Abstract
Antithrombotics have been shown to decrease the risk of stroke in patients with atrial fibrillation (AF). However they are associated with an increased risk of bleeding. We assessed the frequency and appropriateness of antithrombotic therapy in patients admitted to our service with stroke and AF. A retrospective case study of 219 patients (mean age 77.2 years) admitted between January 1999 and 31 December 2001 with a diagnosis of stroke and AF was done. Patient characteristics, presence of comorbid conditions, knowledge of preadmission AF, medication history and appropriateness of antithrombotic treatment were recorded. One hundred and fifty patients were known to have had AF prior to admission. Forty-one presented with an intracranial hemorrhage (19 on warfarin, 10 on aspirin). Of those patients with known AF only 43 were on treatment consistent with the guidelines. Warfarin was recommended in 144 of the whole cohort, but only 39 were taking it. Fifty-three patients were receiving aspirin although warfarin was the recommended treatment. Fifty-four with known AF were not on any antithrombotic treatment. Factors significantly associated with the use of antithrombotic treatment were history of AF (p = 0.0004), valvular heart disease (p = 0.02), venous thromboembolism (p = 0.04), risk of thromboembolism (p = 0.003) and presentation with a nonischemic infarct (p = 0.008). Antithrombotic therapy use in our patients differs significantly from guideline recommendations.
Keywords: aspirin, warfarin, atrial fibrillation
Introduction
Atrial fibrillation (AF) is associated with an increased risk of stroke (Atrial Fibrillation Investigators 1994). The risk is increased in patients who have had a previous stroke or transient ischemic attack (TIA), are hypertensive, have congestive cardiac failure, diabetes or are older than 65 years (Laupacis et al 1998; Gage et al 2001). A number of studies have shown that antithrombotic therapy with either warfarin or aspirin is successful in reducing the risk of stroke (Petersen et al 1989; Stroke Prevention in Atrial Fibrillation Investigators 1991; Atrial Fibrillation Investigators 1994; Hart et al 1999; Lip and Boos 2006). Most comparative studies favor warfarin, but major bleeding events are usually more frequent with warfarin (Morocutti et al 1997; Hart and Halperin 1999; Taylor et al 2001). However it should be noted that a recent report from DiMarco and colleagues (2005) has shown similar bleeding risks with either warfarin or aspirin. Warfarin is recommended as first line treatment (unless contraindicated) followed by aspirin in patients with AF who are at high risk of stroke (Laupacis et al 1998; Hart and Halperin 1999). Yet the uptake of warfarin is not as great as one would predict from population studies largely because it is perceived as carrying a high risk of bleeding, requires ongoing monitoring and the elderly (who are most likely to benefit from warfarin) have other risk factors such as increased risk of falls or dementia that place them at risk of bleeding complications (Sudlow et al 1998; Bungard et al 2000; Go et al 2003; Laguna et al 2004). Furthermore, in clinical trials anticoagulation is carefully monitored in highly motivated patients, such accuracy might be difficult to achieve in clinical practice.
The subtype of stroke is a further factor to consider when deciding on which agent to employ in at risk patients. Not all ischemic strokes that occur in patients with AF are cardioembolic. It has been estimated that one third of strokes occurring in patients are due to small vessel disease or have an atherosclerotic basis (Evans et al 2000; Hart et al 2000). In these patients antiplatelet therapy may be preferred as there is no difference in mortality or stroke recurrence whether these patients are treated with warfarin or aspirin (Evans et al 2001). In addition it is well known that there is a higher prevalence of AF in older individuals and increasing age is also associated with an increased prevalence of small vessel disease (Atrial Fibrillation Investigators 1994). With an increasing aging population these are necessary factors to consider before commencing therapy. In this study we assessed the frequency and appropriateness of antithrombotic therapy in patients who were admitted to our service with stroke and AF. We decided to include patients who had either ischemic or hemorrhagic strokes as this represents true clinical practice. Unlike the randomized clinical trials, we did not exclude patients in whom warfarin (for example venous thromboembolic disease or valvular heart disease) or aspirin (ischemic heart disease) was clearly indicated.
Methods
The study was a retrospective case study of patients who were admitted to Capital and Coast District Health Board hospitals with a diagnosis of stroke with documented AF on admission. The sample was recruited by identifying all adult admissions between 1 January 1999 to 31 December 2001 with a primary or secondary diagnosis of any form of an intracranial vascular event using the relevant ICD-10 codes, these being I600–I679 or G450–G468 (International Statistical Classification of Diseases 2000). Each record was examined and patients were included if the admission met the WHO definition for cerebrovascular accident (CVA) or TIA (Hatano 1976). There also had to be ECG evidence of AF. In addition we extracted the following information from the case record: the basic demographic details, presence of co-morbid conditions, history of the presentation including the occurrence of preadmission AF, medication history on admission, initial examination findings, functional assessment using the Barthel score (Tennant et al 1996) within the first week after admission. Where it was not available the investigators derived a functional grade. This was completed from the patients’ functional level as documented in the case notes from physiotherapy, occupational therapy and nursing assessments. Functional grades of low, medium, high and normal were assessed to correspond to a Barthel score of 0–4, 5–9, 10–19 and 20 respectively. Where available transthoracic echocardiographic findings were noted.
Radiological classification
All CT scans and/or MRI imaging was independently reviewed by an investigator (MN) who was provided with a précis of the relevant clinical features of the presentation. The investigator was blinded to the original radiological report and the antithrombotic treatment status of the patient. The CT scans were classified initially as to whether there was intraparenchymal or extra-axial hemorrhage. Thereafter the lesions were coded according to a modified TOAST classification using a 2 cm cut point (for subcortical and brainstem) instead of 1.5 cm to permit the use of the Miller subtype scheme (Adams Jr et al 1993; Miller et al 1993).
Subtype of ischemic stroke
The classification of the subtype of ischemic stroke was based on the methodology of Miller and colleagues (1993), but was modified as angiography was only very rarely performed in our hospitals. The patients were classified into one of the following six groups: cardioembolic (definite), cardioembolic (probable), lacunar, atherothrombotic, ischemic (miscellaneous) or ischemic (uncertain). Two investigators (CB and TI) independently placed each stroke into one of the above categories. If there was a difference of opinion, the particular case was discussed and a consensus agreement was reached.
Questionnaire
A questionnaire was sent to the primary care physician of each patient requesting information on whether the diagnosis of AF was known prior to the admission and if so whether the patient was on treatment for the same. If not a reason for not using either agent was requested. We also requested information on the preadmission functional status of the patient, prior history of a CVA or TIA or both and history of the relevant comorbidity.
Guideline recommended therapy
Each patient was assigned a guideline recommended therapy of ‘warfarin’, ’aspirin’ or ‘either,’ according to the recommendations of Laupacis et al 1998. This is based on categorization of age (<65 years, 65–75 years, >75 years) and the presence or absence of additional risk factors for stroke (TIA, systemic embolus or stroke, hypertension, poor left ventricular function, rheumatic mitral valve disease or prosthetic heart valve). These guidelines, published in 1998, were available prior to the start of the study period and hence represent best practice at the time. These guidelines are very similar to the CHADS2 Index (Gage et al 2001) that is now more frequently used to assess stroke risk. They differ in the age criterion − >75 years in the CHADS2 index and an additional point for patients who have had a previous stroke or TIA. As 144 of our patients were 75 years or older it is unlikely that the stroke risks score would have changed significantly.
Statistical methods
Simple univariate measures of association and t-tests were used to test the association between potential risk factors for the use versus nonuse of any antithrombotic (aspirin or warfarin), and warfarin use versus aspirin or no antithrombotic therapy.
Logistic regression, using a variety of model selection procedures (backwards selection, and stepwise selection), was used to try and establish independent risk factors for antithrombotic use.
Two derived variables were used in the model building process. One was a variable that counted up the number of risk factors for bleeding (a history of prior bleeding, peptic ulcer disease, cognitive impairment, hepatic disease, alcohol abuse and the risk of falling), with possible scores between 0 and 6. The other was a variable that counted up the number of risk factors for stroke in the presence of AF (hypertension, diabetes mellitus, a history of prior stroke or TIA, congestive heart failure and age over 65 years), with possible scores between 0 and 5. The technique appropriate to ordinal variables, the Wilcoxon test, was also used for these derived variables. Log-normally distributed continuous data (INR) are presented as geometric means with geometric confidence intervals and t-tests. SAS version 8.2 (SAS Institute, Cary NC, 2001) was used for the analysis.
The project was approved by the Wellington Regional Ethics Committee.
Results
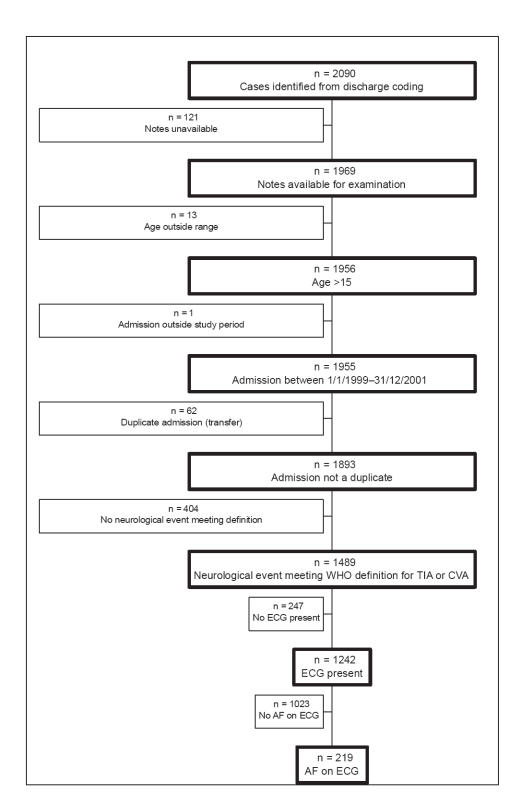
During the period of the audit 2090 patients were identified from discharge coding. Of these 219 met the criteria for the study (Figure 1). One hundred and fifty of these were known to have had AF prior to admission.
Figure 1.
Inclusion criteria flow chart
The characteristics of the patients are shown in Table 1. The mean age of our patients was 77.2 years, 144 of whom were 75 years or older. The risk factors for stroke or bleeding are also shown in Table 1. The mean stroke risk score per individual was 2.19, whereas the mean bleeding risk score was 0.43 per individual.
Table 1.
Patient characteristics
| No. of Patients (%) | ||
|---|---|---|
| Demographics | ||
| Gender: Male: Female | 89:130 | (40.6):(59.4) |
| Age: Mean (±SD) | 77.2 years | ±11.9 |
| Age: Median (Range) | 79.6 years | (31–96 years) |
| Ethnicity: European | 177 | (82.7) |
| Smoker: Current or ex-smoker | 80 | (36.5) |
| Risk factors for stroke | ||
| Age >65 years | 187 | (85.4) |
| Prior stroke or TIA | 91 | (41.6) |
| Congestive heart failure | 55 | (25.1) |
| Hypertension | 107 | (48.9) |
| Diabetes mellitus | 35 | (16.0) |
| Stroke Risk Score (mean ± SD) | 2.19 | ±1.09 |
| Risk factors for bleeding | ||
| Prior bleeding | 12 | (5.5) |
| Peptic ulcer disease | 17 | (7.8) |
| Cognitive impairment | 33 | (15.1) |
| Hepatic disease | 1 | (0.5) |
| Alcohol abuse | 7 | (3.2) |
| Documented risk of falling | 24 | (11.0) |
| Bleeding Risk Score (mean ± SD) | 0.43 | ±0.69 |
Forty-one patients were diagnosed with an intracranial hemorrhage, 19 (46.3%) of these were taking warfarin and 10 (24.4%) aspirin on admission. According to recommended guidelines all bar one of these patients would have been recommended to have warfarin (Laupacis et al 1998). There were no differences in the INR levels between patients with hemorrhagic strokes or ischemic infarcts (geometric mean INR 1.90 and 2.06 respectively, p = 0.57).
The subtypes of stroke are shown in Table 2. One hundred and forty three of 177 ischemic events (80.8%) were categorized as either definite or probable cardioembolic with the rest being lacunar or ischemic (uncertain). Seventy-one of the 143 patients with a definite or possible cardioembolic stroke were on an antithrombotic agent (warfarin, 17 patients (11.9%) or aspirin, 54 patients (37.8%)). Twelve of the 23 patients with lacunar strokes were on either warfarin (4 patients, 17.4%) or aspirin (8 patients, 34.8%).
Table 2.
Stroke details
| Variable | No. of Patients (%) | |
|---|---|---|
| Characteristics of stroke | ||
| Ischemic stroke | 177 | (80.8) |
| Nonischemic | 41 | (18.7) |
| Nonvascular | 1 | (0.5) |
| Ischemic stroke subtype (% of Ischemic) | ||
| Cardioembolic-definite | 104 | (58.8) |
| Cardioembolic-probable | 39 | (22.0) |
| Lacunar | 23 | (13.0) |
| Atherothrombotic | 0 | (0.0) |
| Ischemic uncertain | 11 | (6.2) |
Table 3 shows the actual and recommended treatments for the whole cohort and for those whose AF had been diagnosed prior to the present admission.
Table 3.
Antithrombotic treatment versus guideline recommended therapy
| Actual treatment | Guideline recommendations a | ||||
|---|---|---|---|---|---|
| Warfarin | Warfarin OR Aspirin | Aspirin | Neither | Total | |
| All subjects (n = 219) | |||||
| All Subjects | |||||
| Warfarin AND Aspirin | 3 | 0 | 0 | 0 | 3 |
| Warfarin | 39 | 1 | 0 | 0 | 40 |
| Aspirin | 71 | 3 | 1 | 0 | 75 |
| Neither | 91 | 4 | 6 | 0 | 101 |
| Total | 204 | 8 | 7 | 0 | 219 |
| Subjects with known atrial fibrillation (n = 150) | |||||
| Subjects with known AF | |||||
| Warfarin AND Aspirin | 2 | 0 | 0 | 0 | 2 |
| Warfarin | 37 | 1 | 0 | 0 | 38 |
| Aspirin | 53 | 2 | 1 | 0 | 56 |
| Neither | 52 | 0 | 2 | 0 | 54 |
| Total | 144 | 3 | 3 | 0 | 150 |
Notes: Based on the guidelines presented by Laupacis et al 1998.
Of the 150 patients with known AF, only 43 (28.7%) were on treatment consistent with the guidelines. In 144 of the 219 patients warfarin was the recommended agent, yet only 39 patients (27.1%) were taking it. A further 53 patients (36.8%) for whom warfarin was recommended were receiving aspirin. Among all those patients with known AF, 54 patients (36%) were not on any antithrombotic treatment. For the whole cohort a similar percentage (42%) was not taking any antithrombotic treatment.
The factors associated with a greater or lesser likelihood of using antithrombotic treatment are shown in Table 4. In addition patients who had had a previous CVA or TIA or had a history of ischemic heart disease were more likely to be using some form of antithrombotic treatment. Factors that were associated with warfarin use were a history of venous thromboembolism, a history of AF prior to admission, nonischemic (hemorrhagic stroke syndrome) and older age (Table 5).
Table 4.
Factors associated with the use or nonuse of antithrombotic drugsa
| Variable | Odds ratios (95% CI)b | p value | |
|---|---|---|---|
| History of AF | 3.4 | (1.74–6.65) | 0.0004 |
| History of valvular heart disease | 15.1 | (1.62–141.1) | 0.02 |
| History of venous thrombo-embolism | 4.2 | (1.07–16.4) | 0.04 |
| Risk of bleeding scorec | 0.68 | (0.44–1.05) | 0.08 |
| Risk of thrombo-embolism scorec | 1.58 | (1.16–2.13) | 0.003 |
| Ischemic infarctd | 0.32 | (0.14–0.74) | 0.008 |
Note: Multivariate model
Odds ratio >1, more likely to be using any antithrobotic
per increase in one risk factor
During index admission
Table 5.
Factors associated with the use or nonuse of warfarina
| Variable | Odds ratios (95% CI)b | P value | |
|---|---|---|---|
| History of AF | 16.7 | (4.0–69.7) | 0.0001 |
| History of venous thrombo-embolism | 14.1 | (3.6–54.7) | 0.0001 |
| Agec | 0.96 | (0.93–0.99) | 0.083 |
| Ischemic infarctd | 0.13 | (0.05–0.32) | <0.0001 |
Note: Multivariate model. Warfarin versus aspirin alone or no-antithrombotic
Odds ratio >1, more likely to be using any antithrombotic
per year older
During index admission.
Sixty-seven patients had an echocardiogram performed. The mean (SD) left ventricular ejection fraction was 56.9 (13.8)%; left atrial size 46.6 (10.8 mm) and fractional shortening was 31.2 (8.7)%. Left atrial size was significantly larger among those on antithrombotic agents compared with those not on any antithrombotics (49.9 mm versus 43.6 mm respectively, p = 0.01). No significant differences were observed between LV ejection fraction (p = 0.97) or fractional shortening (p = 0.74).
A bleeding history and a history of peptic ulcer disease were associated with a decreased use of any antithrombotic history (p = 0.04).
Discussion
Our study has shown in an unselected group of patients admitted with a stroke syndrome and AF that antithrombotic treatments with either anticoagulants or antiplatelet agents are underused according to contemporary recommendations (Petersen et al 1989; Atrial Fibrillation Investigators 1994; Stroke Prevention in Atrial Fibrillation (SPAF) Investigators 1991; EAFT Study Group 1993; Laupacis et al 1998; Go et al 2003; Hylek et al 2003; Gage et al 2004; Perez-Gomez et al 2004). Under use was particularly marked with warfarin where only 43 patients (19.6%) were taking the drug prior to admission. According to the guidelines available at the time of the study it would have been indicated in 144 patients. Low uptake has previously been described in the primary care setting where Sudlow and colleagues (1998), noted that only 23% of patients with AF were using anticoagulants. Two more recent studies from the USA (Go et al 2003; Wang et al 2003) showed differing uptake of anticoagulation after AF was diagnosed. Go and colleagues (2003) noted that nearly 55% of their cohort with nonvalvular AF and in whom there was no contraindication to warfarin were prescribed this drug whereas Wang et al 2003 noted in a community based survey that 705 of 861 patients with AF were not treated with warfarin, however it is not clear from this study how many of the patients had contraindications to anticoagulation. Wang and colleagues (2003) point out that using their risk score, stroke rates in patients with low scores (45% of their cohort) were of the order of 1.1 to 1.5 strokes per 100 person years which would suggest that this may be near the threshold for the use of anticoagulants. Our patients seem to have had more severe disease than those of Wang and colleagues (2003) as most of them had co-morbidities that would have placed them in a moderate to high-risk stroke group.
Overall, the uptake of warfarin in patients with nonvalvular AF has remained a problem despite adequate publicity of its effectiveness with quoted prescription rates of 15%–44% (Bungard et al 2000; Iqbal et al 2005). The reasons provided for not using warfarin by the primary care physicians in our study were similar to those enunciated by Bungard and colleagues (2000), in that they included past history of bleeding, noncompliance, cognitive difficulties, frailty and patient choice. Although not mentioned by our practitioners we think that lack of a monitoring centre for anticoagulation probably also played a role in the nonprescription of this drug. Use of warfarin is time consuming for a general practitioner and we think this may have proved a barrier for our prescribers.
Even where anticoagulants are prescribed there might be significant drop out rate. Evans and colleagues (2000), noted in a sample of 288 patients that 74 stopped taking warfarin, 25 because of bleeding complications and 37 for reasons such as choice, compliance or logistics. Similar findings were reported from the much larger AFFIRM Study where 33.6% of the trial participants discontinued warfarin use for some period during the trial. The major reason for withdrawal was maintenance of sinus rhythm, but bleeding was the second most common reason for stopping warfarin (DiMarco et al 2005).
Efforts suggested to improve the use and compliance with warfarin have included home follow-up following initiation of warfarin in hospital. Jackson and colleagues (2004) compared usual care (namely, managed totally by the general practitioner) with a project pharmacist who visited patients at home and adjusted the dose of warfarin accordingly. Using this programme the pharmacist arm of the study achieved a significantly greater proportion of patients with a therapeutic INR early in treatment with less bleeding events as well. A pharmacist led programme is attractive because they are familiar with drug interactions and the difficulties with using warfarin. They are also likely to maintain the INR in the therapeutic range. This will decrease the risk of bleeding and also decrease the risk of stroke as patients who are under anticoagulated have a similar risk of stroke as those who are treated with placebo. Funding for such an arrangement might prove difficult in New Zealand where it would have to come from the local District Health Board. If this were not forthcoming it would be difficult to see where the salaries for the pharmacists would be found.
Other options to make warfarin use more attractive relate to self-monitoring of the INR in a similar fashion as diabetics monitor blood glucose. Two recent studies have shown markedly different outcomes. Gardiner and colleagues (2004) showed that self-testing in 84 patients from an anticoagulation clinic was effective and acceptable. However the trial was open and not controlled. It involved 84 patients who were self selected from 800 who were invited to take part. The average age of the trialists was less than the average age of other patients in the anticoagulation clinic. The second study reported by Murray and colleagues (2004) was a randomized controlled trial involving 608 (of 2586 invited) patients. Three hundred and twenty seven patients were randomized to self-management, but of these 26% were unable to complete training. Factors that were associated with success in training and continuing in the trial were younger age and more education. The authors point out that 76% of the original cohort chose not to undertake self testing and their recommendations were that standardization and dissemination of training would be needed with adequate backup from clinicians if this method was to be successful. In summary some form of central control of anticoagulation seems to be the best method to increase the use of warfarin.
Our study also demonstrated potential problems with the use of antithrombotics. Forty-one of our 219 patients (18.7%) presented with hemorrhage. Nineteen of these were extra-axial (9 patients were taking warfarin, 4 aspirin and 6 neither), the rest were intracerebral (10 patients on warfarin, 5 on aspirin and 6 neither). Although one cannot be sure that the drugs were responsible for the hemorrhage they do seem to be over represented. These findings are of importance and confirm the results of others who have noted that warfarin is associated with a significantly increased risk of bleeding when compared to aspirin or placebo (Taylor et al 2001; Go et al 2003; DiMarco et al 2005). This risk needs to be balanced against the risk of stroke in patients with nonvalvular AF.
Although the potential benefits of warfarin therapy in high-risk populations have been clearly demonstrated (Lip and Boos 2006) the decision to prescribe any agent has to be balanced against the risk (or “perceived risks”) from therapy. These risks are often difficult to quantify in the individual patient and older patients who are likely to gain most benefit are often considered to be at higher risk. Our data suggests that often where warfarin therapy was recommended aspirin was substituted. Aspirin is significantly easier to prescribe as it does not require blood test monitoring, is used in a fixed dose and is considered safer than warfarin in reqard to bleeding complications, despite bleeding risks with either treatment being small (Koudstaal 2000). Furthermore, a significant percentage (28.3%) of our patients may have been on aspirin for ischemic heart disease (IHD), in such patients aspirin would be indicated and our prescribers may not have wished to add warfarin to aspirin for fear of an increased bleeding risk. Yet, as mentioned earlier warfarin is superior to aspirin in preventing stroke in patients with AF.
Investigators have assessed a combination of warfarin and aspirin using a subtherapeutic INR of 1.5–1.9. Such trials have not proved successful in preventing stroke in patients with AF (SPAF III Investigators 1996; Gullov et al 1998; Edvardson et al 2003). A more recent study compared a combination of trifilusal (a cyclooxygenase inhibitor) and therapeutic warfarin against therapeutic warfarin in high-risk patients with AF and in a second group compared trifilusal alone versus a combination of trifilusal and warfarin (INR tritrated to 1.25 to 2) in patients with an intermediate risk of stroke (Perez-Gomez et al 2004). The results showed added benefit in stroke prevention with the combinations with no increase in bleeding risk, but the patients were very closely monitored and the median INR in the intermediate group was 1.93 and 2.17 in the high-risk group respectively. The result particularly with the intermediate risk group would suggest that the INR should be at a level near to 2 to gain any benefit. Therefore this might be an option when using warfarin particularly in intermediate risk patients. We had a small number of patients with lacunar infarcts, this may represent the assessment system that we used, but the placement in this group depended on the clinical picture and the CT scan report. Approximately 50% of these patients were on some form of antithrombotic treatment. Of the 12 patients, 8 were taking aspirin and 4 warfarin. Aspirin may be the preferred agent in these patients as it is equally effective and is safer than warfarin (Evans et al 2001). Although aspirin is recommended in patients with either contraindications to warfarin or small vessel disease it should be noted that for many of our patients this was the first presentation of cerebrovascular disease and thus without prior imaging it would potentially be difficult to know which patients would be suitable for aspirin.
In conclusion, we have shown that in patients presenting to hospital with stroke syndromes and atrial fibrillation, antithrombotic treatment is underused and differs significantly from guidelines. We have suggested changes to correct practice that should make warfarin more frequently used. We believe an anticoagulation service with significant pharmacist input would offer the best solution.
References
- Adams HP, Jr, Bendixen BH, Kappelle J, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- Atrial Fibrillation Investigators. Risk factors for stroke and efficacy of anti-thrombotic therapy in atrial fibrillation: analysis of pooled data from five randomized controlled trials. Am Intern Med. 1994;154:1449–57. [PubMed] [Google Scholar]
- Bungard TJ, Ghali WA, Teo KT, et al. Why do patients with atrial fibrillation not receive warfarin? Am Intern Med. 2000;160:41–6. doi: 10.1001/archinte.160.1.41. [DOI] [PubMed] [Google Scholar]
- DiMarco JP, Flaker G, Waldo AL, et al. Factors affecting bleeding risk during anticoagulant therapy in patients with atrial fibrillation: Observations from the atrial fibrillation follow-up investigation of rhythm management (AFFIRM) Study. Am Heart J. 2005;149:650–6. doi: 10.1016/j.ahj.2004.11.015. [DOI] [PubMed] [Google Scholar]
- [EAFT] European Atrial Fibrillation Trial Study Group. Secondary prevention in non-rheumatic atrial fibrillation after transient ischemic attack or minor stroke. Lancet. 1993;342:1255–62. [PubMed] [Google Scholar]
- Evans A, Perez I, Yu G, et al. Secondary stroke prevention in atrial fibrillation: lessons from clinical practice. Stroke. 2000;32:2106–11. doi: 10.1161/01.str.31.9.2106. [DOI] [PubMed] [Google Scholar]
- Evans A, Perez I, Yu G, et al. Should stroke subtype influence anticoagulation decisions to prevent recurrence in stroke patients with atrial fibrillation. Stroke. 2001;32:2828–32. doi: 10.1161/hs1201.099520. [DOI] [PubMed] [Google Scholar]
- Gage BF, Boechler M, Doggette AL, et al. Adverse outcomes and predictors of underuse of antithrombotic therapy in medicare beneficiaries with chronic atrial fibrillation. Stroke. 2000;31:822–7. doi: 10.1161/01.str.31.4.822. [DOI] [PubMed] [Google Scholar]
- Gage BF, van Walraven C, Pearce L, et al. Selecting patient with atrial fibrillation for anticoagulation. Stroke risk stratification in patients taking aspirin. Circulation. 2004;110:2287–92. doi: 10.1161/01.CIR.0000145172.55640.93. [DOI] [PubMed] [Google Scholar]
- Gage BF, Waterman AD, Shannen W, et al. Validation of clinical classification schemes for predicting stroke. Researchers from the National registry of atrial fibrillation. JAMA. 2001;285:2864–70. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- Gardiner C, Williams K, Mackie IJ, et al. Patient self-testing is a reliable and acceptable alternative to laboratory INR monitoring. Brit J Hematology. 2004;128:242–7. doi: 10.1111/j.1365-2141.2004.05300.x. [DOI] [PubMed] [Google Scholar]
- Go AS, Hylek EM, Chang Y, et al. Anticoagulation therapy for stroke prevention in atrial fibrillation. How well do randomized trials translate into clinical practice? JAMA. 2003;290:2685–92. doi: 10.1001/jama.290.20.2685. [DOI] [PubMed] [Google Scholar]
- Gullov AL, Koefoed BG, Petersen P, et al. Fixed mini dose warfarin and aspirin alone and in combination versus adjusted-dose warfarin for stroke prevention in atrial fibrillation; 2nd Copenhagen Atrial Fibrillation, Aspirin and Anticoagulation Study. Arch Intern Med. 1998;158:1513–21. doi: 10.1001/archinte.158.14.1513. [DOI] [PubMed] [Google Scholar]
- Hart RG, Halperin JL. Atrial fibrillation and thromboembolism: a decade of progress in stroke prevention. Am Intern Med. 1999;131:688–95. doi: 10.7326/0003-4819-131-9-199911020-00010. [DOI] [PubMed] [Google Scholar]
- Hart RG, Pearce LA, McBride R, et al. Factors associated with ischemic stroke during aspirin therapy in atrial fibrillation: analysis of 2012 participants in the SPAF I-III clinical trials. Stroke. 1999;30:1223–29. doi: 10.1161/01.str.30.6.1223. [DOI] [PubMed] [Google Scholar]
- Hart RG, Pearce LA, Miller VJ, et al. Cardioembolic vs. noncardioembolic strokes in atrial fibrillation: frequency and effect of antithrombotic agents in the Stroke Prevention in Atrial Fibrillation Studies. Cerebrovasc Dis. 2000;10:39–43. doi: 10.1159/000016023. [DOI] [PubMed] [Google Scholar]
- Hatano S. Experience from a multicenter stroke register: a preliminary report. Bull WHO. 1976;54:541–53. [PMC free article] [PubMed] [Google Scholar]
- Hylek EM, Go AS, Chang Y, et al. Effect of intensity of all anticoagulation on stroke severity and mortality in atrial fibrillation. N Engl J Med. 2003;349:1019–26. doi: 10.1056/NEJMoa022913. [DOI] [PubMed] [Google Scholar]
- International Statistical Classification of Diseases. Related health problems. 2000 10th Review, Australian modification. Vol.1–5. Copyright of Commonwealth of Australia. [Google Scholar]
- Iqbal MB, Taneja AK, Lip GYH, et al. Recent developments in atrial fibrillation. BMJ. 2005;330:238–43. doi: 10.1136/bmj.330.7485.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson SL, Peterson GM, Vial, Jupe DML. Improving the outcomes of anticoagulation: An evaluation of home follow-up of warfarin initiation. J Int Med. 2004;256:137–44. doi: 10.1111/j.1365-2796.2004.01352.x. [DOI] [PubMed] [Google Scholar]
- Koudstraal PJ. Anticoagulants versus antiplatelet therapy for preventing stroke in patients with nonrheumatic atrial fibrillation and a history of stroke or transient ischemic attacks. Cochrane Database Syst Rev 2. 2000:CD000187. doi: 10.1002/14651858.CD000187. [DOI] [PubMed] [Google Scholar]
- Laguna P, Martin A, del Arco C, et al. Risk factors for stroke and thromboprophylaxis in atrial fibrillation: what happens in daily clinical practice? The GEFAUR-1 Study. Ann Emerg Med. 2004;44:3–11. doi: 10.1016/S0196064404000587. [DOI] [PubMed] [Google Scholar]
- Laupacis A, Albers G, Dalen J, et al. Antithrombotic therapy in atrial fibrillation. Chest. 1998;114(suppl):5795–895. doi: 10.1378/chest.114.5_supplement.579s. [DOI] [PubMed] [Google Scholar]
- Lip GYH, Boos CJ. Antithrombotic treatment in atrial fibrillation. Heart. 2006;92:155–61. doi: 10.1136/hrt.2005.066944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller VT, Rothrock JF, Pearce LA, et al. Ischemic stroke in patients with atrial fibrillation. Effect of aspirin according to stroke mechanism. Neurology. 1993;43:32–6. doi: 10.1212/wnl.43.1_part_1.32. [DOI] [PubMed] [Google Scholar]
- Morocutti C, Amabile G, Fattapposta F, et al. Indobufen versus warfarin in the secondary prevention of major vascular events in non-rheumatic atrial fibrillation. Stroke. 1997;28:1015–21. doi: 10.1161/01.str.28.5.1015. [DOI] [PubMed] [Google Scholar]
- Murray E, Fitzmaurice D, McCahon D, et al. Training for patients in a randomized control trial of self management of warfarin treatment. BMJ. 2004;328:437–8. doi: 10.1136/bmj.328.7437.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Gomez F, Alegria E, Berjŏn J, et al. Comparative of antiplatelet, anticoagulant, or combined therapy in patients with valvular and non valvular atrial fibrillation. J Am Coll Cardiol. 2004;44:1557–66. doi: 10.1016/j.jacc.2004.05.084. [DOI] [PubMed] [Google Scholar]
- Petersen P, Boysen G, Godtfredsen J, et al. Placebo-controlled, randomized trial of warfarin and aspirin for prevention of thromboembolic complications in chronic atrial fibrillation: The copenhagen AFASAK study. Lancet. 1989;i:175–9. doi: 10.1016/s0140-6736(89)91200-2. [DOI] [PubMed] [Google Scholar]
- Stroke Prevention in Atrial Fibrillation III Investigators. Adjusted-dose warfarin versus low-intensity, fixed-dose warfarin plus aspirin for high-risk patients with atrial fibrilliation. Lancet. 1996;348:633–8. [PubMed] [Google Scholar]
- Stroke Prevention in Atrial Fibrillation Investigators. The stroke preventions in atrial fibrillation study: final results. Circulation. 1991;84:527–39. doi: 10.1161/01.cir.84.2.527. [DOI] [PubMed] [Google Scholar]
- Stroke Prevention in Atrial Fibrillation Investigators. Bleeding during anti-thrombotic therapy in patients with atrial fibrillation. Arch Intern Med. 1996;156:409–16. [PubMed] [Google Scholar]
- Sudlow M, Thomson R, Thwaites B, et al. Prevalence of atrial fibrillation and eligibility for anticoagulants in the community. Lancet. 1998;352:1167–71. doi: 10.1016/S0140-6736(98)01401-9. [DOI] [PubMed] [Google Scholar]
- Taylor FC, Cohen H, Ebrahim S. Systemic review of long term anticoagulation or antiplatelet treatment in patients with non-rheumatic atrial fibrillation. BMJ. 2001;322:321–6. doi: 10.1136/bmj.322.7282.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant A, Geddes JM, Chamberlain MA. The Barthel Index an ordinal score or interval level measure. Clin Rehab. 1996;10:301–8. [Google Scholar]
- Wang TK, Massaro JM, Levy D, et al. A risk score for predicting stroke or death in individuals with new-onset atrial fibrillation in the community. JAMA. 2003;290:1049–56. doi: 10.1001/jama.290.8.1049. [DOI] [PubMed] [Google Scholar]