Abstract
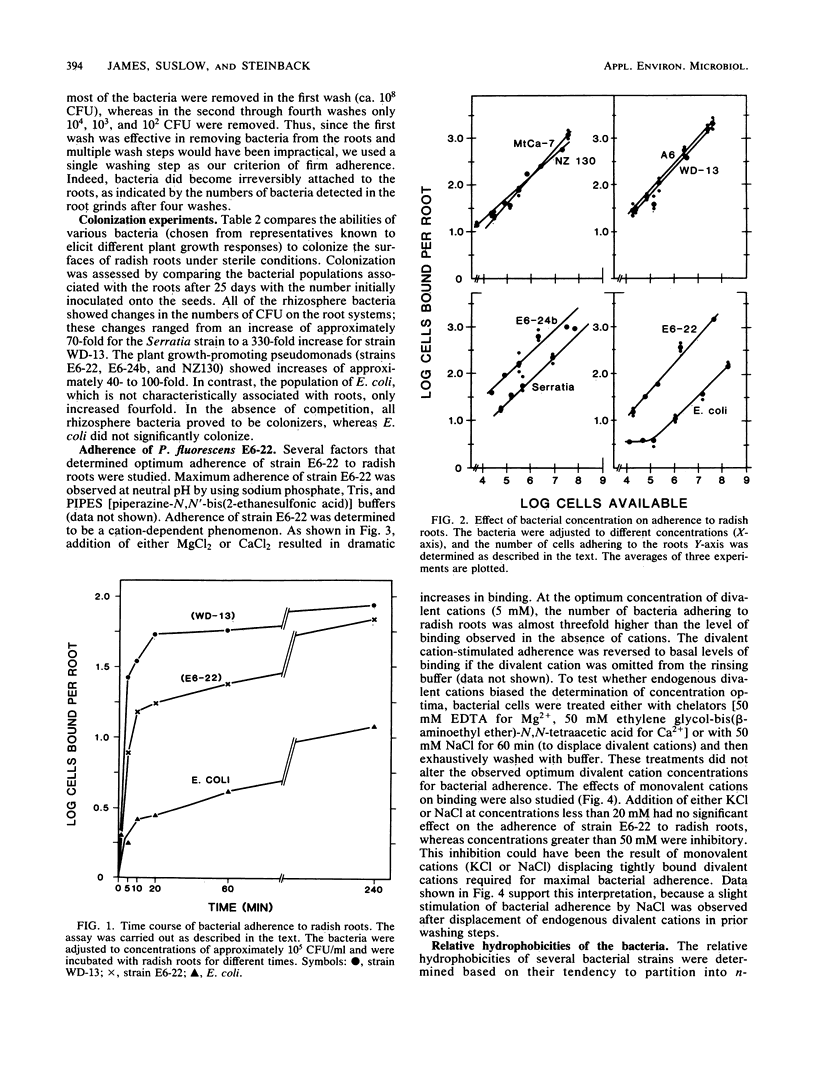
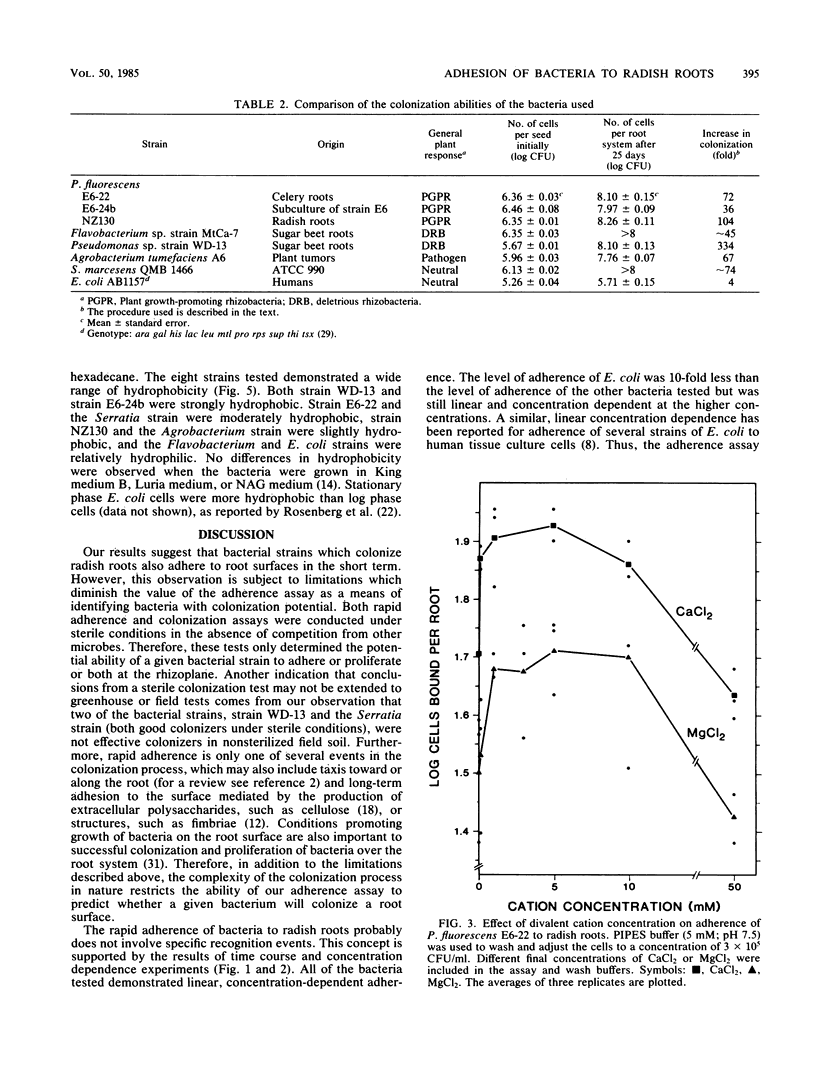
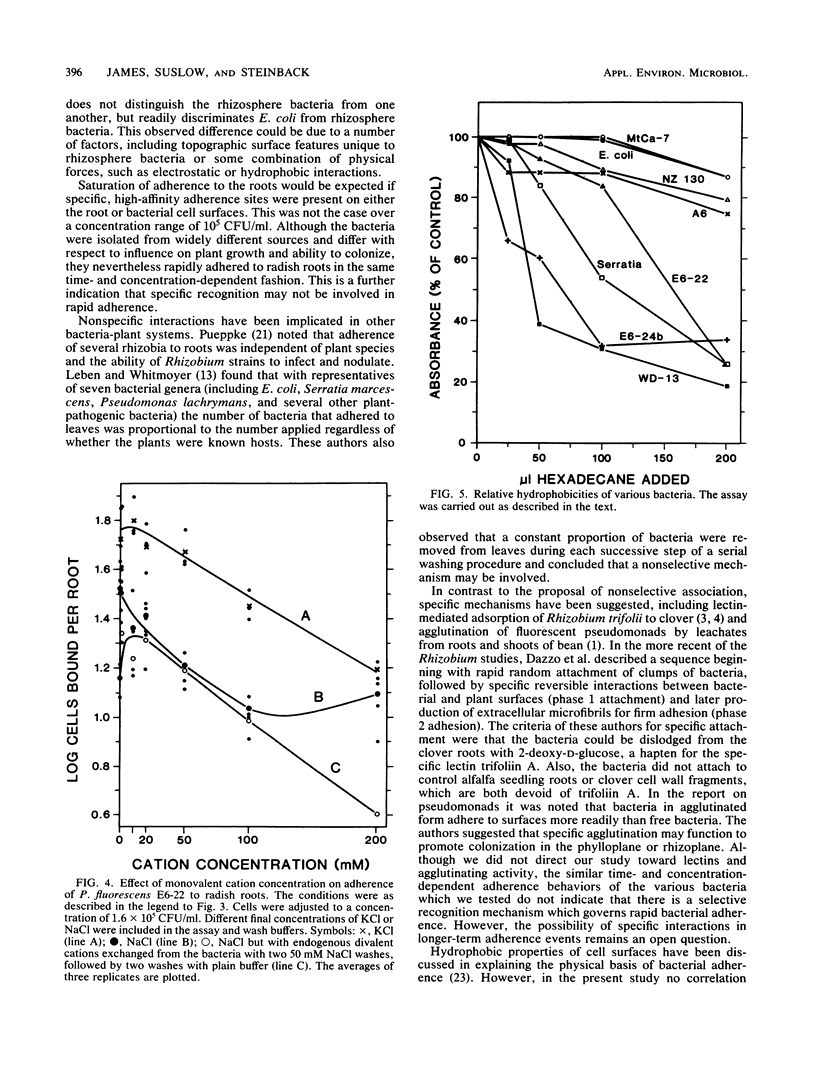
For rhizobacteria to exert physiological effects on plant growth, the bacteria must first effectively colonize the root surface. To examine the relationship between long-term colonization of root systems and adherence to roots in the short term, a binding assay was developed. Adherence was determined by incubating roots of intact radish seedlings with bacteria, washing and homogenizing the roots, and dilution plating the resulting homogenate. Irreversible binding of bacteria was rapid, reaching half-maximum by 5 min. All of the rhizosphere bacteria tested showed similar, concentration-dependent binding (ranging from 104 to 108 CFU/ml), as well as long-term colonization of radish roots under sterile conditions. Escherichia coli, which is not a root colonizer, showed about 10-fold less binding, but still demonstrated concentration-dependent binding and rapid kinetics of adherence at high concentrations (106 to 108 CFU/ml). The bacteria tested were very different with respect to source or habitat and plant response, yet they showed similar concentration-dependent binding. There was no correlation between the relative hydrophobicities of the cell surfaces of strains and the adherence of the strains to roots. Binding of Pseudomonas fluorescens E6-22 was promoted by divalent cations (Ca2+ and Mg2+) at concentrations of 5 to 10 mM, whereas monovalent cations (Na+ and K+) had little effect; electrostatic phenomena may partially explain adherence in the short term, an important prelude to long-term colonization of root surfaces.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dazzo F. B., Napoli C. A., Hubbell D. H. Adsorption of bacteria to roots as related to host specificity in the Rhizobium-clover symbiosis. Appl Environ Microbiol. 1976 Jul;32(1):166–171. doi: 10.1128/aem.32.1.166-171.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzo F. B., Truchet G. L., Sherwood J. E., Hrabak E. M., Abe M., Pankratz S. H. Specific phases of root hair attachment in the Rhizobium trifolii-clover symbiosis. Appl Environ Microbiol. 1984 Dec;48(6):1140–1150. doi: 10.1128/aem.48.6.1140-1150.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher M., Loeb G. I. Influence of substratum characteristics on the attachment of a marine pseudomonad to solid surfaces. Appl Environ Microbiol. 1979 Jan;37(1):67–72. doi: 10.1128/aem.37.1.67-72.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley C. L., Robbins C. M., Richmond M. H. Quantitative assessment of bacterial adhesion to eukaryotic cells of human origin. J Appl Bacteriol. 1978 Aug;45(1):91–97. doi: 10.1111/j.1365-2672.1978.tb04202.x. [DOI] [PubMed] [Google Scholar]
- KING E. O., WARD M. K., RANEY D. E. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med. 1954 Aug;44(2):301–307. [PubMed] [Google Scholar]
- Korhonen T. K., Tarkka E., Ranta H., Haahtela K. Type 3 fimbriae of Klebsiella sp.: molecular characterization and role in bacterial adhesion to plant roots. J Bacteriol. 1983 Aug;155(2):860–865. doi: 10.1128/jb.155.2.860-865.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leben C., Whitmoyer R. E. Adherence of bacteria to leaves. Can J Microbiol. 1979 Aug;25(8):896–901. doi: 10.1139/m79-133. [DOI] [PubMed] [Google Scholar]
- Lindow S. E., Arny D. C., Upper C. D. Distribution of ice nucleation-active bacteria on plants in nature. Appl Environ Microbiol. 1978 Dec;36(6):831–838. doi: 10.1128/aem.36.6.831-838.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthysse A. G., Wyman P. M., Holmes K. V. Plasmid-dependent attachment of Agrobacterium tumefaciens to plant tissue culture cells. Infect Immun. 1978 Nov;22(2):516–522. doi: 10.1128/iai.22.2.516-522.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pueppke S. G. Adsorption of slow- and fast-growing rhizobia to soybean and cowpea roots. Plant Physiol. 1984 Aug;75(4):924–928. doi: 10.1104/pp.75.4.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroth M. N., Hancock J. G. Disease-suppressive soil and root-colonizing bacteria. Science. 1982 Jun 25;216(4553):1376–1381. doi: 10.1126/science.216.4553.1376. [DOI] [PubMed] [Google Scholar]
- Stanley P. M. Factors affecting the irreversible attachment of Pseudomonas aeruginosa to stainless steel. Can J Microbiol. 1983 Nov;29(11):1493–1499. doi: 10.1139/m83-230. [DOI] [PubMed] [Google Scholar]
- Warren G. J., Clark A. J. Sequence-specific recombination of plasmid ColE1. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6724–6728. doi: 10.1073/pnas.77.11.6724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller D. M. Distribution of a Take-All Suppressive Strain of Pseudomonas fluorescens on Seminal Roots of Winter Wheat. Appl Environ Microbiol. 1984 Oct;48(4):897–899. doi: 10.1128/aem.48.4.897-899.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D. H., Kauss H. Adhesion of Colletotrichum lindemuthianum Spores to Phaseolus vulgaris Hypocotyls and to Polystyrene. Appl Environ Microbiol. 1984 Apr;47(4):616–619. doi: 10.1128/aem.47.4.616-619.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]


