Abstract
Objective
To report on a 30-year prospective study of deep infection in 1993 consecutive total hip arthroplasties performed by a single surgeon.
Methods
The relations of numerous variables to the incidence of deep infection were studied.
Results
The cumulative infection rate after the index total hip arthroplasties rose from 0.8% at 2 years to 1.4% at 20 years; 9.6% of the index operations required further surgery. When infections attributed to these secondary procedures were included, the infection rate rose from 0.9% at 2 years to 2% at 20 years. Although the usual variables increased the incidence of infection, the significant and most precise predictors of infection were radiologic diagnoses of upper pole grade III and protrusio acetabuli, an elevated erythrocyte sedimentation rate, alcoholism and units of blood transfused.
Conclusion
From 2–20 years, the incidence of deep infection doubled. Preoperative recognition of the first 4 risk factors permits the use of additional prophylactic measures. Spinal or epidural anesthesia reduced the units of blood transfused (the fifth risk factor) and, hence, the risk of infection. Although most deep infections are seeded while the wound is open, there are many possible postoperative causes. In this study, fewer than one-third of the infections that presented after 2 years were related to hematogenous spread. The efficacy of clean air technology was supported, and it is recommended that all measures that may reduce the incidence of deep infection be employed.
Abstract
Objectif
Faire état des résultats d'une étude prospective de 30 ans sur l'infection profonde dans 1993 arthroplasties totales de la hanche consécutives pratiquées par un même chirurgien.
Méthodes
On a étudié les liens entre de nombreuses variables et l'incidence de l'infection profonde.
Résultats
Le taux cumulatif d'infection après les arthroplasties totales de la hanche de référence est passé de 0,8 % à 2 ans à 1,4 % à 20 ans, et 9,6 % des patients de référence ont dû subir une autre intervention chirurgicale. Lorsqu'on a inclus les infections attribuées à ces interventions secondaires, le taux d'infection est passé de 0,9 % à 2 ans à 2 % à 20 ans. Même si les variables habituelles ont augmenté l'incidence de l'infection, les prédicteurs importants et les plus précis de l'infection étaient les diagnostics radiologiques d'extrémité supérieure de grade III et de protrusion acétabulaire, un taux élevé de sédimentation des érythrocytes, l'alcoolisme et le nombre d'unités de sang transfusées.
Conclusion
De 2 à 20 ans, l'incidence d'infection profonde a doublé. La reconnaissance préopératoire des quatre premiers facteurs de risque permet de recourir à des mesures prophylactiques supplémentaires. L'anesthésie rachidienne ou péridurale réduit le nombre d'unités de sang transfusées (le cinquième facteur de risque) et, par conséquent, le risque d'infection. Même si la plupart des infections profondes s'installent pendant que la plaie est ouverte, il y a de nombreuses causes postopératoires possibles. Au cours de cette étude, moins du tiers des infections survenues après deux jours étaient reliées à la transmission hématogène. On a appuyé l'efficacité de la technologie de l'air propre et on recommande de recourir à toutes les mesures susceptibles de réduire l'incidence d'infections profondes.
This article reports on a prospective study of deep infections after total hip arthroplasty (THA) performed in a community hospital. One surgeon (H.H.) used 1 implant and and 1 technique for 27.5 years. The operations were performed in a hospital in northwestern Ontario that serves a population of 250 000 in an area the size of France.
In 1972, when this study started, Charnley and Eftekhar1 had published their experiences with a clean air unit at Wrightington Centre for Hip Surgery, and there was intense interest in this new technology. A clean air enclosure, the second to be installed in Canada, was used in this study, and it seemed important to document its effectiveness. To do this, numerous variables were measured and recorded in an attempt to determine their influence on the incidence of deep infection.
Methods
From October 1972 to April 2000, 2003 low-friction arthroplasties were performed, following the procedure as taught by Sir John Charnley.2
These index THAs were identified by sequential numbers. Patients who had bilateral THAs had 2 numbers. When a further operation was required, the original number was retained. Data were entered prospectively into a custom-made computer program.3 This was supplemented by a chart review to complete missing entries and to correct errors. The first 10 operations were carried out in a conventional operating room; the subsequent 1993 cases were performed in a Charnley-Howorth clean air enclosure with body exhaust gowns.4,5 This study concerns the 1993 operations in which clean air technology was used (hip nos. 11–2003).
Systemic prophylactic antibiotics were used (usually cephamandol 2 g given intravenously with induction of anesthesia, followed by 1 g in the recovery room). They were administered once in the first 190 cases, randomly in the next 600 cases and almost universally in the last 1203 cases. Antibiotic-loaded cement (tobramycin and vancomycin) was used in the second half of the series (hip nos. 1001–2003) in cases that had undergone previous ipsilateral hip surgery. Ultraviolet (UV) radiation was not used in the first 490 cases. It was used randomly in the next 460 cases and routinely in the remaining 1043 cases.
Deep infections were defined as infections that involved the implant. Infections that remained superficial to the deep fascia were excluded. The indicators of contamination were as follows: growth from 2 swabs taken from the wound just before closure and placed in a nutrient medium, growth on 2 blood agar settle plates placed on the floor beside the operating room table while the wound was open, and growth from the contents of 2 hemovacs, removed at 48 hours. In cases where there had been previous surgery, early cultures were obtained to determine whether there was evidence of an occult infection. Contamination was defined as the presence of any organism.
Because of the long follow-up period, infections were divided into those attributed to the index operation and those attributed to subsequent operations.
We examined the relation of numerous variables to the incidence of deep infection, grouped as follows. First, we considered strategies that might destroy bacteria, for example, the use of prophylactic antibiotics and UV radiation. A second group of variables included circumstances that might increase contamination of the wound, for example, the time the incision was open, perforation of the surgeon's inner gloves (assessed by filling the glove with water, a routine used by Charnley in 1971–1972 and followed by H.H. throughout this study), a history of septic arthritis of the hip, psoriasis, a positive preoperative mid-stream urine sample (MSU), perioperative blood transfusions6 and type of anesthetic (i.e., general, spinal or epidural).7–9 A third group of variables included factors that might compromise the body's defence mechanisms, for example, age, sex, obesity (BMI ≥ 30), white blood cell count, erythrocyte sedimentation rate (ESR), history of hematological malignancies or solid tumours with metastases or pathological fractures, radiotherapy or chemotherapy, primary or secondary immunodeficiencies, use of immunosuppressive drugs or systemic steroids, clinical diagnosis of rheumatoid arthritis, diabetes mellitus or alcoholism, pathological diagnosis as defined by Mitchell and Cruess10 and radiologic diagnosis as defined by Charnley.11,12
An attempt was made to reassess every patient at 6 months, at 1 year and then annually. The assessments were carried out by the surgeon (H.H.) and included an examination and an anteroposterior radiograph of both hips.
Data were analyzed with SPSS 12.0 (SPSS Inc., Chicago, Ill.). All variables were examined individually for their relation to deep infection. For relations that were scores, independent t tests were used to compare means for those with and without a deep infection. For variables that were categories, χ2 tests were used to look for the relation of the category to the presence or absence of deep infection. A liberal significance level of 0.1 with no correction for type 1 error was used to permit identification of the subset of variables with possible relations to deep infection. These variables were then examined according to Cox regession (survival analysis), a more conservative statistical approach, to identify independent (unique contribution) predictors of deep infection.
Results
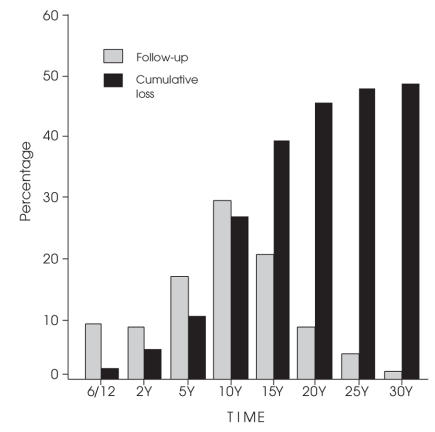
Figure 1 divides cases into those successfully followed and those lost to death at intervals from 6 months to 30 years; 16 cases were never reviewed after discharge from hospital. By the summer of 2006, 973 THAs were lost to death, 797 were known to be alive, and 223 had been lost for 18 months or longer (some of these, if alive, would be more than 100 years old).
FIG. 1. Total hip arthroplasties followed, and cumulative losses to death.
Of the index THAs, 318 (16.0%) had undergone previous hip surgery. These cases had a lower incidence of infection (3 cases, 0.9%) than the 1675 that had not undergone prior operations (26 cases, 1.6%).
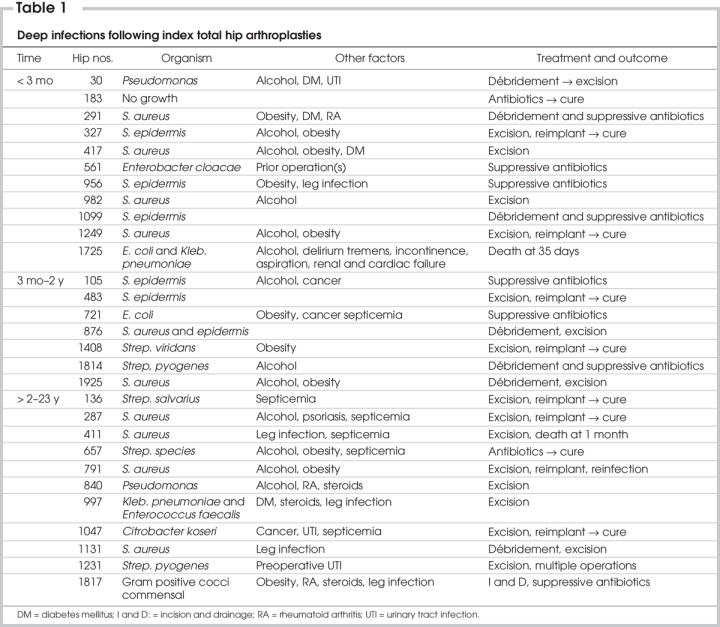
Of the index THAs, 192 (9.6%) required 1 or more subsequent operations; 22 (11.5%) of these subsequent operations were for infections. The remaining 170 (88.5%) operations were for problems other than sepsis, for example, aseptic loosening, dislocation and trochanter reattachment. The 29 (1.5%) cases that developed an infection after the index THA are summarized in Table 1. The predominant infecting microorganisms were gram-positive Staphylococcus aureus (31%) and Staphylococcus epidermidis (21%), and there was no obvious difference between early (< 2 y) and late (2–23 y) cases.
Table 1
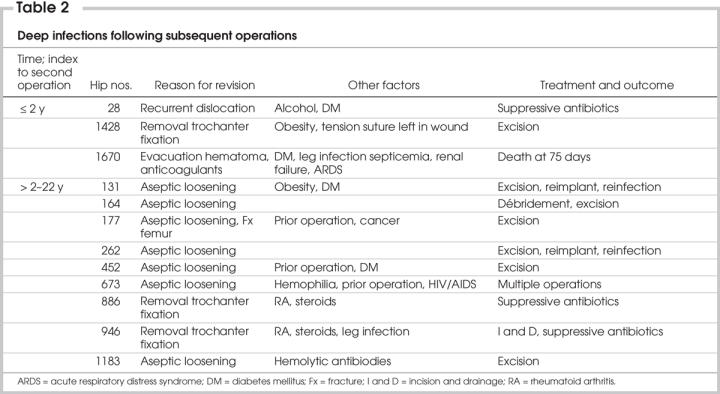
Of the 170 subsequent operations for problems other than sepsis, 12 (7.1%) developed an infection (summarized in Table 2).
Table 2
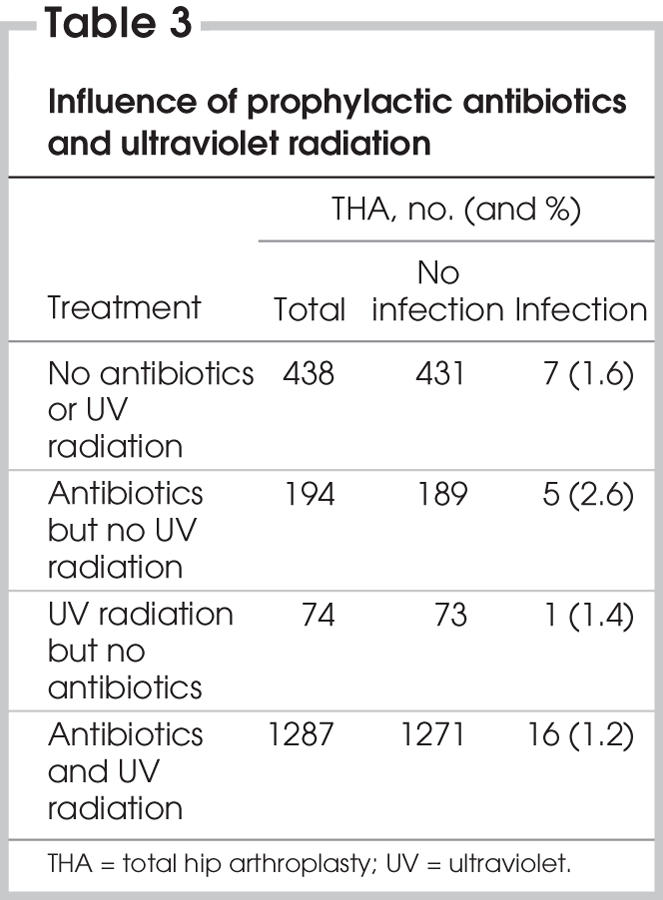
The combined use of prophylactic antibiotics and UV radiation in a clean air enclosure with body exhaust gowns was associated with the lowest infection rate. The use of prophylactic antibiotics or UV radiation, or both, had no statistically significant influence on the incidence of infection (Table 3).
Table 3
Positive cultures obtained from settle plates (15.1%), closing swabs (8.8%) or hemovac contents (4.8%) were associated with an increased infection rate, but this was not statistically significant.
The mean time that the incision was open — 114.9 minutes in cases that developed an infection — was not significantly longer than the mean of 111.2 minutes in those that did not develop an infection.
Perforation of the surgeon's inner glove was detected in 16.5% of cases. These perforations did not increase the infection rate. When an outer glove perforation was recognized during an operation, the glove was replaced. When the remaining unused gloves were tested, 4% were found to be perforated.
A history of septic arthritis of the hip (1% of cases), psoriasis (2.3%) or a positive MSU (14.1%) increased in the incidence of infection, but this was not statistically significant.
Blood transfusions may be contaminated by microorganisms, which may affect the immune system.6 The use of perioperative blood transfusions was associated with an increase in the incidence of infections. On average, the group with infections received 2.76 units of blood, whereas the group without infections received 1.71 units. The difference was significant at p = 0.005. The type of anesthesia may affect blood loss and the need for transfusion. The first spinal or epidural anesthetic was used in hip number 693. In the first 682 cases, 73.3% of the patients received blood, and in the subsequent 1311 cases, 66.4% patients received blood. The decrease in the use of blood transfusions was significant (p < 0.001). This decrease in the use of blood transfusions could have been due to a change in transfusion policy. To avoid this problem, a comparison was made between the 1311 cases (nos. 693–2003) occurring during the same time period when any change in transfusion policy would affect both groups. Patients having a spinal or epidural anesthetic were significantly less likely to receive a blood transfusion (250/459, 54.5%) than those having a general anesthetic (606/827, 73.3%), p = < 0.001. The average number of units transfused in spinal or epidural cases (1.23) was significantly less than in general anesthetic cases (2.06), p = < 0.001.
Age, sex, obesity (BMI ≥ 30) or an elevated white blood cell count were not significantly related to the incidence of infection. The mean ESR in cases that developed an infection (32.6) was significantly higher than that in cases with no infection (23.6), p = 0.03.
The incidence of infection according to the month of the year did not indicate that there were significant seasonal variations in the patients' immune systems.
The type of anesthetic used (general, 76.0%; spinal or epidural, 23.1%; both, 0.9%) did not influence the infection rate.
Hematological malignancies or solid tumours with metastases or pathological fractures (3.2% of cases), radiotherapy or chemotherapy or both (4.7% of cases), primary or secondary immunodeficiencies (0.4% of cases), immunosuppressive drugs (4.8% of cases) or systemic steroids (8.3% of cases) may adversely affect defence mechanisms, but the number of cases was insufficient to show a statistically significant difference.
Rheumatoid arthritis (7.6% of cases) and diabetes mellitus (11.5% of cases) were associated with an increased incidence of infection, but this was not statistically significant. Alcoholism (20.8% of cases) was associated with an infection rate of 3.4%, compared with 1.0% in those who did not suffer from alcoholism, with the difference being significant at p < 0.001.
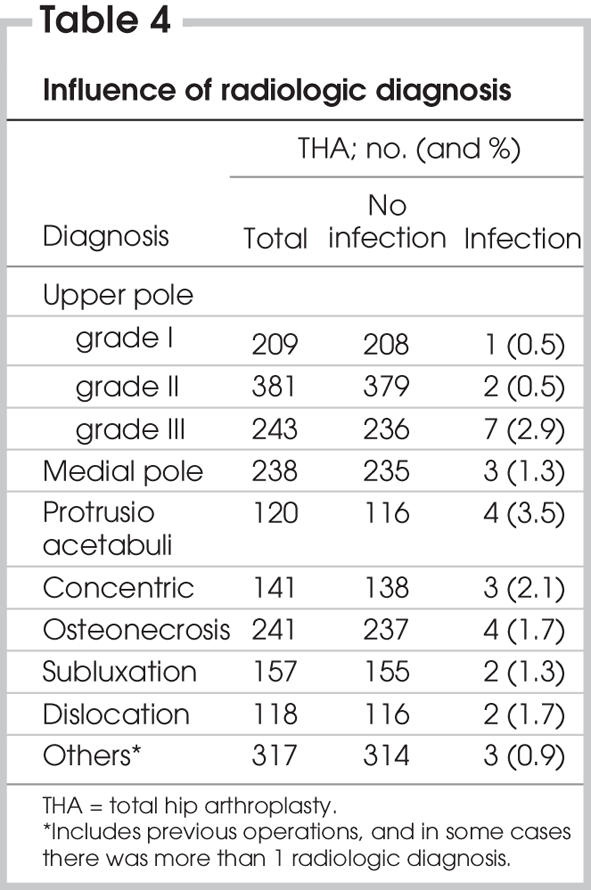
Pathological diagnoses10,11 such as generalized osteoarthritis with Heberden's nodes, idiopathic monoarticular osteoarthrosis and osteonecrosis showed no increased incidence of infection. Radiologic diagnoses12 — upper pole grade III, protrusio acetabuli and concentric — were associated with a higher incidence of infection. The difference was significant for upper pole grade III (p = 0.05) and approached significance for protrusio acetabuli (p = 0.059) (Table 4). Among the 1993 index THAs, rheumatoid arthritis was diagnosed in 19.1% with protrusio acetabuli and in 45.4% with concentric arthritis.
Table 4
Risk prediction
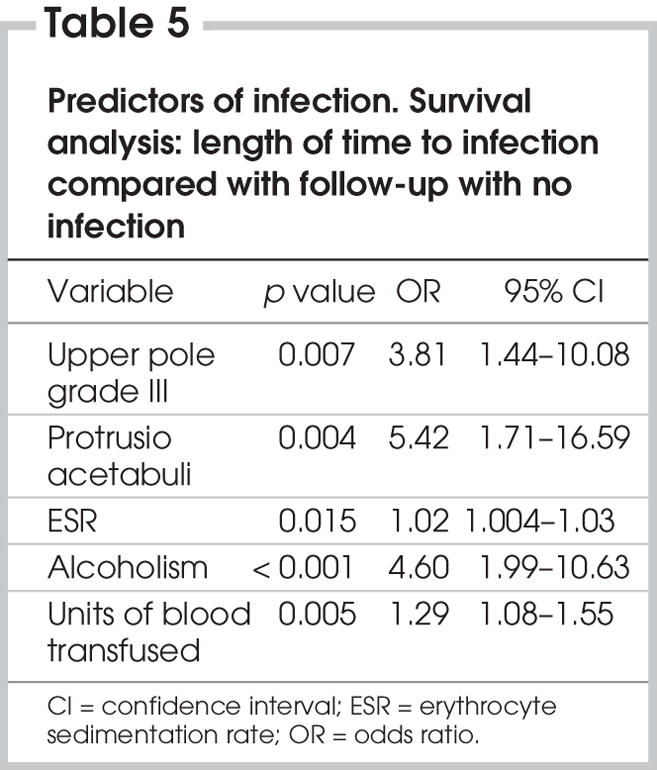
To provide an overall picture of the predictors of infection, a survival analysis (Cox regression) of either the length of time to infection or follow-up with no infection was conducted on the 5 variables (radiologic diagnosis upper pole grade III, radiologic diagnosis protrusio acetabuli, elevated ESR, alcoholism and units of blood transfused) that were either significant (p < 0.05) or approaching significance (p = 0.10). This analysis was based on only 1831 cases (24 with infection) because of missing values for some of the variables. Incorporation of the time dimension gave more precise findings than logistic regression. The overall prediction of infection from these 5 variables was significant at p < 0.001 (Table 5).
Table 5
Time
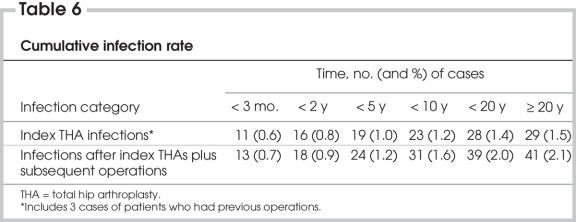
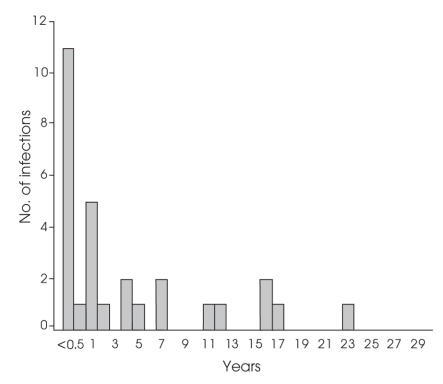
The cumulative infection rate after the index operations increased from 0.8% at 2 years to 1.4% at 20 years (Table 6); 9.6% of the index operations required further procedures. When infections attributed to these secondary operations were included, the infection rate rose from 0.9% at 2 years to 2.0% at 20 years. The incidence of the onset of deep infections declined with time (Fig. 2), but the numbers were not sufficient to show that this was statistically significant.
Table 6
FIG. 2. Time of onset of total hip arthroplasty deep infections.
Discussion
In this study, the incidence of infection declined with time (Table 6, Fig. 2), but infections can present after 10 or even 20 years. The cumulative infection rate after the index THAs rose from 0.8% at 2 years to 1.4% at 20 years. When infections attributed to secondary procedures were included, the infection rate rose from 0.9% at 2 years to 2.0% at 20 years. Thus, from 2 to 20 years, the incidence of deep infections doubled.
A review of Table 1 suggests that 12 of the 29 deep infections (41.4%) could have been due to postoperative factors that included hematogenous infection (nos. 136, 287, 411, 657 and 721), leg infection, that is, ulceration or cellulitis (nos. 956, 997, 1131, 1231 and 1817), urinary tract infection (no. 1047) and delirium tremens with fecal and urine incontinence (no. 1725). Three of these infections occurred early (< 2 y), and the rest occurred 4–23 years after surgery. It is sometimes implied that implant infections occurring after 2 years are hematogenous in origin.13 However, reports of hematogenous infections in which an identical organism was isolated from the implant and the septic site account for a minority of cases. Of the deep infections documented in this study (Table 1), 5 (17.2%) could have been hematogenous; 4 occurred after 2 years, representing a maximum of 30.8% late-onset infections with hematogenous origin.
Nelson's meta-analysis14 indicated that a 5.8% infection rate in a regular operating room was reduced to 1.3% with prophylactic antibiotics, to 0.7% with clean air technology and to 1% with UV radiation. These intraoperative measures all significantly reduced the infection rate by at least 80%, which suggests that intraoperative contamination is much more frequent than later hematogenous contamination. Anecdotal as well as theoretical evidence supports the view that bacterial colonies protected in a biofilm15–18 can survive indefinitely before causing an overt infection. In 1916, a First World War veteran suffered an open fracture of his tibia that became infected but healed. Fifty years later, he was admitted to the Edinburgh Western General Hospital with a respiratory tract infection. H.H. attended him during this admission because, after lying dormant for 50 years, his leg wound broke down and discharged Staphylococcus aureus, a different organism from that isolated from his respiratory tract. Vreeland and colleagues19 reported a microorganism found in a New Mexico salt crystal that was 250 million years old. It was isolated, grown, and found to be Bacillus species 2-9-3. Convincing evidence that most implant infections occurring after 2 years are due to hematogenous seeding is lacking.
The strengths of this study are as follows: its length, that there was 1 surgeon, that 1 type of operation was performed electively, that a fixed protocol was used, that all operations were performed at 1 site and that geographical constraints minimized losses to follow-up. It was made clear from the outset that all patients were to be followed indefinitely. This feature distinguishes the present study from studies that make an attempt to recall all patients after 10 or 20 years. Variables that may reduce the risk of infection — prophylactic antibiotics and UV radiation — were deliberately introduced and studied. Multiple uncontrollable variables, found in any consecutive series, were studied. The study's weaknesses are that other existing variables could have been included and were not and that the number of operations complicated by an infection was not large enough to detect factors that might have had a small effect on the infection rate.
We were unable to show, as did Nelson,14 that the increased infection rate in contaminated cases was statistically significant. This might have been because the number of cases was insufficient.
Prophylactic antibiotics were initially not used because of doubts as to their value20,21 and because of an attempt to demonstrate the value of clean air technology without introducing another variable. The combined use of a clean air enclosure and body exhaust gowns, prophylactic antibiotics and UV radiation was associated with the lowest incidence of infection, but the addition of prophylactic antibiotics and UV radiation, or both, made no statistically significant difference. A larger number of cases might have proved otherwise. It is of interest that there were 7 deep infections (1.6%) in 438 of the index THAs in which no antibiotics or ultraviolet radiation was used, a low incidence for a study of this duration.
In the setting of a clean air enclosure with body exhaust gowns, the length of time the wound was open did not significantly influence the incidence of infection. In a conventional operating room, where the air is always contaminated, time is likely to be an important factor.
With the exceptions of alcoholism, an elevated ESR and, possibly, blood transfusions, we had difficulty in showing that a compromised immune status contributed to the incidence of infection. In 3 patients (hip nos. 28 and 30, 876 and 1131, 1231 and 1183), there were bilateral deep infections. The organisms isolated from the left and right hips were more often than not different. One of the 3 patients suffered from alcoholism and had diabetes mellitus. These cases may be examples of a compromised immune system.
Most of the variables usually associated with an increased risk of deep infection did show an increased incidence of deep infection, but it was not statistically significant. We attribute this to the fact that, even with 1993 cases, the infection rate was too low to have sufficient statistical power to detect effects of small magnitude.
The most precise predictors of infection were obtained by incorporating time as a survival analysis (Cox regression) expressed as the increased risk of infection for 5 variables (Table 5). The first 4 predictors — radiologic diagnosis of upper pole grade III, radiologic diagnosis of protrusio acetabuli, elevated ESR and alcoholism — should be known before surgery and merit extra precautions. The fifth predictor, the number of blood units transfused, is mitigated by use of spinal or epidural anesthesia, which reduces the need for blood.
Thirty-seven years after Charnley and Eftekhar's publication, most joint implant surgery is performed in operating rooms that are less sterile than the Wrightington prototype. Whyte and colleagues (1983)22 suggested bacterial standards for ultraclean operating rooms. The published national standards are not particularly helpful.23–27 This study supports the effectiveness of clean air technology, and at a time when we are concerned about the emergence of resistant strains of microorganisms, it would seem prudent to employ all measures likely to reduce the risk of infection.
Acknowledgments
The authors thank Ms. Sara Hamilton for her work on chart reviews.
Contributors: Dr. Hamilton designed the study and acquired the data, which Drs. Hamilton and Jamieson analyzed. Drs. Hamilton and Jamieson wrote and reviewed the article and gave final approval for its publication.
Competing interests: None declared.
Accepted for publication Oct. 2, 2006
Correspondence to: Dr. H. Hamilton, Port Arthur Health Centre, 194 North Court St., Thunder Bay ON P7A 4V7; drhenryhamilton@hotmail.com
References
- 1.Charnley J, Eftekhar N. Postoperative infection in total prosthetic replacement arthroplasty of the hip joint. Br J Surg 1969;56:641-9. [DOI] [PubMed]
- 2.Charnley J. Low friction arthroplasty of the hip: theory and practice. Berlin: Springer-Verlag; 1979. p. 185-289.
- 3.Hamilton HW, Lai A. A computer program suitable for routine hip replacement follow-up and clinical research. Orthop Rev 1988;17:116-20. [PubMed]
- 4.Charnley J. Low friction arthroplasty of the hip: theory and practice. Berlin: Springer-Verlag 1979. p. 152-183.
- 5.Howorth H. The evolution and development of clean air system for surgery. Health Estate J 1993;47:14-5, 18,20 passim. [PubMed]
- 6.Tartter PI. Immune consequences of blood transfusion in the surgical patient. Surg Immun 1989;2:13-34.
- 7.Moudgil GC, Wade AG. Anaesthesia and immunocompetence. Br J Anaesth 1976;48:31-9. [DOI] [PubMed]
- 8.Nakagawara M, Takeshige K, Takamatsu J, et al. Inhibition of superoxide production and Ca2+ mobilization in human neutrophils by halothane, enfurane, and isofurane. Anesthesiology 1986;64:4-12. [DOI] [PubMed]
- 9.Liu S, Carpenter RL, Neal JM. Epidural anesthesia and analgesia. Anesthesiology 1995;82:1474-96. [DOI] [PubMed]
- 10.Mitchell NS, Cruess RL. Classification of degenerative arthritis. Can Med Assoc J 1977;117:763-5. [PMC free article] [PubMed]
- 11.Hamilton HW, Joyce M. Long term results of low friction arthroplasty performed in a community hospital, including a radiological review. Clin Orthop Relat Res 1986;211:55-64. [PubMed]
- 12.Wroblewski BM, Charnley J. Radiographic morphology of the osteoarthritic hip. J Bone Joint Surg Br 1982;64:568-9. [DOI] [PubMed]
- 13.Fitzgerald RH. Infected total hip arthroplasty: diagnosis and treatment. J Am Acad Orthop Surg 1995;3:249-62. [DOI] [PubMed]
- 14.Nelson JP. The operating room environment and its influence on deep wound infection. In: Murray WR, editor. The Hip. Proceedings of the Fifth Open Scientific Meeting of the Hip Society; St. Louis (MO): CV Mosby; 1977. p. 129-46.
- 15.Costerton JW, Geesey GG, Cheng J-K. How bacteria stick. Sci Am 1978;238:86-93. [DOI] [PubMed]
- 16.Gristina AG. Biomaterial-centered infection: microbial adhesion versus tissue integration. Science 1987;237:1588-95. [DOI] [PubMed]
- 17.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science 1999;284:1318-22. [DOI] [PubMed]
- 18.Trampuz A, Osmon DR, Hanssen AD, et al. Molecular and anti-biofilm approaches to prosthetic joint infection. Clin Orthop Relat Res 2003;414:69-88. [DOI] [PubMed]
- 19.Vreeland RH, Rozenzweig WD, Powers DW. Isolation of a 250 million-year-old halotolerant bacterium from a primary salt crystal [letter]. Nature 2000;407:897-900. [DOI] [PubMed]
- 20.Tachdjian MO, Compere EL. Postoperative wound infections in orthopaedic surgery. J Int Coll Surg 1957;6:797-805. [PubMed]
- 21.Aseptic methods in the operating suite. 3. Preparation of the patient and performance of the operation. Lancet 1968;1:831-9. [PubMed]
- 22.Whyte W, Lidwell OM, Lowbury EJL, et al. Suggested bacteriological standards for air in ultraclean operating rooms. J Hosp Infect 1983;4:133-9. [DOI] [PubMed]
- 23.Ultra-clean ventilation systems. In: Ventilation in healthcare premises. Scottish Health Technical Memorandum 2025 Section 5.8 (2001). Available: www.hfs.scot.nhs.uk/guest/decontamination/CDVer2/SHTM%202025/2025%20Part%203%20Ver2.pdf (accessed 2008 Mar 7).
- 24.Institute of Environmental Sciences. Federal Standard 209E. Airborne particulate cleanliness classes in cleanrooms and clean zones. Mount Prospect (IL): the Institute; 1992.
- 25.Mangram AJ, Horan TC, Pearson ML, et al. Guidelines for prevention of surgical site infection, 1999. Infect Control Hosp Epidemiol 1999;20:247-78. [DOI] [PubMed]
- 26.Sehulster L, Chinn RYW. Infection control and ventilation requirements for operating rooms. In: Guidelines for Environmental Infection Control Health-Care Facilities. Recommendations of CDC and the Healthcare Infection Control Practices Advisor Committee (HICPAC). MMWR Morb Mortal Wkly Rep 2003;52(RR-10):15. [PubMed]
- 27.CDC issues new environmental guidelines. OR Manager 2003;19:20-2. [PubMed]