Abstract
Chronic, noncancer pain such as that associated with osteoarthritis of the hip and knee is typically managed according to American College of Rheumatology guidelines. Patients unresponsive to first-line treatment with acetaminophen receive nonsteroidal antiinflammatory drugs (NSAIDs), including cyclooxygenase-2 (COX-2) inhibitors. However, many patients may have chronic pain that is refractory to these agents, or they may be at risk for the gastrointestinal, renal, and cardiovascular complications associated with their use. Tramadol, a mild opioid agonist and norepinephrine and serotonin reuptake inhibitor, is recommended by current guidelines for the treatment of moderate to moderately severe pain in patients who have not responded to previous oral therapy, or in patients who have contraindications to COX-2 inhibitors and nonselective NSAIDs. An extended-release (ER) formulation of tramadol was approved by the US Food and Drug Administration in September 2005. In contrast with immediate-release (IR) tramadol, this ER formulation allows once-daily dosing, providing around-the-clock analgesia. In clinical studies, tramadol ER has demonstrated a lower incidence of adverse events than that reported for IR tramadol. Unlike nonselective NSAIDs and COX-2 inhibitors, tramadol ER is not associated with gastrointestinal, renal, or cardiovascular complications. Although tramadol is an opioid agonist, significant abuse has not been demonstrated after long-term therapy. It is concluded that tramadol ER has an efficacy and safety profile that warrants its early use for the management of chronic pain, either alone or in conjunction with nonselective NSAIDs and COX-2 inhibitors.
Keywords: chronic pain, COX-2 inhibitor, NSAID, opioid, tramadol
Introduction
Chronic pain was originally defined as pain lasting 3–6 months after onset, but has since been described as pain that extends beyond the healing period, disrupts sleep or normal activities, and is not explained by the low levels of pathology that characterize the disease or condition (JCAHO 2001).
Patients with lower back pain, myofascial pain, and osteoarthritis (OA) are the most likely to suffer from chronic pain, which is one of the leading causes of disability within the work force (Yelin and Callahan 1995; CDC 2001; APF 2002). Over 40% of patients with musculoskeletal disease reported some form of disability, and more than half of working age people with musculoskeletal conditions were unable to work (Yelin and Callahan 1995; CDC 2001). According to the American College of Rheumatologists (ACR), 21 million Americans are affected by OA, which is associated with annual losses of 36 million workdays (Babul et al 2004; ACR 2005). The impact of chronic pain may be even greater; recent estimates by the Center for Disease Control place the number of adults with arthritis and chronic joint symptoms at around 70 million (CDC 2002).
Living with chronic pain significantly reduces patients’quality of life. In a study of 306 patients aged 55–74 years, patients with chronic pain in the hip or knee reported a significantly lower quality of life than a reference group not suffering from chronic pain (p < 0.045) (Hopman-Rock et al 1997). As outlined in Table 1, untreated pain increases anxiety and depression, and is commonly associated with a decreased ability to cope (Eisendrath 1995; Yelin and Callahan 1995; APS 1996; Cohen et al 2000).
Table 1.
Morbidity associated with untreated chronic pain (APS 1996)
| Decreased quality of life |
| Sleep disturbance |
| Adverse impact on: |
| Concentration |
| Ability to work |
| Ability to exercise |
| Physical function |
| Cognitive functions |
| Daily living |
| Social relationships |
| Depression |
| Increased anxiety |
| Inability to cope |
The effects of chronic pain on patients’quality of life are also reflected in the low degree of life satisfaction in patients with this condition (Laborde and Powers 1980). According to the American Pain Foundation, two thirds of chronic pain sufferers were unable to perform routine physical tasks or to enjoy their hobbies, even though they were taking pain medication (APF 2006). The impact of chronic pain is underscored by the finding that past, present, and future satisfaction scores (assessed on Cantril’s self-anchoring scale) showed that patients with severe OA had significantly lower life satisfaction scores than patients on hemodialysis (p < 0.05) (Laborde and Powers 1980).
Sleep disturbance is another major concern of patients with noncancer chronic pain; poor sleep has been reported in 70% of patients in chronic pain clinics and in 60% of patients suffering from arthritis (Menefee, Cohen, et al 2000). A cross-sectional survey of 167 patients with chronic spinal pain showed that high sleep quality and low sleep latency correlated positively with a shorter duration of pain and improved physical functioning (Menefee, Frank, et al 2000). High pain scores were independent indicators of overall sleep quality and sleep latency (Menefee, Frank, et al 2000). A small comparative study between 16 healthy subjects and 14 patients with OA showed an association between chronic pain and changes in EEG sleep patterns (Leigh et al 1988). Significant increases in stage I sleep (drowsiness), accompanied by decreases in stage II sleep (sleep onset), were observed in patients with OA, compared with normal subjects (Leigh et al 1988).
Chronic pain is also associated with considerable economic costs. In the US in 2002, total lost productivity costs due to arthritis and lower back pain were estimated at US $10.3 billion and $19.8 billion, respectively (Stewart et al 2003).
Treatment guidelines for management of chronic pain
For patients with OA of the hip and knee, chronic pain is typically managed according to ACR guidelines, as outlined in Figure 1 (ACR 2000). Acetaminophen is recommended as first-line therapy, although even doses up to 4 g may not provide sufficient pain relief (ACR 2000). Nonselective NSAIDs and COX-2 inhibitors are recommended for patients intolerant or unresponsive to acetaminophen (ACR 2000). The decision to prescribe nonselective NSAIDs is largely determined by a patient’s risk for upper gastrointestinal (GI) disorders following treatment with these agents. These include a history of peptic ulcer disease, increased GI bleeding, use of corticosteroids or anticoagulants (ACR 2000), and generally poor state of health (ACR 2000). Nonselective NSAIDs and COX-2 inhibitors can cause renal toxicity and should be used with caution in patients with mild to moderate renal insufficiency; these agents should not be used in patients with severe renal insufficiency (ACR 2000). Nonselective NSAIDs in combination with gastroprotective agents – misoprostol or a proton pump inhibitor – are recommended for patients unable to take either COX-2 inhibitors or nonselective NSAID monotherapy (ACR 2000). Patients susceptible to NSAID-induced platelet inhibition and bleeding may be prescribed nonacetylated salicylates (ACR 2000).
Figure 1.
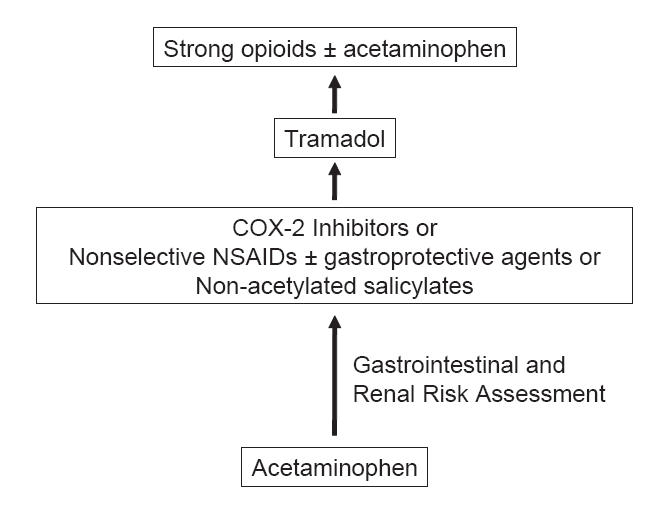
ACR treatment guidelines for pharmacological management of noncancer chronic pain systemic agents (ACR 2000).
Abbreviations: ACR, American College of Rheumatology; COX-2, cyclooxygenase-2; NSAIDs, nonsteroidal antiinflammatory drugs.
Tramadol is a centrally acting oral analgesic that blocks pain through opioid receptor binding and inhibition of norepinephrine and serotonin reuptake. It is currently indicated for the management of moderate to moderately severe pain in adults (Ultram PI 2004). Tramadol may be combined with NSAIDs in patients whose symptoms are poorly controlled by these agents (ACR 2000). Immediate-release (IR) tramadol is initiated at 25 mg and titrated in 25 mg increments over 3 days to achieve 25 mg four times daily (qid), then in 50 mg increments over 3 days to 50 mg qid (Ultram PI 2004). After titration, tramadol may be administered in doses of 50 mg to 100 mg every 4–6 hours as required for pain relief up to a daily maximum of 400 mg; the mean effective daily dosage is between 100 mg and 300 mg (Ultram PI 2004). The most common side effects of tramadol are dizziness, nausea, constipation, and drowsiness (Ultram PI 2004). Despite its mild opioid effects, tramadol has a low potential for abuse and remains the only unscheduled opioid (ACR 2000).
More potent opioid therapy is recommended for patients unresponsive to or intolerant of tramadol (ACR 2000). Joint guidelines have been published by the American Pain Society and American Academy of Pain Medicine on the use of more potent opioids for the management of chronic, noncancer pain. Lifesaving and safe for many patients, a cautious approach with a careful risk assessment should be done in all patients taking opioids (ACR 2000).
Limitations associated with chronic pain treatment
Despite the presence of established treatment guidelines, chronic pain remains an undertreated condition (Weinstein et al 2000; Glajchen 2001). Almost half of all patients in routine clinical practice experience inadequate relief from chronic pain (Cherny and Portenoy 1994; Glajchen 2001). Low patient satisfaction with analgesic therapy is further evidenced by the fact that Americans over age 60 years consult at least 3 physicians about their pain medications (APF 2006).
There are a variety of factors leading to the low effectiveness of chronic pain therapy, including patient factors, physician education, regulatory oversight, and formulary issues. The relatively low effectiveness of chronic pain therapy may be related to various limitations of currently used analgesics (Table 2), as well as conservative dosing by physicians (ACR 2000; Weinstein et al 2000; Stephens et al 2003; Hudson et al 2005; Caldwell et al 2006). Treatment with NSAIDs is restricted by their association with increased risk for GI side effects, renal toxicity, and cardiovascular toxicity, especially in the elderly (ACR 2000). Risk factors for reversible renal failure in patients with intrinsic renal disease receiving nonselective NSAIDs include age (≥65 years), hypertension, and congestive heart failure (Page and Henry 2000). Studies have shown that subjects with a history of heart disease receiving nonselective NSAIDs had a 10.5-fold increased risk for congestive heart failure, compared with non-NSAID users (Page and Henry 2000).
Table 2.
Limitations of pharmacologic therapy for chronic pain
| Therapeutic class | Major limitations |
|---|---|
| Nonselective NSAIDs | Risk for gastrointestinal toxicitya |
| Recommended use of concomitant gastroprotective agents | |
| increases costa | |
| Risk for renal toxicitya | |
| Cardiovascular safety issuesb | |
| COX-2 Inhibitors | Increased risk |
| Cardiovascular safety issuesc | |
| Renal toxicitya | |
| Opioids | Associated with: Reduced cognitive and motor functiond |
| Respiratory depressione | |
| Fear of dependence/tolerance/abusef | |
| Stringent regulatory requirements and potential for scrutiny maylimit adequate prescribingf |
Notes: ACR 2000
Abbreviations: COX-2, cyclooxygenase-2; NSAIDs, nonsteroidal antiinflammatory drugs.
Recent studies have shown that COX-2 inhibitors are similarly associated with cardiovascular safety issues. Using published and unpublished data between 1966 and 2005, a meta-analysis of randomized comparative trials of at least 4 weeks’duration showed both selective COX-2 inhibitors and certain high-dose nonselective NSAIDs significantly increased the risk for cardiovascular events (Kearney et al 2006). Compared with placebo, COX-2 inhibitors showed a relative increase of 42% in serious vascular events (p = 0.003), which was similar to that found with certain nonselective NSAIDs. (Kearney et al 2006).
Formulation, pharmacokinetics, and mechanism of action of tramadol ER
Formulation
Tramadol ER is an ER formulation of (±) cis-2-[(dimethylamino)methyl]-1-(3-methoxyphenyl) cyclohexanol hydrochloride combined with ethyl cellulose, dibutyl sebacate, polyvinylpyrrolidone, sodium stearyl fumarate, colloidal silicon dioxide, and polyvinyl alcohol (Figure 2) (Ultram ER PI 2006). Tramadol ER was recently approved as a nonscheduled analgesic for the control of moderate to moderately severe pain in adults who require treatment over an extended time period (Ultram ER PI 2006).
Figure 2.
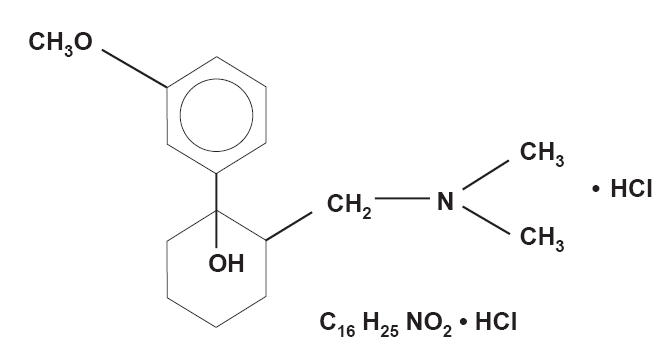
Structure of tramadol extended-release: (±) cis-2-[(dimethylamino) methyl]-1-(3-methoxyphenyl) cyclohexanol hydrochloride (Ultram PI 2004).
Pharmacokinetics
Orally administered tramadol ER 100 mg to 400 mg exhibited dose-proportional pharmacokinetics in healthy human volunteers (Ultram ER PI 2006). Tramadol ER is extensively metabolized through multiple cytochrome P450 pathways (3A4, 2B6, 2D6), of which 2D6 is responsible for formation of the O-demethylated metabolite, M1 (Ultram ER PI 2006). The analgesic activity of tramadol ER is mediated by racemic forms of both tramadol and M1 (Ultram ER PI 2006). However, tramadol has up to a six-fold greater analgesic effect than the M1 metabolite in animal models (Ultram ER PI 2006).
Tramadol ER 200 mg once daily (qd) was compared with tramadol IR 50 mg every six hours (q6h) in healthy subjects. Tramadol ER showed a steady and sustained rise in plasma concentration during the 24-hour period after administration, compared with tramadol IR, which exhibited more frequent fluctuations (Figure 3) (Ultram ER PI 2006). The M1 metabolite exhibited a pharmacokinetic profile similar to that of the parent drug (Ultram ER PI 2006). Tramadol and its M1 metabolite attained mean peak plasma concentrations at 12–15 hours, reaching steady state at 4 days (Ultram ER PI 2006).
Figure 3.
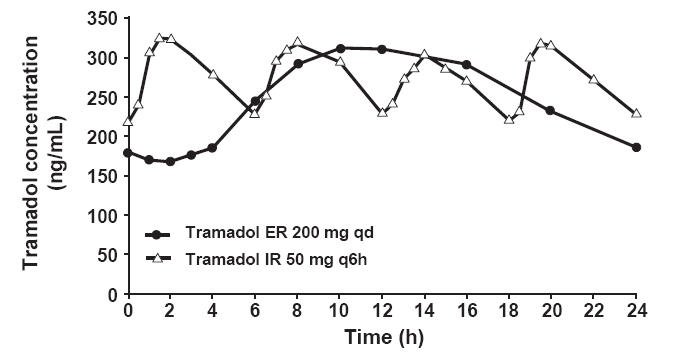
Pharmacokinetics of tramadol ER 200 mg once daily (qd) versus tramadol IR 50 mg every 6 hours (q6h) (mean steady-state tramadol plasma concentrations in healthy subjects on day 8 post dose) (Ultram PI 2006).
Abbreviations: ER, extended-release; IR, immediate-release.
Tramadol ER may be taken without food. A high-fat meal slightly decreased tramadol area under the curve (AUC) and maximum concentration (Cmax) 16% and 28%, respectively, and extended its half-life to 17 hours, compared with 14 hours in fasted conditions (Ultram ER PI 2006).
Mechanism of action
The analgesic efficacy of tramadol and its M1 metabolite has been established in various rodent models of pain (Hennies et al 1988; Kayser et al 1991; Raffa et al 1992, 1995; Mattia et al 1993).
Competitive binding to rodent brain membranes in vitro showed that tramadol is a selective μ-opioid receptor agonist with a 200-fold lower affinity for μ-opioid receptors than its M1 metabolite (Hennies et al 1988; Raffa et al 1995). Opioid agonist activity appeared to be mediated by the dextrorotatory (+) enantiomeric form of tramadol, as opposed to the mirror image levorotatory (–) form (Raffa et al 1995). Tramadol is a relatively weak μ-opioid agonist; its affinity for μ-opioid receptors was 6000, 60, and 10 tmes lower than that of morphine, dextropropoxyphene, and codeine, respectively (Raffa et al 1995). Tramadol’s μ-opioid receptor binding and analgesic effects were only partially blocked by naloxone, suggesting other nonopioid mechanisms of analgesia (Hennies et al 1988; Raffa et al 1992).
In vitro studies, using rodent synaptosomes, showed that tramadol was more potent with respect to norepinephrine and serotonin reuptake inhibition than μ-opioid receptor binding (Hennies et al 1982; Raffa et al 1992). That tramadol mediated its analgesic effects through inhibition of monoamine reuptake in vivo was confirmed in animal and human studies, in which tramadol’s analgesic effects were blocked by yohimbine (Raffa et al 1992; Desmeules et al 1996).
Intrathecal tramadol produced a weak analgesic effect in mice (Mattia et al 1993). In contrast, administration of tramadol by the intracerebroventricular route followed by intrathecal injection resulted in powerful synergistic analgesia, implicating both the brain and spinal cord as the principal sites of action (Mattia et al 1993). Unlike other centrally acting analgesics, tramadol demonstrated limited potential for tolerance in mouse and rat pain models (Kayser et al 1991; Mattia et al 1993).
Efficacy
Effectiveness in clinical practice and clinical trials is the standard by which the true potential of a drug is determined. Tramadol IR has been widely prescribed as an unscheduled opioid in the US for the treatment of chronic pain for more than 10 years, and has demonstrated efficacy and safety in the treatment of moderate to moderately severe pain (ACR 2000; Ultram PI 2004; Mattia and Coluzzi 2005). Although tramadol has opioid analgesic effects, significant abuse has not been demonstrated after long-term therapy (ACR 2000; Cicero, Inciardi, Adams, et al 2005).
Effect on pain measures
The analgesic effects of tramadol ER were initially explored in patients with moderate to moderately severe chronic pain due to osteoarthritis and/or low back pain in 12-week, randomized, double-blind, placebo-controlled trials (Ultram ER PI 2006). Moderate to moderately severe pain was defined by a pain intensity score of ≥ 40 mm, off previous medications, on a 0 to 100 mm visual analog scale (VAS).
In one of these studies, patients with moderate to moderately severe pain due to OA of the knee and/or hip received tramadol ER 100 mg to 400 mg daily (Ultram PI 2006). Treatment was initiated at 100 mg for 4 days, and then increased every 5 days by 100 mg increments up to 400 mg. Pain was measured by the the Western Ontario and McMaster Universities (WOMAC) Pain subscale. Change in baseline pain was assessed at 1, 2, 3, 6, 9, and 12 weeks after treatment.
A responder analysis, based on the percent change in the WOMAC Pain subscale, demonstrated a statistically significant improvement in pain for the 100 mg and 200 mg treatment groups, compared with placebo (Figure 4) (Ultram PI 2006). Surprisingly, the proportion of patients achieving improvement in pain with tramadol ER 300 mg and 400 mg was not significantly different from placebo. A 12-week, randomized, double-blind, placebo-controlled, flexible-dose study of patients with OA f the knee showed improvement in pain based on the Arthritis Pain Intensity VAS (Ultram PI 2006). Significant improvement was observed with a tramadol ER mean daily dose of 270 mg (Ultram PI 2006).
Figure 4.
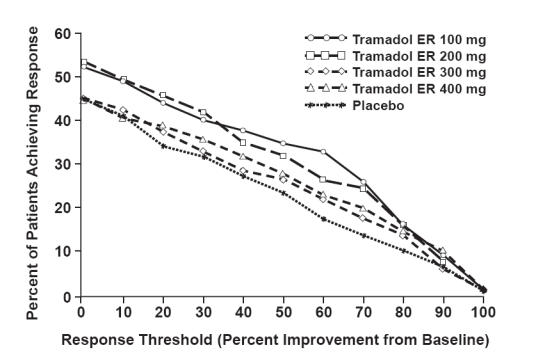
WOMAC pain responder analysis: patients with moderate to moderately severe pain due to osteoarthritis of the knee and/or hip achieving various levels of response with tramadol ER. Patients in the 100 mg and 200 mg treatment groups demonstrated a statistically significant improvement in pain compared with placebo (Ultram PI 2006).
Abbreviations: ER, extended release; WOMAC, Western Ontario and McMaster Universities.
The analgesic efficacy of tramadol ER was also examined in a 12-week, randomized, double-blind, placebo-controlled, flexible-dose study of 246 patients with osteoarthritis of the knee and moderate to severe chronic pain (Babul et al 2004). Patients with a mean age of 61 years and average disease duration of 12–13 years were randomized to tramadol ER (n = 124) or placebo (n = 122) (Babul et al 2004). Tramadol ER 100 mg was administered once daily for the first 4–8 days according to treatment tolerability and then increased to 200 mg (Babul et al 2004). Further titrations up to 300 mg or 400 mg were allowed based on analgesic efficacy and tolerability (Babul et al 2004). The mean tramadol ER dose was 276 mg, close to the highest recommended daily dose of 300 mg (Babul et al 2004; Ultram PI 2006).
Analgesic efficacy was assessed as reduction of pain from baseline through 12 weeks using the Arthritis Pain Intensity VAS (Figure 5) (Babul et al 2004). After 1 week of treatment, VAS scores were decreased by 25% for tramadol ER compared with 14% for placebo (Babul et al 2004). Improvements in pain scores increased over time and were sustained up to 12 weeks; the mean change in VAS from baseline to 12 weeks was significantly greater for tramadol ER (49%) than placebo (27%; p < 0.001) (Babul et al 2004). It should be noted that the placebo response rate of 27% is not uncommon in analgesia trials (Turner et al 1994).
Figure 5.
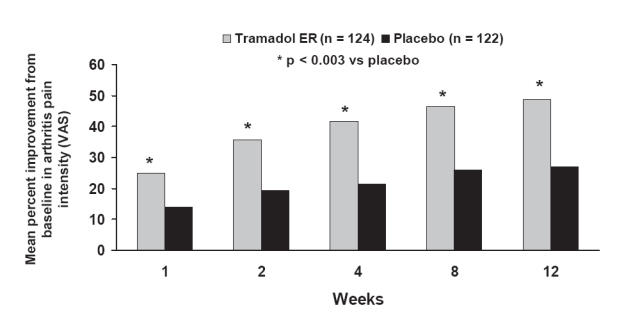
Efficacy of tramadol ER in patients with osteoarthritis: mean change from baseline in Arthritis Pain Intensity VAS assessed through 12 weeks. Reprinted from Babul N, Noveck R, Chipman H, et al. 2004. Efficacy and safety of extended-release, once-daily tramadol in chronic pain: a randomized 12-week clinical trial in osteoarthritis of the knee. J Pain Symptom Management, 28:59–71. Copyright © 2004 with permission from Elsevier.
Abbreviations: ER, extended release; VAS, Visual Analogue Scale.
WOMAC OA subscales were evaluated as a further measure of pain, stiffness, and physical function (Figure 6) (Babul et al 2004). As with the Arthritis Pain Intensity VAS, the WOMAC pain subscale improved significantly from baseline over the 12-week period following treatment with tramadol ER (Babul et al 2004). Tramadol ER significantly improved the mean WOMAC pain score from baseline by 45% compared with 25% for placebo (p < 0.001); an effect consistent with changes observed in the Arthritis Pain Intensity VAS (Babul et al 2004). Similar improvements in the WOMAC stiffness and physical function scores were observed following 12 weeks of tramadol ER therapy. Stiffness and physical function scores were increased by 43% and 44% for tramadol ER compared with 18% and 21% in the placebo group (p < 0.001) (Babul et al 2004).
Figure 6.
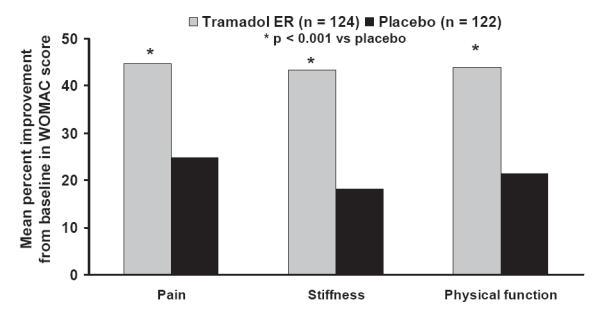
Efficacy of tramadol ER in patients with osteoarthritis using the WOMAC pain, stiffness, and physical function subscales. Mean percentage change in subscales were assessed from baseline to 12 weeks of treatment. Tramadol ER significantly improved pain, stiffness, and physical function subscales, compared with placebo (p < 0.001). Reprinted from Babul N, Noveck R, Chipman H, et al. 2004. Efficacy and safety of extended-release, once-daily tramadol in chronic pain: a randomized 12-week clinical trial in osteoarthritis of the knee. J Pain Symptom Management, 28:59–71. Copyright © 2004 with permission from Elsevier.
Abbreviations: ER, extended release; WOMAC, Western Ontario and McMaster Universities.
Patient Global Assessment and Physician Global Assessment of pain were significantly better for tramadol ER than placebo from baseline to 12 weeks of therapy (p < 0.001) (Babul et al 2004). Treatment discontinuation was significantly lower with tramadol ER (19%) than placebo (38%; p < 0.001) (Babul et al 2004).
Effect of pain management on sleep
Since sleep disturbances are common in patients with chronic pain, the Chronic Pain Sleep Inventory was used to evaluate the effect of chronic pain management on sleep in this study (Menefee, Cohen, et al 2000; Babul et al 2004). Pain relief with tramadol ER was associated with improvements in sleep from baseline, compared with placebo, that were statistically significant from week 2 to week 12 (p < 0.05 vs placebo) (Babul et al 2004). Improvements were noted in specific pain-related sleep parameters: being awakened by pain, trouble falling asleep due to pain, and overall quality of sleep (Babul et al 2004).
Safety
The safety of tramadol ER has been established in several double-blind studies of patients with OA or chronic low back pain, or both and one open-label study in patients with chronic noncancer pain conducted within the US (Ultram PI 2006). These studies included a total of 3108 patients, of whom 901 were at least 65 years of age (Ultram PI 2006).
In two 12-week, randomized, double-blind, placebo-controlled studies of patients with chronic non-cancer pain, adverse events increased with dose from 100 mg to 400 mg (Ultram PI 2006). The most frequently reported adverse events for tramadol ER 100 to 400 mg ranged from 16% to 28% (dizziness), 15% to 26% (nausea), and 12% to 30% (constipation) (Ultram PI 2006). A lower incidence of these adverse events was observed in the placebo group, ranging from 4% to 8% (Ultram PI 2006).
In the 12-week study of 246 patients with OA recently reported by Babul and colleagues (2004), the overall incidence of adverse events over the study period was higher for patients receiving tramadol ER compared with placebo (79.0% vs 63.9%; p = 0.011). Adverse events possibly related to treatment were reported for 63.7% and 32% of patients in the tramadol ER and placebo groups, respectively (p < 0.001) (Babul et al 2004). More than 90% of the adverse events in both treatment groups were of mild or moderate severity (Babul et al 2004). The most frequently reported adverse events in the tramadol ER group were dizziness (33%), constipation (26%), and nausea (24%) (Babul et al 2004). In contrast, the incidence of these adverse events was lower in the placebo group, ranging from 6% to 12% (Babul et al 2004). Although tramadol ER treatment in this study was initiated at a daily dose of 100 mg, certain patients received dose increases up to 400 mg based on the adequacy of pain relief and tolerability of side effects. Therefore, the 400 mg dose of tramadol ER, which is not approved for treatment of chronic pain, may have contributed to the higher rate of adverse events in this study (Ultram PI 2006).
Unlike other agents such as nonselective NSAIDs and COX-2 inhibitors, tramadol has no known association with cardiovascular disease or stomach ulcers.
Tramadol ER should be used with caution in the geriatric population (Tramadol ER PI 2006). A study of 901 patients at least 65 years of age showed that the incidence of adverse events was highest in the group over 75 years of age receiving tramadol ER (Tramadol PI 2006).
Like other opioids, tramadol ER is contraindicated in states of acute intoxication with alcohol, narcotics, hypnotics, centrally acting analgesics, opioids, or psychotropic agents (Ultram PI 2006).
Tramadol ER use may be limited by its potential to increase risk of seizures (Ultram PI 2006). Seizures have been reported in patients taking tramadol within the recommended dose range (Ultram PI 2006). Tramadol increased the risk for seizure in patients taking serotonin reuptake inhibitors, tricyclic antidepressants, and opioids (Ultram PI 2006). Tramadol ER may also increase the risk for seizure if combined with drugs that decrease seizure threshold, such as monoamine oxidase inhibitors (Ultram PI 2006). Furthermore, combination of tramadol ER with central nervous system (CNS) depressants may produce respiratory depression, a reported side effect of opioid therapy (Stephens et al 2003; Ultram PI 2006).
Use of tramadol ER may be limited in patients with renal or hepatic impairment (Ultram PI 2006). Following repeated doses of tramadol ER 100 mg, exposure of the tramadol M1 metabolite was increased by 20%–40% in patients with mild (creatinine clearance = 50–80 mL/min) or moderate (creatinine clearance = 30–50 mL/min) renal impairment, compared with normal subjects (Ultram PI 2006). Additionally, exposure of the M1 metabolite was decreased by approximately 50% in patients with mild and moderate hepatic impairment, compared with normal subjects (Ultram PI 2006). It is recommended that tramadol ER should not be used in patients with severe renal impairment (creatinine clearance <30 mL/min) or severe hepatic impairment (Ultram PI 2006).
Tramadol ER vs tramadol IR
Data from separate studies of tramadol IR and tramadol ER (90 days’and 12 weeks’duration, respectively) suggest that tramadol ER has a lower propensity for adverse events than tramadol IR (Ultram PI 2004; Ultram ER PI 2006). The most frequently reported adverse events for tramadol IR and tramadol ER were in the CNS and the GI system (Ultram PI 2004; Ultram ER PI 2006). Adverse event rates – occurring with an incidence of at least 5% – were higher with tramadol IR than tramadol ER (reported above); event rates for constipation, nausea, and dizziness were 46%, 40%, and 33%, respectively, after 90 days of tramadol IR therapy (Ultram PI 2004; Ultram ER PI 2006).
Like tramadol IR, tramadol ER has the potential for interacting with 2D6- and 3A4- inhibitors that may alter tramadol’s efficacy by lowering M1 metabolite levels or changing tramadol’s exposure (Ultram PI 2006). Metabolism of tramadol ER may also be compromised in patients with 2D6 gene dysfunction, which is present in approximately 8%–10% of the Caucasian population, reducing its analgesic efficacy (Gough et al 1990; Garcia-Quetglas et al 2007).
NSAID dose-sparing potential of tramadol
Combination of tramadol with NSAIDs for the management of chronic pain has the potential to reduce NSAID requirements. In a randomized, double-blind, placebo-controlled study with tramadol IR in patients with OA, in those patients who responded to naproxen, use of tramadol decreased the requirement for naproxen without compromising analgesic efficacy (Schnitzer et al 1999).
Tramadol ER abuse
Tramadol appears to be associated with low rates of abuse (ACR 2000; Cicero, Inciardi, Adams, et al 2005). A pharmacoepidemiologic surveillance study, conducted between 1994 and 2004 among 309 drug abuse experts and 100 police agencies, reported that the rate of tramadol abuse was very low, ranging from 0.5 to 2.0 cases per 100,000 patients over the 10-year study period (Cicero, Inciardi, Adams, et al 2005). Introduction of new branded and cheaper generic formulations in 2002 had no effect on the rate of tramadol abuse (Cicero, Inciardi, Adams, et al 2005). In contrast, the Research Abuse, Diversion, and Addiction-Related Surveillance (RADARS) system showed abuse rates for controlled-release oxycodone of at least 5 cases per 100,000 patients between 2002 and 2004 (Cicero, Inciardi, Munoz, et al 2005). Nevertheless, because tramadol exerts its analgesic effects in part through opioid binding, its potential for abuse should always be considered when prescribed for the treatment of chronic pain.
Optimizing therapy for chronic pain
The goals of managing chronic pain and improving patient quality of life can be achieved by optimizing analgesic therapy. The ideal treatment would be a once-daily formulation with minimum side effects, around-the-clock pain relief, and no potential for end-organ injury.
Of the currently recommended therapies, NSAIDs, including COX-2 inhibitors, may be undesirable choices for long-term treatment due to their potential for end-organ injury. In spite of the proven analgesic efficacy of scheduled opioids, side effects and the need for risk assessment makes prescribing of these agents problematic for a busy provider (Cherny et al 1994; Parrott 1999; Weinstein et al 2000; JCAHO 2001; Stephens et al 2003). In contrast to pure opioid analgesics, tramadol is an unscheduled partial opioid analgesic with a lower potential for tolerance and abuse (ACR 2000; Cicero, Inciardi, Adams, et al 2005). However, because of the short half-life of tramadol IR, daily doses of 50 mg qid are required for maximum effect (Ultram PI 2004). Such a multiple-dose regimen results in frequent daily peak-to-trough fluctuations in plasma drug that may provide less than optimal analgesic activity, especially during the nighttime hours (Ultram PI 2006).
Tramadol ER represents a significant advance over tramadol IR. Once-daily administration of tramadol ER allows for consistent sustained plasma drug levels over a 24-hour time period, providing around-the-clock analgesic effectiveness (Ultram PI 2006). In this respect, tramadol ER has the potential to minimize sleep disturbances, an advantage that could translate into improved patient function and quality of life. Twenty-four hour pain relief could provide added benefits by reducing the overall pill burden and the need for sedatives and hypnotic agents to control sleep disturbance. These effects, combined with a lower incidence of adverse events, suggest tramadol ER would be better tolerated than tramadol IR and offer the potential for increasing patient compliance.
Conclusions
Current ACR treatment guidelines for chronic pain consist of a multistage treatment algorithm based on increasing analgesic potency. Tramadol is currently recommended as an alternative for patients unresponsive to or intolerant of nonselective NSAIDs and COX-2 inhibitors for the relief of moderate to severe chronic pain (ACR 2000). Tramadol ER has benefits that may merit its use earlier in the treatment decision process for moderate to moderately severe chronic pain. Tramadol ER, either as monotherapy or in combination with antiinflammatory agents, as first prescription therapy (following initial use of acetaminophen), for patients who require 24-hour relief from their moderate chronic pain may spare the use of nonselective NSAIDs and COX-2 inhibitors, as well as reduce side effects and improve patient compliance. Tramadol ER may also postpone the need for scheduled opioids and the risks inherent in their use.
References
- [ACR] American College of Rheumatology. Recommendations for the medical management of osteoarthritis of the hip and knee. 2000 update. Arthritis Rheum. 2000;43:1905–15. doi: 10.1002/1529-0131(200009)43:9<1905::AID-ANR1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- [ACR] American College of Rheumatology. Patient education: Osteoarthritis [online] 2005 Accessed 26 June 2006. URL: http://www.rheumatology.org/public/factsheets/oa_new.asp?aud=pat.
- [APF] American Pain Foundation. Pain facts: An overview of American pain surveys [online] 2002 Accessed 18 September 2006. URL: http://www.painfoundation.org/page.asp?file=Library/PainSurveys.htm.
- [APS] American Pain Society. Chronic pain in America – Road-blocks to relief [online] 1996 Accessed 18 Semptember 2006. URL: www.painreliefnetwork.org/wp-content/ uploads/PressPacket/APSRoad-blocksToRelief.pdf.
- Babul N, Noveck R, Chipman H, et al. Efficacy and safety of extended-release, once-daily tramadol in chronic pain: a randomized 12-week clinical trial in osteoarthritis of the knee. J Pain Symptom Management. 2004;28:59–71. doi: 10.1016/j.jpainsymman.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Caldwell B, Aldington S, Weatherall M, et al. Risk of cardiovascular events and celecoxib: a systematic review and meta-analysis. J R Soc Med. 2006;99:132–40. doi: 10.1258/jrsm.99.3.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [CDC] Centers for Disease Control and Prevention. Prevalence of disabilities and associated health conditions among adults–United States, 1999. MMWR Morb Mortal Wkly Rep. 2001;50:120–5. [PubMed] [Google Scholar]
- [CDC] Centers for Disease Control and Prevention. Prevalence of self-reported arthritis or chronic joint symptoms among adults–United States, 2001. MMWR Morb Mortal Wkly Rep. 2002;51:948–50. [PubMed] [Google Scholar]
- Cherny NI, Portenoy RK. The management of cancer pain. CA Cancer J Clin. 1994;44:263–303. doi: 10.3322/canjclin.44.5.263. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Inciardi JA, Adams EH, et al. Rates of abuse of tramadol remain unchanged with the introduction of new branded and generic products: results of an abuse monitoring system, 1994–2004. Pharmacoepidemiol Drug Saf. 2005;14:851–9. doi: 10.1002/pds.1113. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Inciardi JA, Munoz A. Trends in abuse of Oxycontin and other opioid analgesics in the United States: 2002–2004. J Pain. 2005;6:662–72. doi: 10.1016/j.jpain.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Cohen JM, Menefee LA, Doghramji K, et al. Sleep in chronic pain: problems and treatments. Int Rev Psych. 2000;12:115–26. [Google Scholar]
- Desmeules JA, Piguet V, Collart L, et al. Contribution of monoaminergic modulation to the analgesic effect of tramadol. Br J Clinical Pharmacol. 1996;41:7–12. doi: 10.1111/j.1365-2125.1996.tb00152.x. [DOI] [PubMed] [Google Scholar]
- Eisendrath SJ. Psychiatric aspects of chronic pain. Neurology. 1995;45(suppl 9):S26–34. doi: 10.1212/wnl.45.12_suppl_9.s26. [DOI] [PubMed] [Google Scholar]
- Garcia-Quetglas E, Azanza JR, Sadaba B, et al. Pharmacokinetics of tramadol enantiomers and their respective phase I metabolites in relation to CYP2D6 phenotype. Pharmacol Res. 2007;55:122–30. doi: 10.1016/j.phrs.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Glajchen M. Chronic pain: treatment barriers and strategies for clinical practice. J Am Board Fam Pract. 2001;14:211–18. [PubMed] [Google Scholar]
- Gough AC, Miles JS, Spurr NK, et al. Identification of the primary gene defect at the cytochrome P450 CYP2D locus. Nature. 1990;347:773–6. doi: 10.1038/347773a0. [DOI] [PubMed] [Google Scholar]
- Hennies HH, Friderichs E, Schneider J. Receptor binding, analgesic and antitussive potency of tramadol and other selective opioids. Arzneimittlforschung. 1988;38:877–80. [PubMed] [Google Scholar]
- Hennies HH, Friderichs E, Wilsmann K, et al. Effect of the opioid analgesic tramadol on inactivation of norepinephrine and serotonin. Biochem Pharmacol. 1982;31:1654–5. doi: 10.1016/0006-2952(82)90398-7. [DOI] [PubMed] [Google Scholar]
- Hopman-Rock M, Kraaimaat FW, Bijlsma JW. Quality of life in elderly subjects with pain in the hip or knee. Qual Life Res. 1997;6:67–6. doi: 10.1023/a:1026421629416. [DOI] [PubMed] [Google Scholar]
- Hudson M, Richard H, Pilote L. Differences in outcomes of patients with congestive heart failure prescribed celecoxib, rofecoxib, or non-steroidal anti-inflammatory drugs: population based study. BMJ. 2005;330:1370–6. doi: 10.1136/bmj.330.7504.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [JCAHO] Joint Commission on Accreditation of Healthcare Organizations. Pain: current understanding of assessment, management, and treatments [online] 2001 Accessed 3 October 2006. URL: http://www.npcnow.org/resources/PDFs/painmonograph.pdf.
- Kayser V, Besson JM, Guilbaud G. Effects of the analgesic agent tramadol in normal and arthritic rats: comparison with the effects of different opioids, including tolerance and cross-tolerance to morphine. Eur J Pharmacol. 1991;195:37–45. doi: 10.1016/0014-2999(91)90379-5. [DOI] [PubMed] [Google Scholar]
- Kearney PM, Baigent C, Halls H, et al. Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. BMJ. 2006;332:1302–8. doi: 10.1136/bmj.332.7553.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]; Laborde JM, Powers MJ. Satisfaction with life for patients undergoing hemodialysis and patients suffering from osteoarthritis. Res Nurs Health. 1980;3:19–24. doi: 10.1002/nur.4770030105. [DOI] [PubMed] [Google Scholar]
- Leigh TJ, Hindmarch I, Bird HA, et al. Comparison of sleep in osteoarthritic patients and age and sex matched healthy controls. Ann Rheum Dis. 1988;47:40–2. doi: 10.1136/ard.47.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattia C, Coluzzi F. Tramadol. Focus on musculoskeletal and neuropathic pain. Minerva Anestesiol. 2005;71:565–84. [PubMed] [Google Scholar]
- Mattia C, Vanderah T, Raffa RB, et al. Characterization of the unusual antinociceptive profile of tramadol in mice. Drug Dev Res. 1993;31:1932–43. [Google Scholar]
- Menefee LA, Cohen MJ, Anderson WR, et al. Sleep disturbance and nonmalignant chronic pain: a comprehensive review of the literature. Pain Med. 2000;1:156–72. doi: 10.1046/j.1526-4637.2000.00022.x. [DOI] [PubMed] [Google Scholar]
- Menefee LA, Frank ED, Doghramji K, et al. Self-reported sleep quality and quality of life for individuals with chronic pain conditions. Clin J Pain. 2000;16:290–7. doi: 10.1097/00002508-200012000-00003. [DOI] [PubMed] [Google Scholar]; Page J, Henry D. Consumption of NSAIDs and the development of congestive heart failure in elderly patients: an underrecognized public health problem. Arch Intern Med. 2000;160:777–84. doi: 10.1001/archinte.160.6.777. [DOI] [PubMed] [Google Scholar]
- Parrott T. Using opioid analgesics to manage chronic noncancer pain in primary care. J Am Board Fam Pract. 1999;12:293–306. doi: 10.3122/jabfm.12.4.293. [DOI] [PubMed] [Google Scholar]
- Raffa RB, Friderichs E, Reimann W, et al. Opioid and nonopioid components independently contribute to the mechanism of action of tramadol, an‘atypical’opioid analgesic. J Pharmacol Exp Ther. 1992;260:275–85. [PubMed] [Google Scholar]
- Raffa RB, Nayak RK, Liao S, et al. The mechanism of action and pharmacokinetics of tramadol hydrochloride. Rev Cont Pharmacol. 1995;6:485–98. [Google Scholar]
- Schnitzer TJ, Kamin M, Olson WH. Tramadol allows reduction of naproxen dose among patients with naproxen-responsive osteoarthritis pain: a randomized, double-blind, placebo-controlled study. Arthritis Rheum. 1999;42:1370–7. doi: 10.1002/1529-0131(199907)42:7<1370::AID-ANR10>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Stephens J, Laskin B, Pashos C, et al. The burden of acute postoperative pain and the potential role of the COX-2-specific inhibitors. Rheumatology (Oxford) 2003;42(suppl 3):iii40–52. doi: 10.1093/rheumatology/keg497. [DOI] [PubMed] [Google Scholar]
- Stewart WF, Ricci JA, Chee E, et al. Lost productive time and cost due to common pain conditions in the US workforce. JAMA. 2003;290:2443–54. doi: 10.1001/jama.290.18.2443. [DOI] [PubMed] [Google Scholar]
- Turner JA, Deyo RA, Loeser JD, et al. The importance of placebo effects in pain treatment and research. JAMA. 1994;271:1609–14. [PubMed] [Google Scholar]
- Ultram PI. Immediate-release package insert. Raritan, NJ: Ortho-McNeil Inc; 2004. [Google Scholar]
- Ultram PI. Extended-release package insert. Raritan, NJ: Ortho-McNeil Inc; 2006. [Google Scholar]
- Weinstein SM, Laux LF, Thornby JI, et al. Physicians’ attitudes towards pain and the use of opioid analgesics: results of a survey from the Texas Cancer Pain Initiative. South Med J. 2000;93:479–87. [PubMed] [Google Scholar]
- Yelin E, Callahan LF for National Arthritis Data Work Groups. The economic cost and social and psychological impact of musculoskeletal conditions. Arthritis Rheum. 1995;38:1351–62. doi: 10.1002/art.1780381002. [DOI] [PubMed] [Google Scholar]


