findings differed from those for the total study group. (There were no participants with chronic renal failure.) Finally, we conducted stratified analysis in women according whether or not they used estrogen therapy.
Analyses were performed using PC-SAS (version 9.1; SAS Institute, Cary, NC).
Results
Mean age of participants was 64 yr and ranged from 50 to 89 yr (Table 1). Twelve percent of women and 10% of men were current smokers. Prevalence of diabetes was 9% in women and 14% in men, whereas 4% of women and 13% of men had coronary heart disease. Postmenopausal estrogen therapy was used by 39% of women, and osteoporosis medications were used by 12% of women (bisphosphonates, 8%, selective estrogen receptor modulators, 3%, other, 1%). One percent of men used an osteoporosis medication.
Table 1.
Characteristics of women and men in the Framingham Offspring Study, 1996–2001
| Women (n = 1371) | Men (n = 1156) | |
|---|---|---|
| Age (yr), mean (range 50–89) | 64 | 64 |
| Body mass index (kg/m2), mean | 28 | 29 |
| Height (cm), mean | 160 | 175 |
| Current smoker (%) | 12 | 10 |
| Type 2 diabetes mellitus (%) | 9 | 14 |
| Coronary heart disease (%) | 4 | 13 |
| Osteoporosis medications (%) | 12 | 1 |
| Estrogen use (%) | 39 |
Plasma OPG concentration was greater in women (median 5.75 pmol/liter; interquartile range 4.85–6.84) than men (median 5.32; interquartile range 4.38–6.47) and increased with age, smoking, and prevalence of diabetes and heart disease (Table 2). Women in the highest OPG quartile less frequently used estrogen than those in the lower quartiles. Glomerular filtration rate decreased with increasing quartile of OPG, whereas body mass index and frequency of osteoporosis medication use did not differ according to OPG concentration.
Table 2.
Characteristics of women and men according to plasma OPG concentration, the Framingham Offspring Study, 1996–2001
| Women (n = 1371)
|
Men (n = 1156)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| OPG quartile
|
OPG quartile
|
|||||||
| 1 (low) | 2 | 3 | 4 (high) | 1 (low) | 2 | 3 | 4 (high) | |
| n | 340 | 345 | 344 | 342 | 287 | 293 | 287 | 289 |
| OPG (pmol/liter), median | 4.11 | 5.32 | 6.18 | 7.86 | 3.82 | 4.85 | 5.82 | 7.35 |
| OPG (pmol/liter), interquartile range | 0.92 | 0.46 | 0.53 | 1.68 | 0.74 | 0.49 | 0.58 | 1.53 |
| Age (yr), mean | 60 | 62 | 64 | 68a | 59 | 62 | 64 | 69a |
| Body mass index (kg/m2), mean | 27 | 28 | 27 | 28 | 29 | 29 | 29 | 29 |
| Height (cm), mean | 160 | 160 | 160 | 158 | 175 | 175 | 175 | 173 |
| Current smoker (%) | 9 | 12 | 13 | 13a | 8 | 11 | 11 | 12a |
| Type 2 diabetes mellitus (%) | 3 | 7 | 6 | 18a | 7 | 7 | 16 | 26a |
| Coronary heart disease (%) | 2 | 2 | 5 | 9a | 7 | 7 | 14 | 24a |
| Glomerular filtration rate (ml/min per 1.73 m2), mean | 86.52 | 83.11 | 81.20 | 77.08a | 87.01 | 87.33 | 83.97 | 78.55a |
| OPG medications (%) | 11 | 10 | 14 | 11 | 1 | 1 | 1 | 1 |
| Current estrogen use (%) | 47 | 37 | 40 | 33a | ||||
Trend, P < 0.05.
Age-adjusted mean bone density increased with increasing quartile of plasma OPG concentration in women (trend, P = 0.0006) but not in men (trend, P = 0.48; Table 3). Of note, the relatively high bone density value of 0.88 g/cm2 for quartile 4 appeared to account for the positive trend observed in women because mean bone density values were similar for women in quartiles 1 and 2 (0.85 g/cm2) and 3 (0.86 g/cm2).
Table 3.
Age-adjusted mean femoral neck bone mineral density and geometry indices according to sex-specific quartile of plasma OPG concentration, the Framingham Offspring Study, 1996–2001
| Women
|
Men
|
|||||||
|---|---|---|---|---|---|---|---|---|
| OPG quartile | Bone density (g/cm2) | Cross-sectional area (cm2) | Width (cm) | Section modulus (cm3) | Bone density (g/cm2) | Cross-sectional area (cm2) | Width (cm) | Section modulus (cm3) |
| 1 (low) | 0.85 | 2.26 | 3.36 | 1.33 | 0.97 | 2.77 | 3.70 | 1.78 |
| 2 | 0.85 | 2.22 | 3.30 | 1.28 | 0.97 | 2.85 | 3.82 | 1.87 |
| 3 | 0.86 | 2.22 | 3.29 | 1.28 | 0.98 | 2.86 | 3.84 | 1.88 |
| 4 (high) | 0.88 | 2.26 | 3.26 | 1.30 | 0.96 | 2.80 | 3.88 | 1.88 |
| Trend, P | <0.01 | 0.83 | 0.02 | 0.34 | 0.48 | 0.46 | <0.01 | 0.02 |
No consistent pattern was observed in the age-adjusted mean cross-sectional area with increasing OPG in women or men (Table 3). For example, in women, cross-sectional area was 2.26 cm2 for the lowest and highest OPG quartile and 2.22 cm2 for each of the two middle quartiles.
Age-adjusted mean bone width decreased in women from 3.36 cm in the lowest quartile to 3.26 cm in the highest quartile of OPG (trend, P = 0.02; Table 3). In contrast, bone width increased over 4% in men, from 3.70 cm in quartile 1 to 3.88 cm in quartile 4 (trend, P < 0.01).
Section modulus was unassociated with OPG in women (trend, P = 0.34) but positively associated with OPG in men (trend, P = 0.02), increasing 5% from 1.78 to 1.88 cm3, respectively, from the lowest and highest quartiles of OPG (Table 3).
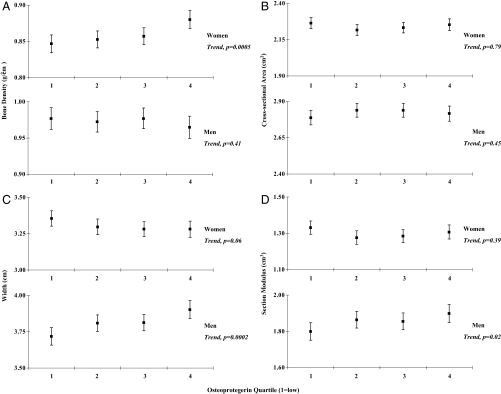
Multivariable-adjusted mean bone density increased with OPG concentration in women but not men (Fig. 1A), whereas bone width (Fig. 1C) and section modulus (Fig. 1D) increased in men but not women. The positive trend in increasing OPG with bone width and section modulus in men was statistically significant whether OPG was treated as a categorical or continuous variable. The trend in decreasing age-adjusted mean bone width observed with increasing OPG in women was no longer significant in multivariable-adjusted models. No significant relation was observed between cross-sectional area and OPG (Fig. 1B).
Figure 1.
Bone mineral density (A), cross-sectional area (B), width (C), and section modulus (D) at the femoral neck for women (top panel) and men (bottom panel) according to quartile of plasma OPG concentration (1 = low). Values presented are least squares means (▪) and 95% confidence intervals (Ι) adjusted for age, height, body mass index, current smoking, osteoporosis medications, and estrogen use (women). P values represent tests for linear trend in bone density and geometry indices across quartile of plasma OPG concentration.
The analysis was repeated excluding participants with diabetes or coronary heart disease; however, the findings were unchanged. We also assessed the relation between OPG and bone density and geometry in women stratified by estrogen use; however, the results did not differ according to whether women were taking estrogen replacement therapy.
Discussion
In this large, population-based study, increased OPG concentration was associated with greater femoral neck bone density in women and with favorable geometry features in men. The associations were independent of age, body size, lifestyle characteristics, chronic diseases, and osteoporosis medication use. The positive association between OPG and age, female gender, and prevalence of diabetes and coronary heart disease confirms previous findings obtained in smaller studies (11,12,13,14).
Our results are consistent with those of Mezquita-Raya et al. (29), who found a positive association between OPG and femoral neck bone density in a clinical study of postmenopausal women conducted in Spain. Our study included participants with similar mean age and used the same OPG assay as Mezquita-Raya et al. (29) Recently Stern et al. (30) reported in the Rancho Bernardo Study that femoral neck bone density was increased with OPG in women using estrogen but found no association in women not using estrogen. Whereas we found no interaction between estrogen use, OPG, and bone density, it is possible that the lower frequency of estrogen use in our study (39 vs. 46% in Rancho Bernardo), or other differences between the study populations, may account for the variation in results.
In contrast, no association between OPG and femoral neck bone density was found in 304 women in the Rochester Epidemiology Project (12) or 180 women in the European Vertebral Osteoporosis Study (31). These studies were notably smaller and used different OPG assays than ours, which may explain, at least in part, the discordance in results. Studies of OPG and bone density, measured for the total hip, have reported varied findings, including an inverse relation in 517 postmenopausal women in Iceland, mean aged 56 yr, (11) and no association in 490 women, aged 65 yr and older, recruited from U.S. clinical centers (14).
Femoral neck bone density did not increase with OPG concentration in men in our study. This appears to be mainly due to increases in the outer diameter in men, which can result in decreased bone density. The null results were consistent with findings reported by MINOS, (13), an osteoporosis study of healthy men in France (n = 252 men), as well as the Icelandic [n = 491 men (11)] and Rancho Bernardo studies [n = 307 (30)]. In the Rochester study, however, Khosla et al. (12) observed an inverse association between OPG and femoral neck bone density in 346 men and concluded that sex steroids may regulate the production of OPG.
In addition to evaluating conventional DXA measures of bone density, the current study assessed femoral neck geometry parameters in relation to circulating OPG. Width and section modulus of the narrow neck increased favorably with OPG in men; however, the positive associations were not present in women. The relation of OPG to femoral neck geometry has not yet been investigated by others; thus, we were unable to evaluate the consistency of our findings with previous studies. However, our findings are consistent with animal models reporting that recombinant OPG increased periosteal circumference of the femoral neck in rats and the proximal tibia and distal radius in monkeys (7,10). On the other hand, improvements in bone geometry observed in animals after pharmacological doses of OPG may be due to secondary changes in PTH secretion or other factors and may not be directly applicable to the interpretation of our findings.
Whereas OPG concentration was positively associated with indices of femoral neck bone strength, the results differed in women and men. Specifically, OPG was positively associated with greater bone mineral density in women (not men) and greater bone width and section modulus in men (not women). These gender-specific results may be explained, at least in part, by a sexual dimorphism in femoral neck fragility. Throughout growth, development, and aging, periosteal apposition increases and displaces the cortex further away from the central axis in men but not (nor nearly to the same magnitude) in women (32). As a result, the periosteal diameter of the femoral neck is wider and bending strength (a function of the distance from the central axis to the cortical rim) is increased in men but not women. These sex differences in bone geometry are consistent with our results of a positive association between OPG and bone width and section modulus (bending strength) in men but not in women. In addition, postmenopausal women have higher rates of bone turnover than men, so a potential effect of OPG on bone density may be easier to detect in women, as found in the current study, compared with men.
Alternatively, the positive association between OPG and geometry indices in men and not women may be attributable to possible lower precision in hip structural analysis measures in women due to smaller bone size, compared with men. Because this investigation used DXA images previously acquired from a scanner no longer in use, we were unable to directly assess precision for the hip structure analysis measures. However, as described earlier in this report, as well as by others (23), reliability was assessed for the bone density measures obtained from the scans used in this study, with a coefficient of variation of 1.7%. The current study is limited by the use of traditional, two-dimensional DXA technology, and three-dimensional imaging methods are clearly needed to help elucidate the clinical significance of circulating OPG on femoral neck bone density and geometry in women and men.
Several important limitations of this study warrant discussion. First, the cross-sectional design prohibits conclusions regarding a casual relation between OPG and bone strength indices examined in this investigation. Moreover, we cannot determine from the present cross-sectional study whether the observed OPG concentrations were pathological or compensatory. Results vary widely from cross-sectional studies of the relation between OPG and markers of bone resorption (31,33,34,35), and prospective studies are required to understand the pathway involving OPG, bone metabolism, and bone strength parameters.
Second, whereas OPG is expressed by the skeleton, it is also produced by nonskeletal courses, including the vascular and immune systems. Circulating OPG concentrations may not reflect production in the bone microenvironment. Thus, caution is warranted in the interpretation of circulating OPG measurements.
Third, the relative expression of OPG to RANKL is a fundamental regulator of bone remodeling. In the Bruneck (Italy) Study (36), participants with decreased RANKL had a 10-fold increased risk of lifetime peripheral and clinical vertebral fracture, compared with those in the highest tertile of circulating RANKL. However, this study was based on few fractures (n = 31), which were ascertained by self-report. In addition to RANKL, numerous factors involved in the regulation of OPG in vitro have been implicated including, estradiol and PTH (37,38). However, information on RANKL, estradiol, PTH as well as a number of other factors was not available for the current study. Future investigations assessing sex hormones and other regulators of bone metabolism as potential mediators in the effect of OPG and the OPG to RANKL ratio on the skeleton will provide better understanding of the mechanisms underlying these pathways.
Finally, measurements on OPG fractions, including monomeric, homodimeric (the active form), and ligand bound OPG, were not available for the current analysis. Because the ratio of OPG to RANKL may be an important determinant of osteoclastogenesis (37), development of reliable and sensitive assays that detect specific fractions of OPG as well as RANKL should be included in future research efforts.
This study had several strengths including characterization of circulating OPG according to bone density and geometry at the femoral neck, the site associated with the greatest morbidity and mortality with respect to osteoporotic fracture. In addition, participants were members of a well-defined, population-based cohort with large numbers of women and men, and information was available on a comprehensive list of potential confounders in our study. Whereas the positive relation between OPG and increased femoral neck bone density in women and greater bone width and bending strength in men must be interpreted with caution, the findings suggest that circulating concentrations of this cytokine may provide clinical and biological clues for treatment and prevention of osteoporosis.
Footnotes
This work was supported by National Institutes of Health Grants K01-AR053118, R01-AR050066, R01-AR/AG 41398, R01-AG028321, and R01-HL076784 and National Heart, Lung, and Blood Institute's Framingham Heart Study Grant N01-HC-25195.
Disclosure Statement: E.J.S., K.E.B., S.D., D.K., S.K., and D.P.K. have nothing to declare. The Hip Structural Analysis Software was licensed to Hologic Inc. by The Johns Hopkins University (T.J.B.'s employer).
First Published Online February 26, 2008
Abbreviations: DXA, Dual-energy x-ray absorptiometry; OPG, osteoprotegerin; RANK, receptor activator of nuclear factor-κβ; RANKL, RANK ligand.
References
- Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ 1998 Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93:165–176 [DOI] [PubMed] [Google Scholar]
- Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay E, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Boyle WJ 1997 Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 89:309–319 [DOI] [PubMed] [Google Scholar]
- Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, Scully S, Tan HL, Xu W, Lacey DL, Boyle WJ, Simonet WS 1998 Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev 12:1260–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno A, Amizuka N, Irie K, Murakami A, Fujise N, Kanno T, Sato Y, Nakagawa N, Yasuda H, Mochizuki S, Gomibuchi T, Yano K, Shima N, Washida N, Tsuda E, Morinaga T, Higashio K, Ozawa H 1998 Severe osteoporosis in mice lacking osteoclastogenesis inhibitory factor/osteoprotegerin. Biochem Biophys Res Commun 247:610–615 [DOI] [PubMed] [Google Scholar]
- Kong YY, Feige U, Sarosi I, Bolon B, Tafuri A, Morony S, Capparelli C, Li J, Elliott R, McCabe S, Wong T, Campagnuolo G, Moran E, Bogoch ER, Van G, Nguyen LT, Ohashi PS, Lacey DL, Fish E, Boyle WJ, Penninger JM 1999 Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature 402:304–309 [DOI] [PubMed] [Google Scholar]
- Bateman TA, Dunstan CR, Ferguson VL, Lacey DL, Ayers RA, Simske SJ 2000 Osteoprotegerin mitigates tail suspension-induced osteopenia. Bone 26:443–449 [DOI] [PubMed] [Google Scholar]
- Ross AB, Bateman TA, Kostenuik PJ, Ferguson VL, Lacey DL, Dunstan CR, Simske SJ 2001 The effects of osteoprotegerin on the mechanical properties of rat bone. J Mater Sci Mater Med 12:583–588 [DOI] [PubMed] [Google Scholar]
- Kostenuik PJ, Capparelli C, Morony S, Adamu S, Shimamoto G, Shen V, Lacey DL, Dunstan CR 2001 OPG and PTH-(1–34) have additive effects on bone density and mechanical strength in osteopenic ovariectomized rats. Endocrinology 142:4295–4304 [DOI] [PubMed] [Google Scholar]
- Ichinose Y, Tanaka H, Inoue M, Mochizuki S, Tsuda E, Seino Y 2004 Osteoclastogenesis inhibitory factor/osteoprotegerin reduced bone loss induced by mechanical unloading. Calcif Tissue Int 75:338–343 [DOI] [PubMed] [Google Scholar]
- Ominsky MS, Kostenuik PJ, Cranmer P, Smith SY, Atkinson JE 2007 The RANKL inhibitor OPG-Fc increases cortical and trabecular bone mass in young gonad-intact cynomolgus monkeys. Osteoporos Int 18:1073–1082 [DOI] [PubMed] [Google Scholar]
- Indridason OS, Franzson L, Sigurdsson G 2005 Serum osteoprotegerin and its relationship with bone mineral density and markers of bone turnover. Osteoporos Int 16:417–423 [DOI] [PubMed] [Google Scholar]
- Khosla S, Arrighi HM, Melton 3rd LJ, Atkinson EJ, O'Fallon WM, Dunstan C, Riggs BL 2002 Correlates of osteoprotegerin levels in women and men. Osteoporos Int 13:394–399 [DOI] [PubMed] [Google Scholar]
- Szulc P, Hofbauer LC, Heufelder AE, Roth S, Delmas PD 2001 Osteoprotegerin serum levels in men: correlation with age, estrogen, and testosterone status. J Clin Endocrinol Metab 86:3162–3165 [DOI] [PubMed] [Google Scholar]
- Browner WS, Lui LY, Cummings SR 2001 Associations of serum osteoprotegerin levels with diabetes, stroke, bone density, fractures, and mortality in elderly women. J Clin Endocrinol Metab 86:631–637 [DOI] [PubMed] [Google Scholar]
- Rogers A, Eastell R 2005 Circulating osteoprotegerin and receptor activator for nuclear factor κB ligand: clinical utility in metabolic bone disease assessment. J Clin Endocrinol Metab 90:6323–6331 [DOI] [PubMed] [Google Scholar]
- Melton 3rd LJ, Gabriel SE, Crowson CS, Tosteson AN, Johnell O, Kanis JA 2003 Cost-equivalence of different osteoporotic fractures. Osteoporos Int 14:383–388 [DOI] [PubMed] [Google Scholar]
- Office of the Surgeon General2004 Bone health and osteoporosis: a report of the Surgeon General. Rockville, MD: United States Department of Health and Human Services, Office of the Surgeon General [Google Scholar]
- Johnell O, Kanis JA 2006 An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 17:1726–1733 [DOI] [PubMed] [Google Scholar]
- Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP 1975 The Framingham Offspring Study. Design and preliminary data. Prev Med 4:518–525 [DOI] [PubMed] [Google Scholar]
- Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP 1979 An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol 110:281–290 [DOI] [PubMed] [Google Scholar]
- Dawber TR, Meadors GF, Moore FE 1951 Epidemiological approaches to heart disease: the Framingham Study. Am J Pub Health 41:279–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demissie S, Dupuis J, Cupples LA, Beck TJ, Kiel DP, Karasik D 2007 Proximal hip geometry is linked to several chromosomal regions: genome-wide linkage results from the Framingham Osteoporosis Study. Bone 40:743–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannan MT, Felson DT, Dawson-Hughes B, Tucker KL, Cupples LA, Wilson PW, Kiel DP 2000 Risk factors for longitudinal bone loss in elderly men and women: the Framingham Osteoporosis Study. J Bone Miner Res 15:710–720 [DOI] [PubMed] [Google Scholar]
- Martin RB, Burr DB 1984 Non-invasive measurement of long bone cross-sectional moment of inertia by photon absorptiometry. J Biomech 17:195–201 [DOI] [PubMed] [Google Scholar]
- Beck TJ, Ruff CB, Warden KE, Scott Jr WW, Rao GU 1990 Predicting femoral neck strength from bone mineral data. A structural approach. Invest Radiol 25:6–18 [DOI] [PubMed] [Google Scholar]
- Khoo BC, Beck TJ, Qiao QH, Parakh P, Semanick L, Prince RL, Singer KP, Price RI 2005 In vivo short-term precision of hip structure analysis variables in comparison with bone mineral density using paired dual-energy X-ray absorptiometry scans from multi-center clinical trials. Bone 37:112–121 [DOI] [PubMed] [Google Scholar]
- Kannel W WP, Garrison R 1998 The Framingham Study, section 35. Survival following initial cardiovascular events. Bethesda, MD: National Institutes of Health [Google Scholar]
- Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D 1999 A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130:461–470 [DOI] [PubMed] [Google Scholar]
- Mezquita-Raya P, de la Higuera M, Garcia DF, Alonso G, Ruiz-Requena ME, de Dios Luna J, Escobar-Jimenez F, Munoz-Torres M 2005 The contribution of serum osteoprotegerin to bone mass and vertebral fractures in postmenopausal women. Osteoporos Int 16:1368–1374 [DOI] [PubMed] [Google Scholar]
- Stern A, Laughlin GA, Bergstrom J, Barrett-Connor E 2007 The sex-specific association of serum osteoprotegerin and receptor activator of nuclear factor κB legend with bone mineral density in older adults: the Rancho Bernardo study. Eur J Endocrinol 156:555–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers A, Saleh G, Hannon RA, Greenfield D, Eastell R 2002 Circulating estradiol and osteoprotegerin as determinants of bone turnover and bone density in postmenopausal women. J Clin Endocrinol Metab 87:4470–4475 [DOI] [PubMed] [Google Scholar]
- Duan Y, Beck TJ, Wang XF, Seeman E 2003 Structural and biomechanical basis of sexual dimorphism in femoral neck fragility has its origins in growth and aging. J Bone Miner Res 18:1766–1774 [DOI] [PubMed] [Google Scholar]
- Oh KW, Rhee EJ, Lee WY, Kim SW, Oh ES, Baek KH, Kang MI, Choi MG, Yoo HJ, Park SW 2004 The relationship between circulating osteoprotegerin levels and bone mineral metabolism in healthy women. Clin Endocrinol (Oxf) 61:244–249 [DOI] [PubMed] [Google Scholar]
- Han KO, Choi JT, Choi HA, Moon IG, Yim CH, Park WK, Yoon HK, Han IK 2005 The changes in circulating osteoprotegerin after hormone therapy in postmenopausal women and their relationship with oestrogen responsiveness on bone. Clin Endocrinol (Oxf) 62:349–353 [DOI] [PubMed] [Google Scholar]
- Uemura H, Yasui T, Umino Y, Niki H, Takikawa M, Saito S, Furumoto H, Irahara M 2003 Circulating osteoprotegerin in women during GnRH-agonist treatment and their relationships with mineral components and biomarkers of bone turnover. Bone 33:860–866 [DOI] [PubMed] [Google Scholar]
- Schett G, Kiechl S, Redlich K, Oberhollenzer F, Weger S, Egger G, Mayr A, Jocher J, Xu Q, Pietschmann P, Teitelbaum S, Smolen J, Willeit J 2004 Soluble RANKL and risk of nontraumatic fracture. JAMA 291:1108–1113 [DOI] [PubMed] [Google Scholar]
- Hofbauer LC, Schoppet M 2004 Clinical implications of the osteoprotegerin/RANKL/RANK system for bone and vascular diseases. JAMA 292:490–495 [DOI] [PubMed] [Google Scholar]
- Khosla S 2001 Minireview: the OPG/RANKL/RANK system. Endocrinology 142:5050–5055 [DOI] [PubMed] [Google Scholar]