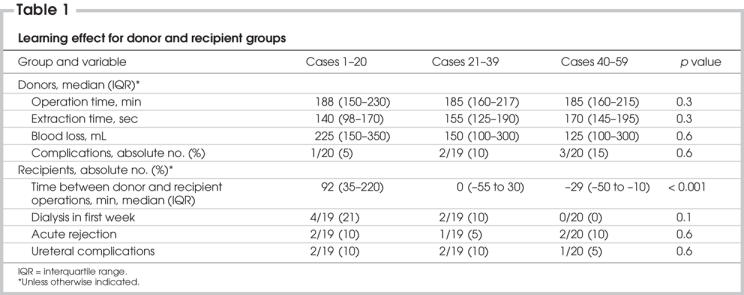
Abstract
Objective
During the learning curve for laparoscopic live donor nephrectomy (LLDN), donor morbidity and poorer graft function may be increased. To minimize these risks, a dedicated team of laparoscopic, urologic and transplant specialists worked together to introduce the technique. This study was undertaken to validate this approach by comparing donor and recipient outcomes and studying our learning curve during the transition from open (OLDN) to LLDN.
Methods
We compared 59 LLDNs with 34 OLDNs performed for adult recipients. Data were collected prospectively for LLDN and retrospectively for OLDN. We compared donor outcomes and recipient graft function in the 2 groups, and we used the cumulative sum (CUSUM) method to generate learning curves; p < 0.05 was considered statistically significant.
Results
From the donor standpoint, the complication rate was 10% in the laparoscopic group, compared with 21% in the open group. Length of stay was shorter after LLDN (3 v. 5 d, p < 0.001). Among the recipients, there were no significant differences in the incidences of ureteral complications, delayed graft function (DGF), creatinine levels, acute rejection or patient and graft survival. When we used the incidence of DGF after OLDN as a benchmark, CUSUM analysis revealed a downward inflection point for DGF after 30 cases, consistent with an improvement in performance.
Conclusion
At our institution, a team approach has allowed the safe introduction of LLDN without a significant negative impact on recipient outcomes and with a reduction in donor length of stay. Using DGF as an outcome, we observed improved performance after 30 cases.
Abstract
Objectif
Au cours de la période d'apprentissage de la néphrectomie par laparoscopie pratiquée sur donneur vivant (NLDV), la morbidité chez le donneur et la prise moins bonne du greffon peuvent augmenter. Afin de minimiser ces risques, des spécialistes en laparoscopie, urologie et transplantation ont conjugué leur efforts en équipe pour implanter la technique. L'étude visait à valider cette approche en comparant les résultats pour les donneurs et les receveurs, et en étudiant notre courbe d'apprentissage pendant la transition de l'intervention ouverte (NODV) à l'intervention NLDV.
Méthodes
Nous avons comparé 59 NLDV et 34 NODV pratiquées pour des receveurs adultes. Nous avons recueilli les données prospectivement pour NLDV et rétrospectivement pout NODV. Nous avons comparé les résultats pour les donneurs et le fonctionnement du greffon chez les receveurs dans les deux groupes et nous avons utilisé la méthode de la somme cumulative (SUMCU) pour générer les courbes d'apprentissage. On a considéré que p < 0,05 était statistiquement significatif.
Résultats
Du point de vue des donneurs, le taux de complications s'est établi à 10 % chez ceux qui ont subi la laparoscopie comparativement à 21 % chez ceux qui ont subi l'intervention ouverte. La durée du séjour était plus courte après une NLDV (3 c. 5 j, p < 0,001). Chez les receveurs, il n'y avait pas de différences significatives au niveau des incidences des complications urétérales, du fonctionnement retardé du greffon (FRG), des taux de créatinine, du rejet aigu ou de la survie du patient et du greffon. Lorsque nous avons utilisé l'incidence du FRG après une NODV comme point de comparaison, l'analyse SUMCU a révélé un point d'inflection à la baisse dans le cas du FRG après 30 cas, ce qui concorde avec une amélioration du rendement.
Conclusion
À notre établissement, une stratégie d'équipe a permis d'implanter sans danger la NLDV sans effet négatif important sur les résultats des receveurs tout en réduisant la durée du séjour du donneur. En utilisant le FRG comme résultat, nous avons observé une amélioration du rendement après 30 cas.
In Canada, the diagnosis of end-stage renal disease increased by almost 60% from 1994 to 2001. In 2001, 4900 new cases were diagnosed, and 75% of patients started hemodialysis, contributing to the total of 16 000 receiving dialysis in Canada that year.1 By the end of 2001, only 1058 patients had received a transplant, and only 3% of those had undergone preemptive transplantation, despite its clear benefit to graft survival.2,3 A total of 3014 patients remained on the waiting list, 2.3% of whom died while awaiting a transplant. The disparity between the demand for kidneys and the current supply is at the source of this growing problem.
To cope with the demand, there have been large increases in the rates of living kidney donation in the United States, coincident with the introduction of laparoscopic live donor nephrectomy (LLDN). First performed in 1995, LLDN has become widely accepted and now represents almost 50% of all living donor procedures in the United States.4 Canadian transplant centres have not uniformly achieved the same success in developing living kidney donation. For example, in Quebec, the proportion of transplanted kidneys procured from live donors has increased from 12% in 1995 to 22% in 2005, representing just 50 live donors in the population of 7.6 million people in that year.1
The acceptance of LLDN may be hindered by safety concerns during the “learning curve.” Although the experience of large individual centres has been very positive, the pioneers in this field have warned that “early experience with laparoscopic nephrectomy is associated with donor complications, recipient graft loss, and ureteral problems”5 and that “the laparoscopic approach to living donor nephrectomy has the potential to expose a large number of patients to the learning curve of each physician offering this technique.”6 Some of these concerns were confirmed by the United Network for Organ Sharing survey,4 which brought to light higher rates of reoperation, of postoperative complications and of readmission when compared with the open procedure. In contrast, meta-analyses of comparative studies demonstrate faster recovery after LLDN without compromising donor safety or recipient outcomes.7
During the implementation of LLDN at our institution, we adopted an approach that we hoped would allow us to minimize the morbidity associated with the learning curve and obtain results that were comparable, from the recipient standpoint, or superior, from the donor standpoint, to those obtained with the well-established open approach.8 A team that included an experienced laparoscopic general surgeon with experience in laparoscopic splenectomy and adrenalectomy, a urologist with experience in laparoscopic nondonor nephrectomy, an anesthetist dedicated to the procedure and a minimally invasive surgery nursing team was created with the support of the transplant surgeons. This team was kept constant to minimize variability. To benefit from the early experience of the pioneering institutions, an unedited video from the University of Maryland was viewed and followed by a visit to that centre. A written operative protocol was then developed and formally reviewed by all members of the team. The procedure was first practised in the animal laboratory, and then the team performed 2 laparoscopic nephrectomies for urologic indications. The first LLDN was performed at our centre in December 2000. Two attending staff surgeons scrub for each case. Laparoscopic fellows have participated actively since about the 40th procedure.
The purpose of this study was to validate the safe adoption of this approach in a Canadian centre with moderate volume, with a secondary focus on the institutional learning curve. We pursured this objective, first, by comparing donor and recipient outcomes of LLDN with those of open live donor nephrectomy (OLDN) at our institution; second, by comparing donor and recipient laparoscopic outcomes with themselves over time; and third, by comparing our laparoscopic recipient outcomes with the benchmarks published by high-volume centres.
Methods
This is a retrospective study of consecutive LLDN donor–recipient pairs done between December 2000 and December 2004 and consecutive OLDN donor–recipient pairs done between January 1998 and December 2004. Pediatric recipients (aged < 18 years) and their respective donors (n = 10) were excluded from the analysis. The laparoscopic data were collected prospectively, whereas the open data were collected retrospectively by chart review.
LLDN operative technique
All patients underwent left LLDN according to the method described by Flowers and colleagues9 The donor is positioned in the right lateral decubitus position and a 12–15 mm Hg carbon dioxide pneumoperitoneum is created by means of an open technique via a left subcostal incision. With an ultrasonic scalpel (Johnson and Johnson, New Brunswick, NJ), the left colon is mobilized medially with the spleen and tail of the pancreas. The renal vein and artery, as well as the ureter with periureteral tissue (including the gonadal vein), are dissected, and the kidney is freed of its lateral and superior attachments. Intravenous mannitol is given at the begining and end of the vascular dissection. Topical papaverine is administered to the renal artery. After ureteral division, intravenous heparin is administered, and the renal artery and vein are divided. The kidney is prebagged and extracted through an incision of 6–8 cm in the left iliac fossa. Anticoagulation is then reversed with protamine sulfate. The organ is cooled and perfused with Belzer solution.
OLDN operative technique
In the lateral decubitus position, a transverse incision is made from the tip of the 12th rib toward the umbilicus and the retroperitoneal space is entered through the external, internal, and transversus muscles. Gerota's fascia is then opened, and the ureter and its periureteral tissue are dissected down to the level of the iliac vessels. After the renal vein and artery are dissected free, the ureter is divided. Mannitol, furosemide and heparin are then administered. Topical papaverine is administered when hypoperfusion due to vasospasm is suspected. The artery and vein are then divided. The kidney is extracted, cooled and perfused with Belzer solution. Anticoagulation is then reversed with protamine sulfate.
Study parameters
Demographic and perioperative data were collected for the donors and recipients of both groups, as were data on mortality and any major complications (i.e., complications requiring pharmacologic or surgical treatment and classified as grade II or more according to the Clavien classification10). Recipient-specific parameters that were recorded included the time between surgeries (time between the end of the LLDN and the start of the transplant operation) and the warm ischemia time. Postoperative serum creatinine levels were collected daily during admission and at 1, 3, 6 and 12 months. Primary outcomes were as follows: delayed graft function (defined as the need for dialysis within the first postoperative week), ureteral complications (leak or stricture), acute biopsy-proven graft rejection and graft loss (defined as a permanent return to dialysis or retransplant).
Statistical analysis
Data are expressed as medians (and interquartile range [IQR]) and were analyzed with the Mann–Whitney U test for continuous data and Yates'corrected χ2 test for categorical data, unless otherwise specified. The log-rank test was used to compare the open and laparoscopic groups with respect to cumulative survival to first rejection episode, cumulative graft survival and cumulative patient survival. Patients who died with grafts that were free of rejection or still functioning were censored at the time of their death. For the multiple groups' analysis outlined in Table 1, the Kruskall–Wallis analysis of variance was used for continuous data and the χ2 test for categorical data. Analysis was performed with GB-Stat 6.5.4 (Dynamic Microsystems Inc, Silver Spring, Md.). The criterion for statistical significance was p < 0.05.
Table 1
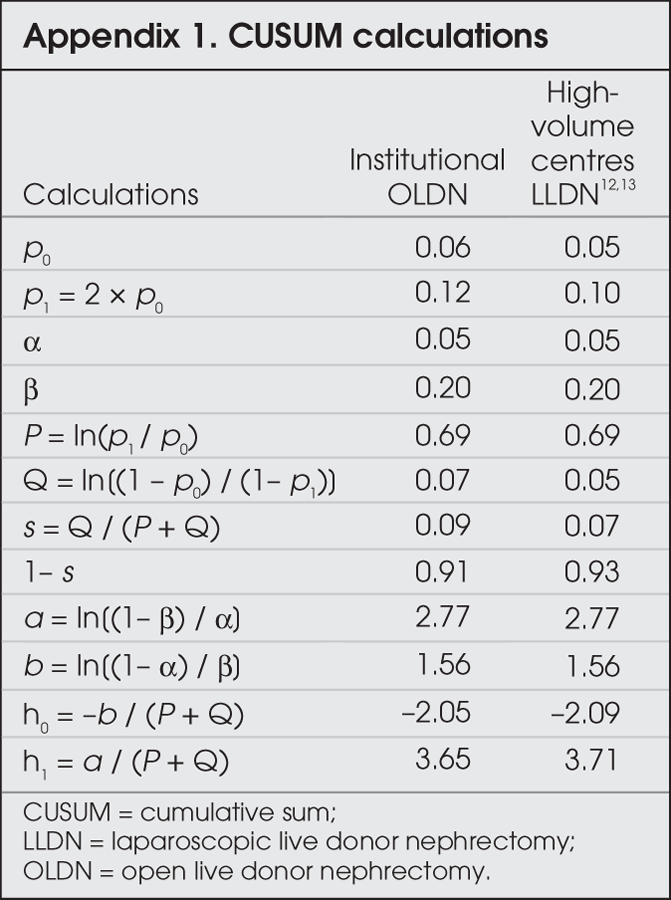
The cumulative sum (CUSUM) method was used to analyze the learning curves for LLDN, with both delayed graft function and ureteral complications used as outcomes, according to the method described by de Oliveira Filho.11 Calculations are described in Appendix 1. For each outcome, a patient with delayed graft function (DGF) or a ureteral complications (UC) was considered a “failure,” whereas a patient who did not suffer these outcomes was considered a “success.” Two sets of curves were generated. The first set was generated with our institutional OLDN data as the benchmark. The acceptable failure rate was therefore set to equal the incidence of DGF and UC reported for the open donors included in this study (6%). The second set was generated with the LLDN data from high-volume centres as the benchmarks.12,13 The acceptable failure rate was set at 5%. The probability of α and β errors was set at 5% and 20%. For each graph, 2 decision limits (h1 and h0) are calculated. For each success, the amount s is subtracted from the previous CUSUM score. For each failure, the amount 1 – s is added to the previous CUSUM score. When the curve crosses the upper decision limit (h1) from below, the actual failure rate is significantly greater than the acceptable failure rate, with a probability of type I error equal to α (i.e., the chance that an acceptable performance is erroneously labelled as unacceptable). When the curve crosses the lower decision limit (h0) from above, the actual failure rate does not differ significantly from the acceptable failure rate, with a probability of type II error equal to β (i.e., the chance that unacceptable performance is erroneously considered acceptable). When the curve is situated between h1 and h0, no statistical inference can be drawn, owing to lack of power.
Results
Between December 2000 and December 2004, 69 patients underwent LLDN. Of those, 10 were donating their kidney to a pediatric recipient, and we excluded those donor–recipient pairs from the analysis. Between January 1998 and December 2004, 34 OLDNs were performed (1 donor–recipient pair was excluded because charts were missing); of these, 9 occurred after the December 2000 introduction of LLDN at our centre. Since then, the increased number of live-donor kidneys, as well as a constant cadaveric source, have led to an increase in the total number of renal transplants performed at our centre, from a yearly average of 46 transplanted kidneys in the 3 years preceding the introduction of LLDN to 65 transplants yearly from 2001 to 2004.
Comparison of laparoscopic and open outcomes
The donor
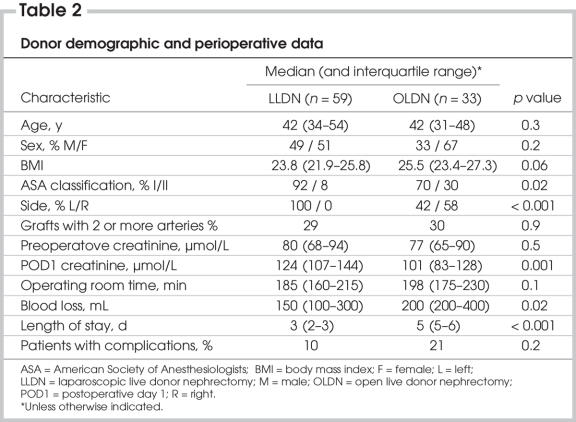
Table 2 summarizes donor demographic and perioperative data. Donor demographic data were equivalent between both groups with respect to age, sex distribution and body mass index. Of open donors, 30% had an American Society of Anesthesiologists (ASA) classification of 2, as compared with 8% in the laparoscopic group (p = 0.02). All the nephrectomies performed laparoscopically were left-sided, whereas 42% of kidneys procured in the open fashion were left-sided (p < 0.001). This is because, since the introduction of the laparoscopic technique, the indication for open nephrectomy was the need to procure the right kidney for anatomic reasons. In both groups, 30% of patients had kidneys with multiple arteries, and operative time was equivalent. The laparoscopic donors' creatinine was higher on the first postoperative day. Of the donors, 6 (10%) in the laparoscopic group suffered complications, as did 7 patients (21%) in the open group (p = 0.2). In the laparoscopic group, 1 patient had intraoperative bleeding that required conversion and transfusion, and another had postoperative abdominal wall bleeding that required transfusion. One patient had pulmonary edema requiring reintubation, 1 had a right pneumothorax requiring a thoracic drain, 1 patient's stay was prolonged owing to an allergic reaction to cefazolin, and 1 patient developed a major depressive episode in the weeks following the operation. In the open group, 2 patients received blood transfusions, 2 developed pulmonary edema, 2 had wound infection, and 2 developed pneumonia. Blood loss was significantly less in the laparoscopic group. Finally, length of hospital stay was 3 days in the laparoscopic group, compared with 5 days in the open group (p < 0.001).
Table 2
The recipient
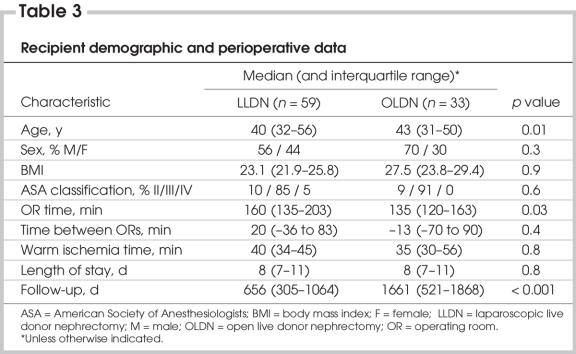
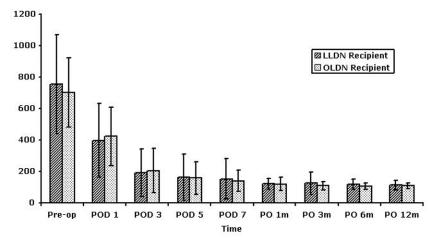
Table 3 summarizes recipient demographic and perioperative data. Patients in the open group were slightly older, but sex distribution, body mass index and ASA score were equivalent. Operative time was 25 minutes shorter in the open group (p = 0.03), but warm ischemia time was equivalent. Length of stay was 8 days in both groups. Follow-up was significantly longer in the open group: 1661 days versus 656 days (p < 0.001). Serum creatinine levels for both groups of recipients were equivalent up to 1 year after transplantation (Fig. 1). Although patients who required dialysis in the first postoperative week were excluded, this did not change the level of statistical significance (data not shown).
Table 3
FIG. 1. Recipient postoperative serum creatinine levels. Data are presented as means with standard deviation and analyzed with Student's t test. LLDN = laparascopic live donor nephrectomy; OLDN = open live donor nephrectomy; POD = postoperative day.
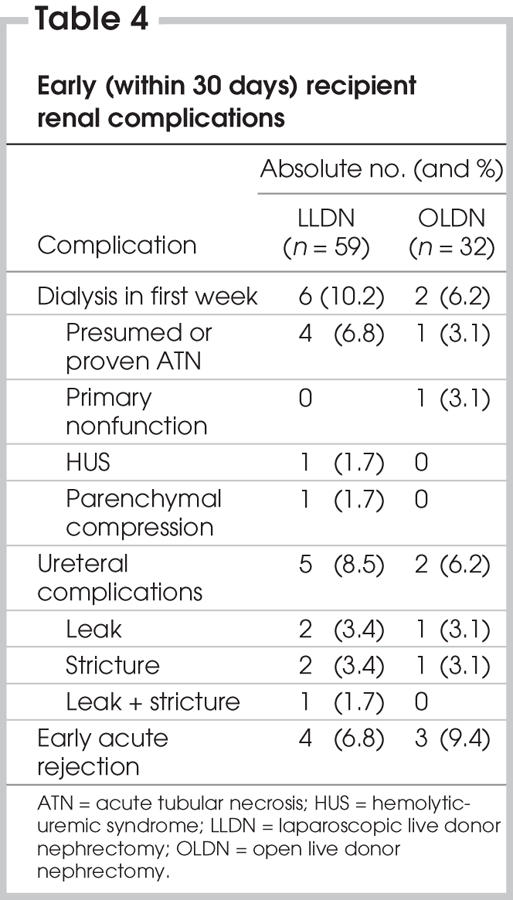
Table 4 summarizes early (within 30 d) recipient renal complications. One patient in the open group was not included owing to missing data. In the laparoscopic group, 6 patients (10.2%) required dialysis in the first week because of presumed or proven acute tubular necrosis (4 patients), parenchymal compression following closure or hemolytic-uremic syndrome. In the open group, 2 patients (6.2%) required dialysis in the first week (p = 0.8) because of acute tubular necrosis and primary nonfunction. In the laparoscopic group, 5 patients (8.5%) had a ureteral complication (urinary leak in 2, ureteral stricture in 2 and both leak and stricture in 1), compared with 2 patients (6.2%) in the open group (1 leak and 1 stricture) (p = 0.07). There were 5 patients with acute rejection in the laparoscopic group (4 had early acute rejection) and 6 in the open group (3 had early acute rejection), giving an equivalent cumulative survival to first rejection episode (p = 0.2). There was 1 failed graft in the laparoscopic group and 3 in the open group, 1 of which was due to primary nonunction, giving an equivalent cumulative graft survival (p = 0.2). Finally, there were 3 mortalities in the laparoscopic group (from postoperative hemorrhage in a Jehovah's witness who had refused transfusions, lung cancer and end-stage cystic fibrosis) and 1 in the open group (owing to sepsis from acute peritonitis in a patient on peritoneal dialysis following graft failure), but cumulative patient survival was equivalent (p = 0.4). For patients with adequate follow-up in the laparoscopic group (n = 32), 2-year patient and graft survival was 91% and 88%, respectively. It was 97% and 91%, respectively, in the open group (n = 33).
Table 4
Comparison of laparoscopic outcomes over time
Table 1 divides our laparoscopic experience into 3 groups: cases 1–20, cases 21–39 and cases 40–59. There was no significant difference in operative time, extraction time, estimated blood loss or in number of major complications in the donor population. In the recipient population, the incidence of delayed graft function, ureteral complications and acute rejection were also statistically similar among the 3 groups. The incidence of DGF, however, trended downward with experience. The only measured outcome that significantly differed among the groups was the time interval between donor and recipient operations: the median time was 92 minutes, 0 minutes and –29 minutes (p < 0.001) in groups 1, 2 and 3, respectively.
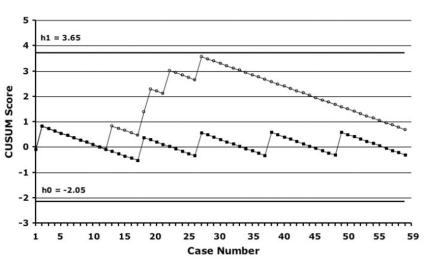
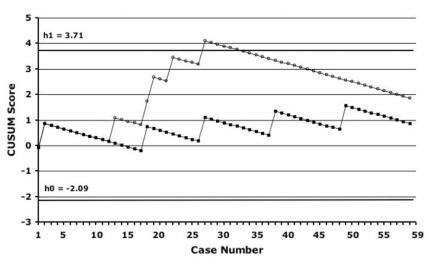
Figure 2 demonstrates our institutional learning curves, which were generated according to the CUSUM method, with DGF (dialysis in the first week) and UC as outcomes. The incidence of these complications in our own population of OLDN patients (6% for DGF and 6% for UC) was used as the benchmark for “acceptable” failure rates. For DGF, there was an upward slope of the curve until case 29, after which performance began to improve. No such pattern was evident for rates of UC. Both curves remain between h0 and h1, meaning that no statistical inference can be drawn. The analysis was also done with the use of a 5% incidence of DGF and UC based on published data from high-volume laparoscopic donor nephrectomy centres instead of our institutional open data. With these benchmarks for DGF, the CUSUM learning curves revealed an early failure rate significantly higher than the preset acceptable failure rate until case 29, after which performance improved. For UC, performance remained between h0 and h1 throughout (Fig. 3).
FIG. 2. CUSUM learning curves using delayed graft function and ureteral complications as outcomes, with institutional open donor nephrectomy data as the benchmarks. CUSUM = cumulative sum; black squares = ureteral complications; white circles = delayed graft function.
FIG. 3. CUSUM learning curves using delayed graft function and ureteral complications as outcomes, with high-volume centre laparoscopic donor data as the benchmarks.12,13 CUSUM = cumulative sum; black squares = ureteral complications; white circles = delayed graft function.
Discussion
LLDN was introduced at our institution according to a systematic and collaborative approach that included an extensive literature review, a site visit to the University of Maryland, animal work and laparoscopic (nondonor) nephrectomy. Other lower-volume centres have validated similar approaches by demonstrating very favourable outcomes during their transition from OLDN to LLDN.14,15 Further, in our centre, the introduction of LLDN has been associated with an increase in the volume of living kidney donors and renal transplantation in general, as has been previously demonstrated by others.16,17
From the donor standpoint, our data are in keeping with what has been demonstrated by other studies comparing laparoscopic and open nephrectomy: shorter hospital stays, less blood loss and an equivalent number of complications. Conversely, as opposed to most studies, our operative times are equivalent, not higher.18 Finally, our conversion rate of 1.7% is equivalent to that reported in series of more than 200 cases.19,20 Other studies have also demonstrated that the laparoscopic approach is associated with reduced narcotic requirements, superior postoperative quality of life and a quicker return to regular activities, compared with the open approach.9,21,22
From the recipient standpoint, graft function, as determined by serum creatinine levels, was equivalent at 1 year, and the incidence of graft complications such as delayed graft function, ureteral complications, and rejection between the recipients of laparoscopic vs. open donors was also equivalent. The other mentioned outcomes have generally been equivalent when open and laparoscopic donors are compared, with rates ranging from 0%–10% for DGF, 0%–11% for UC and 2%–30% for acute rejection in the laparoscopic arm.18 Finally, in agreement with most large studies,12,23,24 we demonstrated equivalent patient and graft survival.
As with other complex procedures, several authors have demonstrated the existence of a learning curve for LLDN. Leventhal and colleagues25 reported that all their complications with this procedure occurred in the first 30 cases, with none occurring in the next 50 cases. Jacobs and colleagues19 reported significantly higher blood loss, complications and UC in their first 100 cases, compared with the next 220 cases, and Nogueira and colleagues26 demonstrated a learning curve for warm ischemia time.
Analyzing our laparoscopic outcomes over time did not demonstrate any significant differences in donor operative time, extraction time, donor blood loss and donor complications. For the recipients, the only statistically significant difference seen was in the time elapsed between the donor and recipient surgeries. In our first 20 cases, there was a delay of over 90 minutes between the end of the donor operation and the beginning of the recipient procedure, whereas in our last 20 cases, the recipient operation was already underway when the donor procedure ended. This is due to the transition from sequential to simultaneous donor and recipient operations that has occurred at our institution. This is only a surrogate outcome for cold ischemia time (CIT), which was not available in our study. Nevertheless, whether such a small difference in CIT has a clinical impact is not known. In a small study, Baverstock and colleagues27 showed equivalent graft function between simultaneous (CIT = 23.6 min) and sequential (CIT = 191.7 min) donor–recipient procedures. Indeed, we did not notice any statistically significant differences in DGF, acute rejection or UC. However, the incidence of DGF was highest in the first 20 cases and then declined.
Because the number of patients is too small to find statistically significant differences with standard statistical analysis, we also used the CUSUM method because this approach may be a more sensitive way to find patterns of failure or success.28 The CUSUM curve for DGF had a clear upward trend followed by an inflection point seen at case 30. In this study, although the causes of DGF are not all clearly related to the procurement (or even implantation), this early cluster is suggestive of the presence of an institutional learning curve effect. Although improvement occurred at 30 cases, this took 18 months to accrue; hence, these data may not necessarily be applicable to centres with lower or higher volumes. In CUSUM analysis, how one defines the acceptable failure rate affects whether the comparison with the actual failure rate is statistically significant. For example, the CUSUM curve remained between h0 and h1 when the acceptable DGF rate was set at 6% (our institutional open rate), but it crossed the h0 line from below when it was set at 5% (high-volume benchmark), indicating that it was significantly higher than this failure rate.
When UC is used as the outcome, both in comparison with our institutional open donor data and in comparison with that of high-volume centres, no such learning curve effect is seen. Indeed, although, Jacobs and colleagues19 reported a significantly higher rate of ureteral complications early in their centre's experience, this issue was overcome with a technical change in which the gonadal vein was kept intact with the ureter and a significant amount of fat around the lower pole was maintained.13 This is the approach to ureteral dissection used in our series.
Some limitations must be acknowledged. First, although the laparoscopic data were collected prospectively, this is essentially a retrospective study and most of the open data are historical in nature. For this reason, follow-up was significantly longer in the open group, although the impact was likely minimal because most adverse events occurred within the first postoperative year. Over the course of the 7 years spanning this study, there might have been minor differences in immunosuppression protocols and perioperative management of recipients. Finally, laparoscopic fellows started actively participating in the procedures at about case 40, although there were no readily identifiable changes in the laparoscopic technique over time and 2 attending staff surgeons have continued to scrub on all cases. Second, there were discrepancies between the groups, such as higher ASA status and proportion of right-sided kidneys in the open donor group, although this difference would be unlikely to account for the longer hospital stay noted between the 2 groups. In addition, operative time was shorter in the open recipient group, most likely because all open donor and recipient operations were sequential, as opposed to those in the laparoscopic group, which were partly simultaneous. It is impossible to determine what effect, if any, these minor differences might have had on the reported outcomes. Third, the number of patients in each group was relatively small, and one could question whether we had sufficient power to detect small differences in relatively rare outcomes such as graft complications. This is demonstrated by the fact that the CUSUM curves remained between the h0 and h1 lines, suggesting that more observations are required to draw statistical inferences. Fourth, from the donor standpoint, although we showed a difference in length of stay, this is not the best outcome with which to compare these 2 groups. Outcomes such as quality of life and functional status, for which we had only incomplete data, might have been more appropriate and informative.29 Finally, although we do not believe that the higher donor laparoscopic postoperative creatinine levels are clinically significant, ultimately, longer follow-up is required. Others have demonstrated that long-term serum creatinine levels (> 1 y) are comparable after open and laparoscopic donation.30
The laparoscopic approach to live donor nephrectomy was safely introduced in a moderate-volume Canadian centre, with several outcomes comparing favourably with the open approach and without evidence of a learning curve for most outcomes. Improvement in the incidence of DGF, however, was seen after about 30 cases, although this was never statistically unacceptable when our institutional open donor data were used as the benchmark. These results were made possible by our learning from pioneering institutions to avoid their early pitfalls (particularly ureteral problems) and by our use of a collaborative, multidisciplinary approach to benefit from all team members' expertise and reduce variability.
Acknowledgments
This study was supported by an unrestricted educational grant from Tyco Healthcare Canada. The authors thank Donna Stanbridge, RN, for data collection and maintenance of the database.
Appendix 1.
Presented at the Canadian Surgical Forum, Montréal, September 2005.
Contributors: Drs. Bergman and Feldman designed the study. Drs. Bergman, Feldman, Anidjar, Demyttenaere, Metrakos, Tchervenkov and Paraskevas acquired the data, which Drs. Bergman, Feldman, Carli and Fried analyzed. Dr. Bergman wrote the article. All the authors reviewed the article and gave final approval for its publication.
Competing interests: None declared.
Accepted for publication Apr. 4, 2007
Correspondence to: Dr. L.S. Feldman, 1650 Cedar Ave., Rm. L9-316, Montréal QC H3G 1A4; liane.feldman@muhc.mcgill.ca
References
- 1.Canadian Organ Replacement Register data 1997-2003 [website of the Canadian Institute of Health]. Available: http://secure.cihi.ca/cihiweb/dispPage.jsp?cw_page=statistics_results_source_corr_e (accessed 2007 Jan 7).
- 2.Mange KC, Joffe MM, Feldman HI. Effect of the use or nonuse of long-term dialysis on the subsequent survival of renal transplants from living donors. N Engl J Med 2001;344:726-31. [DOI] [PubMed]
- 3.Meier-Kriesche HU, Kaplan B. Waiting time on dialysis as the strongest modifiable risk factor for renal transplant outcomes: a paired donor kidney analysis. Transplantation 2002;74:1377-81. [DOI] [PubMed]
- 4.Matas AJ, Bartlett ST, Leichtman AB, et al. Morbidity and mortality after living kidney donation, 1999-2001: survey of United States transplant centers. Am J Transplant 2003;3:830-4. [PubMed]
- 5.Kim FJ, Ratner LE, Kavoussi LR. Renal transplantation: laparoscopic live donor nephrectomy. Urol Clin North Am 2000;27:777-85. [DOI] [PubMed]
- 6.Fabrizio MD, Ratner LE, Montgomery RA, et al. Laparoscopic live donor nephrectomy. Urol Clin North Am 1999;26:247-56. [DOI] [PubMed]
- 7.Nanidis TG, Antcliffe D, Kokkinos C, et al. Laparascopic versus open live donor nephrectomy in renal tranplantation: a meta-analysis. Ann Surg 2008;247:58-70. [DOI] [PubMed]
- 8.Rawlins MC, Hefty TL, Brown SL, et al. Learning laparoscopic donor nephrectomy safely: a report on 100 cases. Arch Surg 2002;137:531-4. [DOI] [PubMed]
- 9.Flowers JL, Jacobs S, Cho E, et al. Comparison of open and laparoscopic live donor nephrectomy. Ann Surg 1997;226:483-9. [DOI] [PMC free article] [PubMed]
- 10.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [DOI] [PMC free article] [PubMed]
- 11.de Oliveira Filho GR. The construction of learning curves for basic skills in anesthetic procedures: an application of the cumulative sum method. Anesth Analg 2002;95:411-6. [DOI] [PubMed]
- 12.Ratner LE, Montgomery RA, Kavoussi LR. Laparoscopic live donor nephrectomy: the four year Johns Hopkins University experience. Nephrol Dial Transplant 1999;14:2090-3. [DOI] [PubMed]
- 13.Jacobs SC, Cho E, Foster C, et al. Laparoscopic donor nephrectomy: the University of Maryland 6-year experience. J Urol 2004;171:47-51. [DOI] [PubMed]
- 14.Duchene DA, Johnson DB, Li S, et al. Laparoscopic donor nephrectomy at a low volume living donor transplant center: successful outcomes can be expected. J Urol 2003;170:731-3. [DOI] [PubMed]
- 15.Muthu C, McCall J, Windsor J, et al. The Auckland experience with laparoscopic donor nephrectomy. N Z Med J 2003;116:U516. [PubMed]
- 16.Schweitzer EJ, Wilson J, Jacobs S, et al. Increased rates of donation with laparoscopic donor nephrectomy. Ann Surg 2000;232:392-400. [DOI] [PMC free article] [PubMed]
- 17.Kuo PC, Johnson LB. Laparoscopic donor nephrectomy increases the supply of living donor kidneys: a center-specific microeconomic analysis. Transplantation 2000;69:2211-3. [DOI] [PubMed]
- 18.Handschin AE, Weber M, Demartines N, et al. Laparoscopic donor nephrectomy. Br J Surg 2003;90:1323-32. [DOI] [PubMed]
- 19.Jacobs SC, Cho E, Dunkin BJ, et al. Laparoscopic live donor nephrectomy: the University of Maryland 3-year experience. J Urol 2000;164:1494-9. [PubMed]
- 20.Montgomery RA, Kavoussi LR, Su L, et al. Improved recipient results after 5 years of performing laparoscopic donor nephrectomy. Transplant Proc 2001;33:1108-10. [DOI] [PubMed]
- 21.Perry KT, Freedland SJ, Hu JC, et al. Quality of life, pain and return to normal activities following laparoscopic donor nephrectomy versus open mini-incision donor nephrectomy. J Urol 2003;169:2018-21. [DOI] [PubMed]
- 22.Wolf JS Jr, Merion RM, Leichtman AB, et al. Randomized controlled trial of hand-assisted laparoscopic versus open surgical live donor nephrectomy. Transplantation 2001;72:284-90. [DOI] [PubMed]
- 23.Philosophe B, Kuo PC, Schweitzer EJ, et al. Laparoscopic versus open donor nephrectomy: comparing ureteral complications in the recipients and improving the laparoscopic technique. Transplantation 1999;68:497-502. [DOI] [PubMed]
- 24.Troppmann C, Ormond DB, Perez RV. Laparoscopic (vs open) live donor nephrectomy: a UNOS database analysis of early graft function and survival. Am J Transplant 2003;3:1295-301. [DOI] [PubMed]
- 25.Leventhal JR, Deeik RK, Joehl RJ, et al. Laparoscopic live donor nephrectomy–is it safe? Transplantation 2000;70:602-6. [DOI] [PubMed]
- 26.Nogueira JM, Cangro CB, Fink JC, et al. A comparison of recipient renal outcomes with laparoscopic versus open live donor nephrectomy. Transplantation 1999;67:722-8. [DOI] [PubMed]
- 27.Baverstock RJ, Manson AD, Liu L, et al. A prospective comparison of simultaneous and sequential live-donor renal transplantation. Transplantation 2002;74:1194-7. [DOI] [PubMed]
- 28. Novick RJ, Fox SA, Stitt LW, et al. Assessing the learning curve in off-pump coronary artery surgery via CUSUM failure analysis. Ann Thorac Surg 2002;73:5358-62. [DOI] [PubMed]
- 29.Bergman S, Feldman LS, Mayo NE, et al. Measuring surgical recovery: the study of laparoscopic live donor nephrectomy. Am J Transplant 2005;5:2489-95. [DOI] [PubMed]
- 30.Lind MY, Zur Borg IM, Hazebroek EJ, et al. The effect of laparoscopic and open donor nephrectomy on the long-term renal function in donor and recipient: a retrospective study. Transplantation 2005;80:700-3. [DOI] [PubMed]