Abstract
Despite tremendous progress in medicine during last couple of decades, cancer still remains the most horrifying diagnosis for anybody due to its almost inevitable futility. According to American Cancer Society Statistics, it is estimated that only in the United States more than half a million people will die from cancer in 2006. For those who survive, probably the most fearsome symptom regardless of cancer type will be the pain. Although most pain specialists and oncologists worldwide are well aware of the importance to adequately treat the pain, it was yet established that more than half of cancer patients have insufficient pain control, and about quarter of them actually die in pain. Therefore, in this review article we attempted to provide the comprehensive information about different options available nowadays for treating cancer pain focusing on most widely used pharmacologic agents, surgical modalities for intractable pain control, their potential for adverse effects, and ways to increase the effectiveness of treatment maximally optimizing analgesic regimen and improving compliance.
Keywords: analgesic ladder, opioids, adjuvant, neuromodulation
General consideration in cancer pain management
Despite all advances in prevention, early detection, and newer, more effective treatment modalities, cancer in general remains one of the most debilitating and deadly diseases nowadays, and is the second leading cause of mortality of all Americans (Jemal et al 2004). The sheer potential for suffering from cancer can be a horrifying experience for anyone bearing this diagnosis, while pain is probably one of the most frightening of all cancer symptoms for patients and their families (Valdimarsdottir et al 2002; Winslow et al 2005). According to statistics published by the American Cancer Society in 2002, “50%–70% of people with cancer experience some degree of pain” (ACS 2002), which usually only intensifies as the disease progresses. Less than half get adequate relief of their pain, which negatively impacts their quality of life. The incidence of pain in advanced stages of invasive cancer approaches 80% and it is 90% in patients with metastases to osseous structures (Pharo and Zhou 2005).
Suboptimal pain control can be very debilitating. Patients and their families tend to be under great distress after the diagnosis of cancer. Although many of these patients carry a very poor prognosis, prompt and effective pain control can prevent needless suffering, may significantly improve the quality of their lives, and may potentially spare families the feeling of helplessness and despair. Although cancer can be a terminal disease, there should be no reason to deny a patient the opportunity to live productively and free of pain. Severe pain can interfere with physical rehabilitation, mobility, and proper nutrition. A significant number of cancer patients are subsequently diagnosed with depression. Therefore, the goals of pain control in any patient with cancer should be to optimize the patient’s comfort and function while avoiding unnecessary adverse effects from medications (Cherny 2000).
Several practice guidelines exist for the treatment of cancer pain (Jacox et al 1994; Wilson et al 1997; Benedetti et al 2000; Panchal et al 2005). From these, probably the most widely used are the guidelines developed by the World Health Organization (WHO) 20 years ago, which include the 3-step “analgesic ladder” designed to facilitate and standardize pharmacologic cancer pain management and advise physicians worldwide how to better provide pain management to their patients (Figure 1). According to the ladder algorithm, selection of nonopioid, opioid, and adjuvant analgesic therapy should be individualized, as directed by the intensity of the pain. This approach has been shown to provide good to satisfactory pain relief over a 10-year observation period in 88% of cancer patients in an over-2000-patient anesthesiology-based pain service (Zech et al 1995). On the other hand, it was estimated in 1994 that less than half of cancer patients in general practice get adequate relief of their pain, and 25% actually die in pain (Jacox et al 1994). This is particularly disappointing because the pain endured by 90% of these patients could have been well managed with relatively simple interventions (Friedman and Rodgers 2004).
Figure 1.
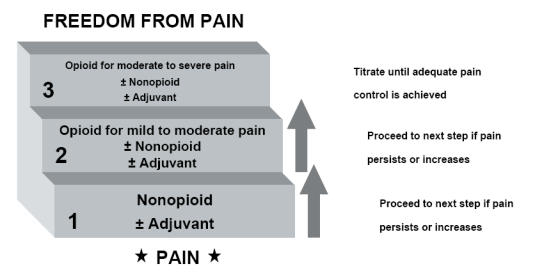
The World Health Organization cancer pain treatment step ladder.
With ongoing progress in the pain management field, a number of palliative care specialists argue that the WHO program, even though updated in 1990, had not kept pace with the rapidly changing developments in oncology and pain research (Meldrum 2005). It was reported that the current ladder method consistently failed to provide sufficient relief to 10%–20% of advanced cancer patients with pain, particularly in cases of neuropathic pain and pain associated with bone involvement (Ahmedzai 1997). Therefore, it was suggested that a fourth, “interventional”, step be added to the 3-step WHO analgesic ladder once opioids and other drugs fail, which will incorporate nerve blocks, intrathecal drug delivery systems, and other surgical interventions (Miguel 2000). However, it may be reasonable to further adjust the WHO pain management ladder from its current approach to a more sophisticated 5-step algorithm that would separate potentially reversible neuromodulation (electrical or chemical) from virtually irreparable destructive procedures, such as cordotomy, rhizotomy, or thalamotomy, and would also include physical and psychological modalities at every step along the entire continuum of care (Figure 2).
Figure 2.
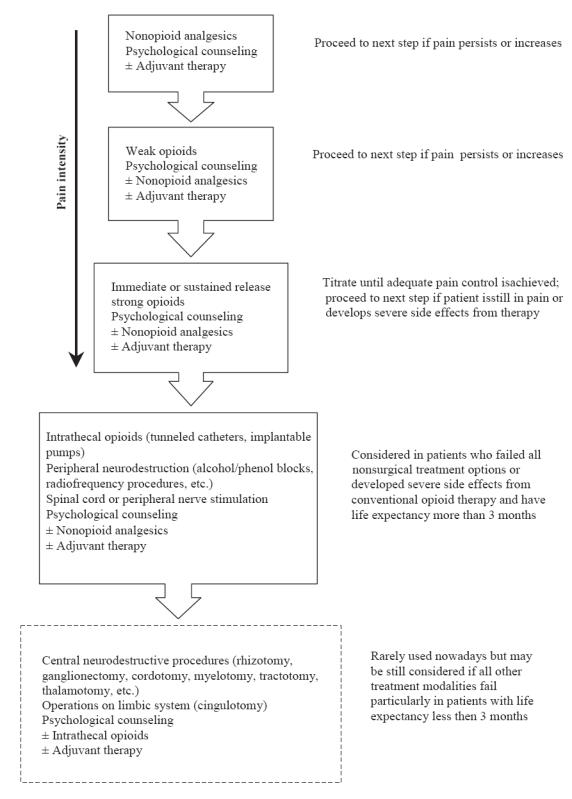
Modified analgesic ladder for the treatment of cancer pain.
There are many challenges that physicians may encounter in the treatment of cancer pain. Generally, pain is a subjective feeling that has not to date been easily and universally quantified (Noble et al 2005). Patients with similar cancer types may experience different intensities of pain, may respond to the same analgesic in different ways, and may exhibit varying sensitivities to the adverse effects from many of the drugs used. Because cancer pain is usually multifaceted, a single analgesic may not be sufficient enough to alleviate all the aspects of pain that the patient is experiencing, thus complicating the pharmacological regimen. Depending on the type and extent of the cancer, the administration routes may be limited for some patients and more innovative methods of drug delivery may need to be utilized. Society has also placed limitations on adequate pain control because of some unfounded concerns, including fear of physical dependency and addiction to opioids, as well as discomforting side effects that may occur with long-term analgesic treatment (Bloodworth 2005). Although important in considering opioid regimen in patients with nonmalignant chronic pain (Nedeljkovic et al 2002), it has been highlighted, however, that neither physical dependency nor addiction are significant problems in the management of cancer patients (Portenoy 1996).
Another challenge to the treatment of cancer pain is the paucity of good clinical trials providing objective data that can be extrapolated to individual patients. Some of the limitations with the clinical trials found in the literature today include the heterogeneity of cancer pain types, the limited number of patients enrolled, the spectrum of available analgesics and doses used for optimal pain control, the lack of a single objective pain scale, and the variable duration of treatment provided in different textbooks or guidelines. In addition, inadequate pain assessment, lack of knowledge about modern modalities for chronic pain management, and fear of potential liability and censure by regulatory agencies for prescribing opioids among physicians in general practice may play another role in undertreating the cancer pain (McCarberg and Barkin 2001; NCCN 2005).
Initial assessment of cancer pain
Comprehensive pain assessment is one of the most important initial steps for successful management of cancer pain. It is recommended that pain should be evaluated at every clinical visit and incorporated as the “fifth vital sign” (Benedetti et al 2000). Ideally, the assessment should target the severity, duration, quality, and location of the pain (Turk et al 2002). Reports of pain made by the patient should be the primary source of pain assessment and should be obtained at periodic intervals. Periodic monitoring may include, but is not limited to a patient’s verbal report of treatment efficacy and any side effects associated with pain management. Analyses of aggregated outcomes are essential to continuous quality improvement of chronic pain management in the clinical setting (Wilson et al 1997). In addition, it is important for the clinician to inquire about how the pain has affected patient’s daily activities and relationships with others.
To help introduce objectivity in the evaluation, a number of pain scales have been utilized to quantify pain intensity. Currently, it is recommended that pain should be measured using a numerical rating scales (from 0–10, where 0 indicating no pain and a 10 indicating the worst imaginable pain) (Benedetti et al 2000). In cases of children, the elderly, and patients with language differences, facial expression scales, ie, Wong-Baker scale, should be considered (Wong and Baker 1988). This enables clinicians to make a continuous objective assessment of pain intensity throughout the course of the treatment. To assess the quality of the painful stimulus, it is best to allow the patients to describe the pain themselves, which very often helps healthcare practitioners get a better understanding of the source and the type of pain. Clinicians should attempt to obtain more information about the pain by conducting pain histories to determine a cause and the best treatment modality (Benedetti et al 2000). It has also been suggested that clinicians pay more attention to psychological factors because fear and anxiety may have significant effect on the perception and experience of pain (Turk et al 2002).
There are two types of pain: nociceptive pain and neuropathic pain. Nociceptive pain stimulus is transmitted by peripheral nerves from specialized pain receptors, called nociceptors, whose function is to report any injury, which in cancer patients is usually secondary to invasion of tumor into bone, joints, or connective tissue. Nociceptive pain can be somatic (usually sharp or dull well-localized aching or squeezing sensation), visceral (usually poor-localized deep pressure-like sensation), and associated with invasive procedures, ie, lumbar puncture, biopsy, surgical intervention. Neuropathic pain, on the other hand, results from mechanical or metabolic injury to the nervous system itself, either centrally or peripherally, and is generally associated with mishandling of incoming somatosensory stimuli. In patients with advanced cancer this can be a result of tumor infiltration of nerves or nerve roots, as well as iatrogenic in nature as a result of exposure to chemotherapeutic agents (ie, vinca alkaloids) or radiation therapy.
In general, nociceptive pain responds relatively well to traditional analgesics, including nonsteroidal anti-inflammatory drugs (NSAIDs) and opioids, whereas neuropathic pain remains more difficult to treat, and more commonly is better alleviated by antiepileptic drugs or tricyclic antidepressant agents, which modulate action potential propagation and the availability of chemical neurotransmitters such as norepinephrine and serotonin. It is important to keep in mind that cancer patients will generally experience a combination of pain types, and the treatment of the disease, ie, surgery, radiation, chemotherapy, may be an important source of the painful stimuli along with progression of the disease itself.
Pharmacological management of cancer pain
According to WHO cancer pain treatment ladder the initial step in any pain management is consisted of using nonopioid analgesics, which include acetaminophen, aspirin, NSAIDs, such as ibuprofen or ketorolac, and the most recent addition, the selective cyclooxygenase type 2 (COX-2) inhibitors, such as rofecoxib, celecoxib and valdecoxib. Most nonopioid analgesics used for cancer pain treatment are summarized in Table 1.
Table 1.
Most commonly used nonopioid analgesics in United States
| Drug | Preparation | Dose | Maximum | Comments |
|---|---|---|---|---|
| Acetaminophen | tablets – 325, 500, 650 mg suspension – 160, 325 mg/5 ml suppository – 80, 120, 125, 200, 300, 325, 600, 650 mg |
325–1000 mg PO q4–6h PRN |
4000 mg/day | No anti-inflammatory effect. Hepatotoxic if overdosed |
| Aspirin | tablets – 81, 162, 325, 500, 650, 975 mg suppository – 60, 120, 200, 300, 600 mg |
325–650 mg PO q4h PRN |
4000 mg/day | Bleeding risk is the most significant concern |
| Diclofenac | tablets – 50 mg delayed-release – 25, 50, 75, 100 mg SR |
50 mg PO BID-TID |
150 mg/day | Alt dose for SR form is 100 mg PO daily |
| Etodolac | tablets – 400, 500 mg extended-release – 400, 500, 600 mg SR capsules – 200, 300 mg |
200–400 mg PO q6–8h |
1200 mg/day | The dose for SR is 400–1000 mg once daily |
| Ibuprofen | tablets – 100, 200, 400, 600, 800 mg suspension – 40 mg/ml, 100 mg/5 ml |
400–600 mg PO q4–6h PRN |
3200 mg/day | Use with caution in patients with history of peptic ulcer |
| Indomethacin | capsules – 25, 50 mg extended-release – 75 mg SR suspension – 25 mg/5 ml suppository – 50 mg |
25–50 mg PO TID |
200 mg/day | Use with caution in patients with history of peptic ulcer |
| Ketoprofen | tablets – 12.5 mg capsules – 25, 50, 75 mg extended-release – 100, 150, 200 mg SR |
25–50 mg PO q6h–q8h |
300 mg/day | Maximum dose for SR form is 200 mg/day |
| Ketorolac | tablets – 10 mg parenteral – 15 mg/ml, 30 mg/ml |
10 mg PO q4–6h PRN or 30 mg IV/IM q6h |
40 mg/day PO or 120 mg/day IV/IM |
IV therapy should not exceed 5 days |
| Meclofenamate | capsules – 50, 100 mg | 50–100 mg PO q4–6h PRN |
400 mg/day | |
| Mefenamic acid | capsules – 250 mg | 250 mg PO q4–6 PRN |
For therapy ≤ 1 wk in duration |
Recommended first dose is 500 mg PO |
| Meloxicam | tablets – 7.5, 15 mg suspension – 7.5 mg/5 mL |
7.5–15 mg PO once daily |
15 mg/day | COX-2 preferential NSAID |
| Nabumetone | tablets – 500, 750 mg 2000 mg/day |
100–2000 mg once daily | May divide daily dose to BID | |
| Naproxen | tablets – 250, 375, 500 mg SR – 375, 500 mg suspension – 125 mg/5 ml |
250–500 mg PO BID | 1500 mg/day for 3–5days |
Maintenance dose is maximum 1000 mg/d for 6 months |
| Oxaprozin | tablets – 600mg | 1200 mg PO once daily |
1800 mg/day | |
| Piroxicam | capsules – 10, 20 mg | 20 mg PO once daily |
20 mg/day | May divide daily dose BID |
| Sulindac | tablets – 150, 200 mg | 150–200 mg PO BID | 400 mg/day | |
| Tolmetin | capsules – 400 mg tablets – 200, 600 mg |
200–600 mg PO BID-TID |
1800 mg/day | |
| Tramadol | Tablets – 50 mg extended-release – 100, 200, 300 mg SR |
50–100 mg PO q4–6h PRN; 100– 300 mg QD for SR |
400 mg/day | Maximum dose for SR form is 300 mg/day |
| Celecoxib | capsules – 100, 200, 400 mg | 200 mg PO BID | 400 mg/day | COX-2 inhibitor |
| Rofecoxib* | tablets – 12.5, 25, 50 mg suspension – 12.5 mg/5 ml, |
50 mg PO daily for 5 days, then 25 mg PO 25 mg/5 ml daily |
Withdrawn from the market in 2004 | |
| Valdecoxib* | tablets – 10, 20 mg | 10-20 mg PO BID | Withdrawn from the market in 2005 | |
Abbreviations: PO, oral; IM, intramuscular; IV, intravenous; PRN, as needed; BID, twice daily; TID, three times daily; NSAID, nonsteroidal anti-inflammatory drug; SR, sustained release.
Note: COX-2 inhibitors Rofecoxib (Vioxx) and Valdecoxib (Bextra) were removed from the market due to increased cardiovascular and dermatological risks.
Acetaminophen (paracetamol) is recommended as a first step analgesic for mild to moderate pain. Although its mechanism of action is not fully understood, it is thought to inhibit central prostaglandin synthesis in the central nervous system, which explains its analgesic and antipyretic activity without any effects on inflammation. Acetaminophen is not generally used alone for cancer pain, but rather in combination with opioids (ie, hydrocodone, codeine, etc) Although acetaminophen is effective and well tolerated by most of the patients, its use is limited by a maximum daily dose of 4000 mg (2000 mg/day in patients with hepatic dysfunction) due to potential hepatic toxicity. On the other hand, the gastro-intestinal toxicities seen with chronic NSAIDs use are not seen with acetaminophen. Acetaminophen is excreted by kidneys and dosing must be adjusted in patients with significant renal insufficiency.
Aspirin is another drug from this group that can be used for mild to moderate pain control. Unlike acetaminophen, aspirin serves not only as an analgesic and antipyretic but also as an anti-inflammatory agent, which may be an important addition to the therapeutic effect in patients who have severe inflammatory pain. It is a safe over-the-counter drug widely used for noncancerous acute pain control and for management and prophylaxis of myocardial infarction due to its well-established anti-platelet action. However, it has to be used very cautiously in cancer patients, as in high doses required for adequate pain control (650–1000 mg orally every 4–6 hours) aspirin can cause a number of unwanted side effects, such as tinnitus, vertigo, hyperventilation, as well as increased risk of peptic ulcer disease and gastrointestinal (GI) bleedings. If overdosed, aspirin can cause cardiovascular instability, exacerbate underlying renal insufficiency, and even lead to coma with renal failure, metabolic acidosis, and respiratory arrest.
NSAIDs are potent analgesics, antipyretics, and anti-inflammatory agents, which makes them useful for cancer related pain of musculoskeletal origin. They work through nonspecific inhibition of cyclooxygenase (COX), an enzyme that mediates prostaglandin synthesis from arachidonic acid. Because of nonspecific inhibition of both isoenzymes of cyclooxygenase (COX-1 and COX-2), all nonselective NSAIDs have significant adverse effects on gastric mucosa and renal parenchyma, and some inhibit platelet function. With chronic use, they can cause serious gastric ulcerations and bleeding, which is a result of the inhibition of COX-1 isoenzyme. Therefore, NSAIDs may not be an optimal choice in patients who are experiencing nausea and vomiting associated with receiving chemotherapy or who have a history of GI bleeding in the past. In addition, care must be taken in patients that may already have renal insufficiency related to advanced age or disease progression because of the potential to exacerbate these conditions due to modulation of prostaglandin activity on renal blood flow (Dunn 1984). The NSAIDs have maximum daily doses that limit their utility in moderate to severe cancer pain management. All of the NSAIDs are available orally, but only ketorolac is available in parenteral form for pain control. Indomethacin, like aspirin, is available in suppository forms for rectal administration.
COX-2 inhibitors (rofecoxib, celecoxib, and valdecoxib) have less potential for GI and hematological side effects seen with the traditional NSAIDs, a factor that makes them more attractive for cancer pain management. These drugs specifically inhibit the COX-2 isoenzyme, which is considered the inducible isoenzyme during painful stimuli. This selectivity spares the inhibition of COX-1, which is thought to be constitutive in the GI tract and required for normal gastrointestinal function. In addition, there are emerging studies that show an antitumoral effect with these agents due to inhibition of cytokine production seen in many solid tumors (Rouff and Lema 2003). This class of drugs is an attractive option in those patients with cancer involving inflammation and those who are at high risk for GI bleeding or platelet dysfunction. COX-2 inhibitors may also be considered as one of the most effective agents for patients with bone metastasis as prostaglandins appear to play an important role in pathogenesis of bone pain (Haegerstam 2001). In addition smaller doses of opioids can be used with COX-2 inhibitors thereby minimizing potential risk for opioid side effects. Because of their relatively short half-lives, they are also capable of treating breakthrough pain.
However, like NSAIDs, COX-2 inhibitors should be used with caution with those at risk for renal failure, and case reports have emerged documenting this severe adverse effect (Morales and Mucksavage 2002). Moreover, the overall safety of COX-2 inhibitors, particularly rofecoxib, has recently come into question due to increased risk of acute myocardial infarction and sudden cardiac death among high-dose chronic users of this drug (Bombardier et al 2000; Mukherjee et al 2001), which led to voluntarily withdrawal of rofecoxib (Vioxx®) from the U.S. market in 2004 (Merck 2004; Singh 2004). On the other hand, our experience shows that the majority of patients with disseminated metastatic bone disease will accept the risk of having a heart attack while on COX-2 inhibitors as opposed to living with unbearable pain or experiencing severe side effects from high-dose opioid therapy. Therefore, COX-2 inhibitors may still serve as a good option for relief of musculoskeletal pain in patients with terminal cancer. No parenteral forms of COX-2 inhibitors are commercially available at present in the Uniteds States.
Tramadol is a centrally acting nonopiate analgesic with low affinity for μ-opioid receptors, and is effective in the treatment of moderate to severe pain. It has been also shown to inhibit reuptake of serotonin and norepinephrine, which synergistically enhances its weak opioid mechanism of action (Raffa et al 1992; Desmeules et al 1996). This may explain the reduced incidences of abuse, respiratory depression and other adverse effects of traditional opioids in patients on long-term tramadol therapy (Raffa 2001). Tramadol can be beneficial in patients who fail nonopioid therapy and wish to delay taking opioids avoiding the common side effects of constipation, somnolence, and fatigue. It is shown to be effective in such nonmalignant opioid-resistant chronic pain states as fibromyalgia and diabetic neuropathy (Harati et al 1998; Russell et al 2000), and had marginal to moderate success in the treatment of chronic cancer pain (Grond et al 1999). Unlike the NSAIDs, tramadol has no anti-inflammatory activity, extensively metabolized in the liver and is available in tablet form only.
As pain progresses, nonopioid regimens alone may not be sufficient to provide necessary analgesia or may be approaching maximum recommended daily doses. At this point, a trial of opioid and nonopioid analgesic combination may be instituted. A variety of fixed combinations with acetaminophen are available on the market, which usually include codeine, hydrocodone, oxycodone, or propoxyphene (Raffa 2001). Based on extensive evidence of their efficacy, these combinations are recommended in the second step of WHO analgesic ladder for the management of moderate to severe pain (Schug et al 1990). Another attractive choice for long-term pain treatment is the combination of acetaminophen with tramadol, which has been demonstrated in humans to be more effective with a faster onset and longer duration of action than either component alone, without increasing the incidence of adverse events (Raffa 2001). Nevertheless, this phase requires frequent and constant evaluation of the patient to titrate each drug to a successful dose, which is in general limited by the nonopioid component. Once the limit is reached for these agents (eg, >4 g/day of acetaminophen), the next step is to advance to pure opioid therapy.
The opioids are typically the most common drug class used in the treatment of cancer pain. They work by binding to μ-opioid receptors within the central nervous system, which are responsible for opioid-mediated analgesia, respiratory depression, sedation, physiological dependence, and tolerance (Gutstein and Akil 2001). Analgesic effect of opioids is largely dependent on μ-receptor saturation and is thus influenced by the type and severity of the pain, prior exposure to opioids, and individual distribution of receptors (Friedman and Rodgers 2004). There is no maximum dose for these agents; they are only limited by the development of side effects that are patient specific in their onset and severity. Common opioid side effects include nausea, constipation, sedation, and confusion, and they can be often managed without compromising pain control by adjusting the daily dose of the drug or in persistent cases by instituting additional medications, such as metoclopramide for nausea, laxatives for constipation, or methylphenidate for sedation.
Prolonged use of opioids may lead to development of tolerance (the need to increase opioid dose with time to maintain equipotent analgesic effects) and opioid-induced abnormal hypersensitivity to pain (so-called pro-nociceptive sensitization). Experimental studies suggest that both phenomena could be related to N-methyl-D-aspartate (NMDA) receptor mediated changes in central nervous system (Trujillo and Akil 1991; Mao et al 1994, 1995; Alvarez et al 2001). Opioid desensitization and hypersensitization of NMDA receptors from prolonged opioid therapy may both contribute to an apparent decrease in analgesic efficacy, regardless of the progression of the pain. Thus, in some instances, treating increasing pain with increasing doses of same opioid may be futile (Ballantyne and Mao 2003). Although this has not been shown conclusively in the clinical setting, NMDA receptor antagonists (ketamine, dextromethorphan, memantine, amantadine) and low-dose opioid antagonists (naloxone, naltrexone) might partially reverse opioid tolerance. In addition, because the cross-tolerance to opioids is incomplete, opioid rotation (switching from one opioid to another) can be also used to overcome the unwanted adverse effects of opioid receptor desensitization (Mercadante 1999a; Lussier and Pappagallo 2004).
Exogenous opioids may also affect hormonal and immune systems with prolonged use, leading to reduced fertility, libido, and drive, along with moderate immunosuppression (Finch et al 2000; Ballantyne and Mao 2003). However, in cases of chronic cancer pain these adverse effects of opioid therapy, along with fear of physical dependence and addiction, may be considered as not very important, and should not prevent the physician from providing adequate pain control to the patient.
A wide variety of opioids are currently available in the market (Table 2), and are roughly categorized into controlled (or sustained) release (SR), such as MS Contin®, Avinza®, Kadian®, Oxycontin®, Duragesic®, and immediate release (IR) formulations, such as MSIR, Oxycodone, Hydromorphone, Actiq®, etc.
Table 2.
Most commonly used opioid analgesics in United States
| Drug name | Formulation | Duration of action (hours) | Recommended analgesic dose |
|---|---|---|---|
| Morphine sulfate | Tablets (IR): 15 and 30 mg | 2–4 | 10–30 mg q3–4h |
| Rectal suppositories: 5, 10, 20, and 30 mg | 10–20 mg q4h | ||
| Parenteral (SC, IM, IV): 2, 4 and 20 mg/ml | 2.5–10 mg q2–6h1 | ||
| Epidural | 3–5 mg once, then 0.1–0.7 mg/hr | ||
| Intrathecal | Start 100:1 IT-to-IV, then titrate to pain | ||
| MS Contin® | Tablets (CR): 15, 30, 60, 100, 200 mg | 8–12 | 15–30 mg q8–12h |
| Oramorph®SR | Tablets (SR): 15, 30, 60, 100 mg | 8–12 | 15–30 mg q8–12h |
| Kadian® | Capsules (ER): 20, 30, 50, 60, 100 mg | 24 | 20 mg q24h, may increase by 20 mg increments every other day |
| Avinza® | Capsules (ER): 30, 60, 90, 120 mg | 24 | 30 mg q24h, may increase by 30 mg increments q4days (max 1600 mg/d) |
| Codeine | Tablets: 15, 30, 60 mg Oral solution: 15 mg/5 ml, 30 mg/5 ml |
2–4 | 15–60 mg q4–6h (max 60 mg/d) |
| Dilaudid® (hydromorphone) | Tablets (IR): 2, 4, 8 mg; Oral solution: 5 mg/5 mL; Suppositories: 3 mg | 2–4 | 2–8 mg q3-4h for PO and PR |
| Parenteral (SC, IM, IV): 1, 2, 4, 8 mg/ml; | 1–4 mg q4–6h2 | ||
| Intrathecal | Start 100:1 IT-to-IV, then titrate to pain | ||
| Palladone® | Capsules (ER): 12, 16, 24, 32 mg | 24 | Withdrawn from the market in 2005 |
| Roxycodone® (oxycodone) | Tablets (IR3): 5, 15, 30 mg, capsules: 5 mg; Oral solution: 5 mg/5 ml, 20 mg/ml | 2–4 | 5–30 mg q4h |
| OxyContin® | Tablets (SR): 10, 20, 40, 80, 160 mg | 12 | 10–160 mg q12h4 |
| Opana® (oxymorphone) | Tablets (IR) 5, 10 mg | 4-8 | 5–10 mg q4–6h |
| Opana®ER | Tablets (ER) 5, 10, 20, 40 mg | 12 | 5–40 mg q12h |
| Propoxyphene HCl | Capsules: 65 mg5 | 2–4 | 65 mg q4h (max 390 mg/24h) |
| Methadone | Oral solution: 5 mg/5 ml, 10 mg/5 ml, 10 mg/ml; Tablets: 5, 10, 40 mg |
4–8 | 2.5–10 mg q3-6h Has very long half-life (8–60 hours) |
| Meperidine (Demerol®) | Oral solution: 50 mg/5 ml Tablets: 50, 100 mg |
2–4 | 50–150 mg q3–4h (decrease dose if given IV, administer slow or via PCA). |
| Parenteral (SC, IM, IV): 10 mg/ml | Not recommended for chronic use. | ||
| Fentanyl | Parenteral (IM, IV) | 1–3 | 25–100 mcg q1–2h 0.5–1.5 mcg/kg/hr IV infusion via PCA |
| Actiq® | Oral transmucosal lozenge: 200, 400, 600, 800, 1200, 1600 mcg | 2–4 | Start with 200 mcg for breakthrough pain episodes6, then titrate to pain |
| Duragesic® | Transdermal patch: 25, 50, 75, 100 mcg/hr | 72 | 25 mcg/h q72hr, may increase q3–6days |
Abbreviations: IR, immediate release; CR, controlled release; SR, sustained release; ER, extended release.
Notes: Alternative dose for IV morphine: 0.1 mg/kg IV once, then 1-10mg/hr via IV PCA.
Start 0.2-0.6 mg q2-3h (IV), 0.8-1 mg q4-6h (SC/IM) in opiate-naive patients
Oxycodone is also available as OxyIR (5 mg immediate release tablets); doses are similar to Roxycodone
80 mg and 160 mg formulations of Oxycontin should be used in opiate-tolerant patients only
65 mg propoxyphene hydrochloride (Darvon®) = 100 mg propoxyphene napsylate (Darvon-N)
Can be also used IM or SC at 2.5-10 mg q8-12h, but generally PO is recommended for chronic pain
For use only in opiate tolerant patients, recommended maximum dose is 4 units per day
It was suggested that for patients with mild to moderate cancer pain opioid analgesic therapy may start with the trial of codeine or hydrocodone (Fukshansky et al 2005). Codeine is a weak opium alkaloid with a potency 1/10 of morphine. Hydrocodone is a more potent hydrogenated ketone derivative of codeine, which is typically available only as a combination product with acetaminophen (Vicodin, Norco) or ibuprofen (Reprexain, Vicoprofen). Although both these drugs are very well suited for the treatment of different mild to moderate pain syndromes, they have almost no role in the treatment of severe chronic cancer pain.
Morphine is considered the standard opiate and the drug of first choice in the treatment of moderate to severe cancer pain (Schug et al 1990; Wilson et al 1997; Benedetti et al 2000). It should be titrated to maximum tolerability before moving on to another opiate such as fentanyl, hydromorphone, or oxycodone. Morphine, first identified nearly 200 years ago, is available in a variety of formulations (ie, parenteral, oral, rectal) and the oral form is available in a range of preparations, from immediate release to sustained release, allowing it to be precisely titrated to the patient’s response (Table 2). The oral formulation is recommended initially due to its ease of administration and convenience of use. A typical regimen consists of a sustained-release (SR) preparation given every 8–12 hours with breakthrough doses of immediate-release (IR) form given every 3–4 hours in between if needed. As a guide, the cumulative as-needed doses should not exceed the total dose given as a sustained preparation for that interval. Thus, a patient requiring morphine 120 mg SR every 12 hours should receive morphine 30 mg IR every 3 hours for breakthrough pain. Regimens will require frequent adjustments allowing 3–4 days for the patient to respond before initiating a change unless toxicity is apparent.
One double-blind, multi-centered crossover study compared the efficacy, safety, and pharmacokinetics of a novel once-daily morphine formulation and a 12-hour SR morphine formulation in the treatment of chronic cancer pain (Hagen et al 2005). The investigators found that there was no significant difference between treatments for evaluations of overall pain intensity, analgesic efficacy, or adverse events. However, the once-daily formulation showed less fluctuation in plasma morphine concentration to compare with SR form, and most patients chose once-daily morphine dosing for continuing pain management, as it was providing more stable pain control over the day.
The most common adverse effects of morphine include sedation and some degree of cognitive impairment, which usually improves with time in patients taking stable and moderate doses of opioid (Bruera et al 1989; Chapman et al 2002). Nausea and vomiting are frequently seen upon initiation of therapy and after large dose increases, but usually subside with time. Constipation is seen with chronic therapy; patients do not develop tolerance to it and typically require preemptive treatment with laxatives.
One of the important aspects to consider for adequate opioid treatment is that patients may have varying responses to an individual opioid based on various pharmacodynamic and pharmacokinetic interactions. For example, morphine is hepatically glucuronidated to two metabolites: morphine-3-glucuronide (M3G) and morphine-6-glucuronide (M6G). M3G has no analgesic properties but may be involved in certain side effects such as myoclonus. M6G is a more potent analgesic than the parent compound and passes much more readily into the central nervous system. Morphine and its metabolites are excreted by the kidneys and toxicity can be seen in patients with underlying renal insufficiency or failure. In cirrhosis, the bioavailability of morphine is increased due to the lack of first pass metabolism; however, the production of the more potent M6G metabolite may decrease resulting in a less than optimal analgesic effect. In addition, it should be noted that older individuals, who make the vast majority of terminal cancer patients, may have an increased sensitivity to opioids, due to decreased hepatic metabolism and decreased renal excretion, as well as a reduced number of opioid receptors due to brain atrophy (Balducci 2003). Therefore, it is vital to incorporate interpatient differences into the dosing scheme in order to arrive at a tolerable but effective regimen.
The effects of active morphine metabolites can be induced or inhibited by a variety of medications. The anti-epileptic drugs carbamazepine, phenobarbital, and phenytoin, as well as the antibiotic rifampin can accelerate clearance of morphine. Phenothiazines, tricyclic antidepressants, and cimetidine will interfere with morphine metabolism and may potentate its effect if administered simultaneously. Co-administration of morphine and benzodiazepines may produce strong synergistic action resulting in sedation, hypotension, and sometimes delirium (Donnely et al 2002).
Should a patient fail morphine therapy, another opiate should be instituted and dosed according to its morphine equivalency. Initial dosing of the new opioid should be 25%–50% less than the expected equivalent dose of morphine since the patient may not be cross-tolerant to the new agent. Cross-tolerance can be seen particularly when changing from a more potent to a less potent agent and is a result of variable effects of each opioid on the pain receptors.
Hydromorphone (Dilaudid®) is a water-soluble opioid that is several times more potent than morphine allowing for smaller doses to be used. It is available in parenteral, rectal, subcutaneous, and oral formulations. However, hydromorphone can be also administered via epidural and intrathecal routes (Fukshansky et al 2005). Hydromorphone should be considered particularly for patients on morphine who are having side effects of increased confusion or myoclonus (Friedman and Rodgers 2004). When using injectable hydromorphone, clinicians must be aware of its potency. Although IV hydromorphone is six to seven times more potent than IV morphine (Sarhill et al 2001) it could be 20 times more potent than oral morphine. Hydromorphone relieves continuous dull pain more effectively than sharp intermittent pain, and when mixed with epinephrine it provides superior pain relief (Fukshansky et al 2005).
Fentanyl is a quick acting lipophilic opiate available in parenteral, transmucosal, and transdermal formulations. Intravenous fentanyl is 70 to 100 times more potent than IV morphine (Pereira et al 2001) and has very rapid onset of action – 5 minutes to peak analgesia, versus at least 15 minutes for IV morphine (Gutstein and Akil 2001). Fentanyl is most widely used in palliative medicine in the form of a transdermal patch (Duragesic®), which is especially useful in those patients who do not have enteral access for analgesia or for whom nausea and vomiting limit the ingestion of the required dose of opioid. The efficacy and tolerability of transdermal fentanyl for long-term treatment of cancer pain have been extensively studied and very well documented (Grond et al 1997; Payne et al 1998; Radbruch et al 2001; Kornick et al 2003).
The release of fentanyl from the transdermal system is characterized by two distinct phases following initial application: during the first phase, a rapid loading dose is absorbed from the contact adhesive, which is followed by a plateau phase when fentanyl is released from the patch reservoir at a constant rate (Varvel et al 1989). Although it may take 12–24 hours for the initial onset of action to occur (Korte et al 1996), transdermal route eliminates gastrointestinal absorption and subsequently first-pass metabolism of fentanyl, which makes possible to use lower doses of medication and reduce incident of adverse effects (Ahmedzai and Brooks 1997; Donner et al 1998). Fentanyl usually is not recommended for children under 12 years, due to unknown safety profile at this age, and is not suitable for patients with severe cachexia due to limited subcutaneous fat stores. One of the main drawbacks of transdermal fentanyl is that its elimination half-life is up to 18 hours after patch removal, thus patients who experience side effects, especially respiratory depression, will need to be monitored and supported for a full day following discontinuation.
The prevalence of breakthrough pain has been well described in patients with chronic cancer pain (Portenoy and Hagen 1990; Fine and Busch 1998), and IV fentanyl can be successfully used for breakthrough pain in the hospital or hospice care. However, for outpatient management more convenient choice for breakthrough cancer pain will be a rapid-onset opioid Actiq®— the oral transmucosal fentanyl citrate (OTFC) lozenge that patients can dissolve in the buccal space for immediate relief, usually within 5–10 minutes (Farrar et al 1998). One study compared its use with oral immediate-release morphine and found OTFC to be superior for fast pain control (Coluzzi et al 2001). Other study demonstrated that OTFC may be an effective alternative to intravenous opioids in rapidly titrating analgesia in selected opioid-tolerant cancer patients who are in pain crisis (Burton et al 2004a).
Another centrally acting synthetic opioid, transdermal buprenorphine, is now being widely prescribed in Europe and Australia for cancer pain management (Budd 2003; Skaer 2004). Transdermal buprenorphine is contained in a matrix patch as opposed to traditional reservoir patch technology used for transdermal fentanyl, which makes it more robust in handling. In a matrix system, the substance is an integral part of the polymer structure of the patch. Thus, while damaging a reservoir patch might result in ‘dose dumping’ and potentially overdosing the patient, damaging a matrix patch will not interfere with the controlled release of the medication (Evans and Easthope 2003).
Oxycodone is a synthetic opioid that is metabolized hepatically to the active oxymorphone. One study compared controlled-release oxycodone and morphine tablets in 45 cancer patients, and although the authors found that both drugs have provided similar analgesic effects, there were differences in pain relief in those patients who had underlying renal or hepatic dysfunction with better pain control in patients receiving oxycodone (Heiskanen et al 2000). This may be due to the accumulation of active metabolites or differences in the phenotype for CYP2D6 that metabolizes oxycodone. This study stresses the importance of pharmacogenomics in guiding and individualizing pain therapy in the future. In most markets, oxycodone is significantly more expensive than morphine and is thus less attractive as a first-line analgesic (Friedman and Rodgers 2004). CR oxycodone (Oxycontin®), based on special drug delivery system known as AcroContin system, uses a dual-control matrix with two hydrophobic polymers, which are not influenced by pH and therefore are independent of acidity. Oxycontin® is effective in moderate to severe cancer pain and allows for convenience of every 12 hours administration (Fukshansky et al 2005).
Methadone is an inexpensive synthetic opioid agonist that has a very long half-life, no active metabolites, and little tendency to induce tolerance in patients. It has unique properties that make it useful in treating pain which is poorly controlled by other opioids. In addition to binding to the opioid μ-receptor, methadone produces analgesic effects through its antagonism at the NMDA receptor site and by increasing the availability of neurotransmitters serotonin and norepinephrine within the central nervous system (Davis and Walsh 2001). Methadone works particularly well in opioid rotation and may be an effective alternative for cancer patients, although its equianalgesic dosing to morphine has not been firmly established and can vary widely depending on the cumulative dose of morphine (Lawlor et al 1998; Ripamonti et al 1998; Berland 2000). It occurs more frequently in patients previously exposed to high doses of opioids than in patients receiving low dose (Fukshansky et al 2005). Methadone is available for oral, sublingual, rectal, intravenous, and subcutaneous administration, and has relatively low risk for opioid-associated adverse effects.
Another potent opioid with simultaneous action at NMDA receptor site, levorphanol, can be also considered for treatment of cancer-associated pain in some patients. Its main compound dextromethorphan has been shown to be beneficial for adequate analgesia after bone-malignancy resection, especially when used for epidural infusion (Weinbroum et al 2004). However, earlier reports have provided controversial data and showed low efficacy of dextromethorphan in controlling cancer associated pain compared with more traditional opioids, such as morphine (Mercadante et al 1998).
Ketamine also has effects in blocking the NMDA receptors and has found some success in treating neuropathic pain, especially in a situation where large doses of opioids have contributed to the development of severe hyperalgesia (Kannan et al 2002; Hocking and Cousins 2003). Ketamine can be given by multiple routes: IV, IM, SC, oral, rectal, nasal, transdermal, epidural, or even intrathecal, although the optimal route of administration remains unclear due to a lack of good clinical trials and limited experimental studies. Ketamine has been used in a variety of neuropathic pain syndromes that are refractory to high-dose opioids, such as central pain, ischemic pain, and pain associated with posttraumatic nerve or spinal cord injury, as well as in fibromyalgia, refractory facial pain, and post-herpetic neuralgia (Hocking and Cousins 2003). However, there is very limited data on ketamine trials in cancer pain management. In addition, apart from a few cases of complete resolution, ketamine generally did not provide a long-term solution in clinical trials for chronic pain, and the magnitude of reported benefit was often only a little more than a placebo effect (Hocking and Cousins 2003). Nevertheless, ketamine may be still used in refractory cancer pain management as an adjunctive modality for its opioid-sparing benefits, allowing smaller doses of morphine to be given (Mercadante et al 2000). Limiting its use are also the side effects that include sedation, delirium, and hallucinations at higher doses.
There are many other opioids available on the market today. However, they are not usually recommended for routine use in cancer pain management. These include diamorphine (commonly known as heroin), meperidine, propoxyphene, and mixed agonist-antagonist agents (ie, butorphanol, pentazocine, nalbuphine). Heroin is not used in the United States in medical practice and considered as one of the most dangerous street drugs; however, it is still widely used in UK for chronic malignant and nonmalignant pain control. Meperidine is metabolized to a neurotoxic metabolite normeperidine which can induce seizures if accumulated. The effect of propoxyphene can be considered more euphoric than analgesic. The mixed agents have a ceiling effect as well as the potential in reversing analgesic effects of any pre-existing opioid the patient is already taking and, therefore, they are not considered efficacious.
Although cancer pain usually can be relieved in more than 70% of patients using a simple opioid-based regimen, there will be always patients who experience little or no pain relief despite substantial analgesic doses of opiates or who develop intolerable adverse effects. As it was mentioned earlier, unsatisfactory analgesic response may be due to a variety of factors: differences in patient metabolism, multiple pain mechanisms, disease progression, and sensitivity to side effects. Some nonanalgesic medications are found to be very helpful in amplifying the effect of many analgesic drugs, particularly in patients with neuropathic pain. In such cases, a variety of strategies can be implemented to improve the pain control and balance between analgesia and side effects (Vielhaber and Portenoy 2002). Among these strategies is the use of adjuvant analgesics, although very few of these drugs have been actually studied in cancer populations.
The term “adjuvant analgesic” describes any drug with a primary indication other than pain, but with analgesic properties in some painful conditions. They can be added to the regimen at any time depending on the quality of the pain. In some cases, the type of pain suggests the value of one category of adjuvant analgesic over another; in others, the existence of another symptom concurrent with pain favors the use of a specific drug (Lussier et al 2004). There are several major groups of adjuvant analgesics (ie, antidepressants, antiepileptic drugs, muscle relaxants, corticosteroids, etc) that are used nowadays to intensify the effect of opioids and NSAIDs on long-term pain control. For example, pain that is neuropathic in nature is typically not amenable to standard opiate therapy, and the addition of tricyclic antidepressants (TCA) or/and antiepileptic drugs (AED) can offer a very effective treatment strategy in such patients (Collins et al 2000).
TCA such as amitriptyline, imipramine, doxepin, and clomipramine are attractive adjuvant agents in cancer patients due to their positive effects on mood and sleep. The analgesic properties of TCA have been extensively studied in a variety of chronic nonmalignant pain conditions (Onghena and van Houdenhove 1992; Watson 2000). Although only few clinical trials have specifically evaluated these drugs for cancer pain (Walsh 1986; Magni et al 1987), our experience supports their analgesic effects. Early use of antidepressants is also justified when pain is accompanied by depression, which is fairly common in patients with advanced cancer. However, the use of TCA, especially in medically ill or elderly patients may be limited due to frequent side effects similar to those seen with opiates, which include drowsiness, constipation, urinary retention, and dry mouth, as well as such serious adverse effects as orthostatic hypotension, liver function impairment and cardiotoxicity (Glassman and Bigger 1981; Pharo and Zhou 2005). TCA are contraindicated in patients with a known history of glaucoma and should be avoided in patients who are suicidal.
Nontricyclic compounds, such as selective serotonin reuptake inhibitors (SSRI) and serotonin-norepinephrine reuptake inhibitors (SNRI), are generally safer, have fewer side effects than TCA and, therefore, may be considered for patients who have relative contraindications to tricyclics or have experienced severe adverse effects during the treatment (Masand and Gupta 1999). However, there are only limited data supporting the analgesic efficacy in nonmalignant pain management of few SSRI, ie, paroxetine (Sindrup et al 1990) and citalopram (Sindrup et al 1992), and SNRI, ie, venlafaxine (Sindrup et al 2003) and duoxetine (Arnold et al 2004), and no studies have been reported on cancer pain (Saarto and Wiffen 2005). It is thought that norepinephrine reuptake is necessary for an antidepressant to be effective on neuropathic pain, therefore, TCA and SNRI in general may have better results on alleviating neuopathic pain than SSRI (Lynch 2001).
It should be noted that the secondary amines, desipramine and nortriptyline, are less anticholinergic, usually better tolerated than the tertiary amines, and may be more desirable in the elderly (Lussier et al 2004; Maizels and McCarberg 2005). Another nontricyclic antidepressant, trazadone, has been shown to have same effectiveness in cancer-related neuropathic pain as amitriptyline (Carr et al 2004).
There is good evidence that AED are particularly useful as adjuvant therapy in the long-term management of neuropathic pain (Rowbotham et al 1998; Backonja 2000; Tremont-Lukats et al 2000; Rice and Maton 2001; Jensen 2002). Of the all AED, gabapentin (Neurontin) is probably the most widely prescribed medication for the treatment of cancer-related neuropathic pain (Caraceni et al 1999; Oneschuk and al-Shahri 2003), although its specific mechanism of action has not been fully elucidated at this time. Nonetheless, due to its proven analgesic effects, good tolerability, and a rarity of drug-drug interactions, gabapentin is now recommended as a first-line agent for the treatment of neuropathic pain of diverse etiologies, especially in the medically ill population (Dworkin et al 2003a; Caraceni et al 2004). It should be initiated at a daily dose of 100–300 mg and can be increased every 3 days. The usual maximum dose is 3600 mg daily, but can be higher if needed, and an adequate trial should include 1–2 weeks at the maximum-tolerable dose (Lussier et al 2004). Gabapentin is usually well tolerated, and the most common side effects are somnolence, dizziness, and unsteadiness, which are typically not severe if carefully titrated.
There are several other AED, such as carbamazepine (Backonja 2000), phenytoin (Yajnik et al 1992), lamotrigine (Zakrzewska et al 1997; Vestergaard et al 2001), pregabalin (Dworkin et al 2003b), and levetiracetam (Price 2004), that have been reported to be efficacious in alleviating different neuropathic pain syndromes, including cancer-related pain (Yajnik et al 1992; Dunteman 2005). In general, the last three drugs are well-tolerated and lack significant drug-drug interaction, which makes them superior to carbamazepine or phenytoin for the long-term management of neuropathic pain. The most frequent serious adverse effects of long-term therapy with carbamazepine, phenytoin, and lamotrigine are bone marrow suppression and liver toxicity, whereas levetiracetam only rarely causes pancytopenia, and pregabalin has almost no serious adverse effects, except mild thrombocytopenia and some congestive heart failure exacerbation.
Corticosteroids belong to another major group of medications widely used as an adjuvant therapy for cancer- related pain syndromes, which include bone pain, neuropathic pain from infiltration or metastatic compression of neural structures, headache due to increased intracranial pressure, arthralgias, and pain due to obstruction of hollow viscus or distention of an organ capsule (Greenberg et al 1980; Ettinger and Portenoy 1988; Watanabe and Bruera 1994; Lussier et al 2004). They inhibit prostaglandin production, reduce inflammation, decrease capillary permeability, and have membrane stabilization effects, which reduces neuronal excitability (Pharo and Zhou 2005). Corticosteroids can also improve appetite, nausea, malaise, and overall quality of life (Farr 1990; Mercadante et al 2001). However, it should be always taken into consideration that corticosteroids when used for a long time can produce significant adverse effects, such as immunosuppression, hypertension, hyperglycemia, gastric ulcers, and psychosis; although in cancer patients the benefit from corticosteroid administration can often outweigh the potential risk for adverse effects, particularly in cases of central nervous system involvement.
The use of adjuvant medications to treat opiate side effects can also allow an increase in the analgesic dose. The second-generation (atypical) agent olanzapine (Zyprexa) was reported to decrease pain intensity and opioid consumption, and improve cognitive function and anxiety, in a recent case series of cancer patients (Khojainova et al 2002). Stimulants such as methylphenidate or caffeine can increase alertness in patients who are experiencing somnolence on a dose of morphine that provides sufficient pain control (Dalal and Melzack 1998). In addition, it has been shown that in cancer patients, methylphenidate not only can reduce opioid-induced somnolence, but can also significantly improve cognition, treat depression, and alleviate fatigue (Rozans et al 2002). Liberal use of laxatives to treat constipation can also allow an opioid dose to be escalated. Patients who have pain associated with bone metastases may especially benefit from the use of bisphosphonate compounds, such as pamidronate or zolendronate (Rosen et al 2001; Serafini 2001; Gordon 2005). These agents decrease the effect of bone osteoclast resorption and are typically given intravenously every 4 weeks. Calcitonin has also shown some beneficial effect in alleviating the pain associated with bone metastases (Roth and Kolaric 1986; Szanto et al 1992).
Other adjunctive strategies may include topical agents (local anesthetics, capsaicin) useful for mucositis or peripheral neuropathies (Slavin et al 2004); clonidine, an alpha-2 adrenergic agonist usually given intraspinally (to avoid systemic side effects) for the management of severe intractable cancer pain partly responding to opioids (Eisenach et al 1995); amantadine, a noncompetitive NMDA antagonist, which has been shown to reduce surgical neuropathic cancer pain (Pud et al 1998); baclofen, which can be used in case of spasticity and central pain secondary to spinal cord lesions (van Hilten et al 2000); benzodiazepines, which used to reduce patients’ fear and anxiety related to their disease (Pharo and Zhou 2005); as well as antihistamines, antipsychotics, or any other unusual adjuvant analgesics, that may be beneficial for the treatment of severe refractory pain not responsive to traditionally used drug combinations.
Surgical management of cancer pain
Surgery is rarely used for the treatment of cancer pain these days, particularly since longer-acting opioids, such as slow-release oxycodone or morphine, and transcutaneous fentanyl patches became available. In addition to that, prior to considering surgical intervention, one should try a variety of less invasive techniques, such as nerve blocks, radiofrequency ablations or neurolytic destructions, as well as many other procedures available nowadays from the wide pain management arsenal.
When it comes to the choice of pain-relieving surgical procedures, these are usually divided into two broad categories: neurodestruction and neuromodulation. Neurodestructive procedures involve interruption of pain pathways, which can be performed anywhere starting at the level of the nerve, nerve root, ganglion, spinal cord, thalamus, or the brain stem depending on the nature and extent of the pain (Fenstermaker 1999; Lordon 2002). One of the most commonly used procedures in the past was spinal cordotomy that targets the spinothalamic tract on the cervical or upper thoracic level and results in eliminating pain sensation from the opposite side of the body (Jones et al 2003). Although safe and effective if done on one side only, it may be associated with a very high rate of complications if performed bilaterally. Midline myelotomy is reserved for patients with severe bilateral or visceral pain (Nauta et al 2000); it interrupts a nonspecific pain-transmitting pathway located in the vicinity of the central canal of the spinal cord. Thalamotomy is usually aimed at either nuclei involved in somatosensory perception or more anteriorly located centers that relay affective aspects of pain (Whittle and Jenkinson 1995). Cingulotomy targets the part of the limbic system that appears to modulate painful sensations and certain psychological aspects of pain experience; it is usually reserved for patients with intractable cancer pain after failure of antineoplastic and palliative pharmacological treatments and when more conservative analgesic procedures are not applicable (Wong et al 1997).
Among positive sides of neurodestructive procedures are the relative ease of performance and immediate pain relief, making these procedures quite attractive to some patients suffering from intractable cancer associated pain who failed all means of traditional palliative therapy. However, the problems associated with destruction of the nervous tissue include: the irreversibility of action, particularly of the side effects (numbness and weakness that come directly as a result or as a complication of destructive operation may take very long time to recover), inability to test or reliably predict the effect of procedure due to individual anatomical and physiological variability, relatively short duration of the effect (most neurodestructive procedures result in 3 months to 1 year pain relief, mainly due to the plasticity of the central nervous system), and higher risk of complications with bilateral procedures. Also, neurodestructive procedures cannot be performed in patients with coagulopathies, which are developed due to their disease itself or as an unwanted side effect of the treatment. Despite all these, carefully selected and performed neurodestructive procedures may be useful for certain cancer patient populations (Kanpolat et al 1995). For example, a patient with gynecological malignancy who suffers from unilateral pelvic and leg pain due to radiation effect or direct tumor invasion of the lumbar plexus and has a life expectancy of 2–3 months, may be an excellent candidate for a cervical cordotomy, which has a unique chance of rendering the patient painless and free of narcotic medication side effects for the rest of her life.
Chronic abdominal pain associated with pancreatic and other types of intra-abdominal cancer can be successfully treated by celiac plexus block (injection of a neurolytic agent near the celiac plexus). Analysis of the available data regarding the efficacy and safety of this procedure to control cancer pain has shown that celiac block provided long-lasting benefit for 70% to 90% of patients with different types of abdominal cancer with mean pain scores decreased by 40% in average (Eisenberg et al 1995; Shah et al 2003). Orthostatic hypotension, local pain, and diarrhea were some of common side-effects of this procedure, but were conservatively managed with prompt resolution of the symptoms. Only a few patients had been reported to develop severe neurological complications, such as lower extremity weakness and paresthesia, from the procedure (Eisenberg et al 1995). In cases of visceral and pelvic pain associated with extensive gynecologic, colorectal, or genitourinary cancer, neurolytic block of the hypogastric plexus can be used (de Leon-Casasola et al 1993; Plancarte et al 1997). Although the reported results of this procedure in general are less convincing than for celiac plexus block (probably due the character of the cancer and possible more extensive involvement of bone structures in this group of patients), in cases of medically intractable pelvic pain, hypogastric block may be still beneficial to consider, all the more that no serious complications were reported in literature related to this procedure.
In addition, local nerve blocks or neurolysis with phenol or alcohol can be used for treating localized pain (Miguel 2000), and kyphoplasty used for painful vertebral compression fractures in patients with disseminated metastatic cancer (Fourney et al 2003).
Electrical neuromodulation, the electrical stimulation of neural structures (peripheral nerves, dorsal columns of spinal cord, and brain stimulation), although widely used nowadays for successful treatment of intractable neuropathic and central pain, has almost no role in the treatment of cancer-related pain (Taub 2003). Spinal cord stimulation may be helpful for those with primary neuropathic nature of pain, such as patients with arachnoiditis, but is unlikely to eliminate the significant nociceptive component of cancer pain. Although spinal cord stimulation has been tried in patients after pelvic exenteration with severe, intractable pain due to radiation necrosis, and showed up to 60% reduction in pain, decrease in opioid consumption, and overall improvement in quality of life for longer than 3 years (Miguel 2000), this treatment modality is not yet considered as one of the recommended treatments for intractable cancer pain.
Chemical neuromodulation, on the other hand, has become widely accepted in the treatment of cancer pain. Intrathecal opioids (such as morphine and hydromorphone) given alone or in combination with adjuvant medications (alpha-adrenergic agonists, eg, clonidine, or local anesthetics, eg, bupivacaine) are now commonly used for medically intractable cancer pain (Smith et al 2002; Rauck et al 2003). Although these agents may be delivered via variety of catheters and ports, most accepted practice consists of the implantation of a self-contained pump that delivers medication at a specific rate into the subarachnoid space via a dedicated intrathecal catheter (Slavin et al 2002). Intrathecal administration of opioids is an option for those patients whose effective systemic dose cannot be tolerated due to presence of unacceptable side effects or whose pain is refractory to conventional therapy. Intrathecal infusion bypasses the blood–brain barrier and results in much higher cerebrospinal fluid concentrations with less medication. Compared with the epidural route, intrathecal infusion is associated with higher rates of satisfactory pain relief and lower rates of treatment failure and technical complications (Dews and Mekhail 2004).
Morphine has been extensively studied intrathecally for patients with cancer and found to be more effective in relieving nociceptive pain versus neuropathic pain (Becker et al 2000). One multicenter, randomized clinical trial demonstrated that patients with refractory cancer pain are more effectively treated with addition of implantable intrathecal drug delivery system to a comprehensive medical management (Smith et al 2002). In this study, the patients who received intrathecal morphine infusion had significantly better pain relief at four weeks than patients treated by conventional medications alone. In addition, the toxicity scores representing the cumulative analysis of combined side effects from the treatment were reduced by 50% in intrathecal pump group. And finally, patients with implanted intrathecal drug delivery system had significant reduction of fatigue and depressed consciousness, as well as improved rate of survival at six months. Several other clinical studies (retrospective, as well as prospective, randomized multicenter trials) have shown that addition of intrathecal opioid administration through programmable drug delivery system can significantly increase pain control, reduce toxicities, and improve overall survival and quality of life in cancer patients with refractory pain (Gilmer-Hill et al 1999; Rauck et al 2003; Burton et al 2004b).
An intrathecal pump can be implanted into the subcutaneous fat of the abdomen to provide a continuous infusion of medications. The older pumps had preset infusion rates (continuous flow pumps); therefore each dose adjustment had to be done by changing the concentration of the drug inside the pump. More commonly used nowadays, programmable pumps contain an electronic module that allows adjustment of the drug infusion rate using telemetry programming. All pumps have to be refilled at regular time intervals, but patients usually tolerate these refills quite well as they are done every one to three months in office or clinic settings by simple insertion of the needle into the center of the reservoir through the skin. The most common side effects of intrathecal opioid therapy are nausea and vomiting, and the most frequent complications include infection or hematoma at the surgical site.
Other drugs that can be administered intrathecally include hydromorphone, bupivacaine, clonidine, baclofen, and ziconotide (Kedlaya et al 2002). Hydromorphone is one of the first alternatives to consider for intrathecal administration when morphine therapy becomes not suitable for any reason. Fentanyl has been also tried intrathecally, however, due to low solubility in cerebrospinal fluid it does not dissipate far from the site of injection, therefore is not widely used for cancer pain treatment (Lordon 2002).
Clonidine and bupivacaine are the most commonly used nonopioid medications for intrathecal administration in cancer patients. They are both used in combination with morphine to amplify its analgesic effect. Clonidine produces analgesia by its action on alpha-2 receptors on presynaptic primary afferents and postsynaptic dorsal horn neurons of the spinal cord, which causes a decrease in the release of C-fiber neurotransmitters (eg, substance P) and inhibition of preganglionic sympathetic transmission (Hassenbusch et al 2002). Clonidine can produce marked bradycardia, orthostatic hypotension, and sedation at higher doses and should be used cautiously.
Local anesthetic bupivacaine produces its analgesic effect by blocking voltage-sensitive sodium channels and thus, preventing the generation and conduction of nerve impulses. Bupivacaine can be very helpful adjunct to morphine, particularly in the treatment of neuropathic pain (Mercadante 1999b; Kedlaya et al 2002). However, its dosing is limited by potential neurotoxicity, which can result in numbness, motor weakness, and bowel or bladder dysfunction at higher doses. Motor block may be seen at doses as low as 10 mg per day, but slower dose titration can generally overcome this adverse effect.
GABAB agonist baclofen can be used in cancer patients with associated sever spasticity and/or dystonia (Gatscher et al 2002). When administered intrathecally, baclofen inhibits both monosynaptic and postsynaptic reflexes at the spinal level and produces muscle relaxation. In addition, doses ranging 3–20 μg/hr have been shown to be effective in a variety of neuropathic pain syndromes through unclear yet mechanism (Hilten et al 2000). Sedation, hypotonia with weakness, and urinary retention are some of the side effects of intrathecal baclofen therapy. It has been also reported that abrupt discontinuation of intrathecal baclofen, regardless of the cause, can be life-threatening, and may result in high fever, altered mental status, exaggerated rebound spasticity, and even rhabdomyolysis, multiple organ-system failure and death (Rigoli et al 2004; Kao et al 2003; Mohammed and Hussain 2004).
Several years ago Elan Pharmaceuticals introduced a new analgesic drug, ziconotide, which has been recently approved by FDA for intrathecal treatment of persistent neuropathic pain in the United States. Ziconotide binds to specific N-type voltage-sensitive calcium channels found in neural tissue and acts by blocking neurotransmitter release from primary nociceptive afferents terminating in the superficial layers of the dorsal horn of the spinal cord (Miljanich and Ramachandran 1995; McGivern and McDonough 2004). This mechanism of action distinguishes ziconotide from all other analgesics, including opioids. In fact, ziconotide is potently antinociceptive in animal models in which morphine exhibits poor anti-nociceptive activity (Miljanich 2004). The results from few multicenter randomized, double-blind, placebo-controlled trials showed that intrathecal ziconotide provided clinically and statistically significant analgesia in patients with severe pain from cancer or AIDS (Mathur 2000; Staats et al 2004). However, although the safety of ziconotide administered as a continuous intrathecal infusion has been evaluated in over 1000 patients participating in acute and chronic pain clinical trials, lack of long-term prospective studies and high incidence of dose-dependent adverse effects during the initial titration stage of continuous intrathecal infusion of ziconotide currently limit its use as a drug of first choice even in patients with advanced cancer who fail the traditional methods of pain control (Webster et al 2001; Wermeling et al 2003; Doggrell 2004).
Benefits of intrathecal pumps are quite obvious: due to drug delivery route, equianalgesic effect may be reached at doses about 100 times lower than with systemic administration, which significantly decreases the dose-related side effects of opioid medications; the patient does not have to think about constant need to have the oral medication available (with associated reduction of risks related to abuse and mishandling of opioids); continuous drug delivery eliminates fluctuations in the drug level that are inevitable with bolus oral or parenteral dosing (Slavin and Solko 2003). In addition, chemical neuromodulation is both adjustable and reversible, so the side effects of the treatment may be minimized by either changing the rate of infusion or drug composition, or by stopping the therapy altogether without any lasting consequences. The treatment is also testable; the patient and the caregiver may estimate the degree of pain relief from the results of a pre-surgical medication trial.
At the same time, implantable devices are associated with higher upfront costs related to the procedure and the device itself, potential risk for infection and hardware malfunction, need for general anesthesia for system implantation, and similar procedural contraindications (coagulopathy, active systemic infection, etc) as with any other surgical intervention.
Besides adverse effects that are related directly to intrathecal administration of medications, there are not many serious complications reported for intrathecal drug delivery system implantation itself. Some of the common complications are infection, including few reports of associated meningitis, granuloma formation at the tip of the subarachnoid catheter, bleeding or hematoma at the site of the surgery, and malfunctioning of the device. All hardware-related complications are fully reversible without serious consequences and usually easily treated by either surgical revision of the implanted drug delivery system or complete removal of the device. Another consideration that may affect the decision to proceed with permanent implantation of intrathecal drug delivery system could be the cost of the hardware and related expenses, which may be noteworthy. However, several studies showed overall cost-saving benefit of this modality versus, for example, externalized epidural catheter in the treatment of intractable cancer pain when patients have estimated life expectancy of at least 3 to 4 months (Bedder et al 1991; Erdine and Talu 1998; Miguel 2000). Therefore, hard-to-control cancer pain in patients with more than 3 months survival is a well-founded indication for intrathecal drug delivery pump implantation when all conventional medical treatment regimens fail.
Summary
Because of the negative consequences on both patients and their families, and wide variety of pain management techniques available nowadays, patients with cancer should be comforted with maximally achievable pain control and not live in fear of inadequately treated pain. As the survival of patients with cancer becomes longer, reliable pain relief is now a high-priority issue that warrants both scientific research and industrial development of new devices and pharmaceutical agents that would make this pain relief complete, safe, and lasting.
Therefore, current approach to pain control should be individualized for every patient and will require knowledge of the cancer type, the drugs available on the market, the patients’ metabolism, drug tolerances, and even their genetic morphology. Periodical re-evaluation of patient’s medication regimen is essential to finely tune their analgesia and to minimize the exposure to potentially dangerous adverse effects. In addition, the approach must be interdisciplinary in nature: a surgeon, oncologist, pain specialist, pharmacist, psychologist, or physical therapist cannot treat the cancer pain alone; only by working together can these specialists give the cancer patient relief from the most fearsome symptom of their disease—their persistent pain.
References
- Ahmedzai S. New approaches to pain control in patients with cancer. Eur J Can. 1997;33(Suppl 6):S8–14. doi: 10.1016/s0959-8049(97)00205-0. [DOI] [PubMed] [Google Scholar]
- Ahmedzai S, Brooks D. Transdermal fentanyl versus sustained-release oral morphine in cancer pain: preference, efficacy, and quality of life. J Pain Symptom Manage. 1997;13:254–61. doi: 10.1016/s0885-3924(97)00082-1. [DOI] [PubMed] [Google Scholar]
- Alvarez V, Arttamangkul S, Williams JT. A RAVE about opioid withdrawal. Neuron. 2001;32:761–63. doi: 10.1016/s0896-6273(01)00530-x. [DOI] [PubMed] [Google Scholar]
- [ACS] American Cancer Society. Cancer facts and figures 2002 [online] 2002 doi: 10.6004/jadpro.2020.11.2.1. Accessed 4 April 2006. URL: http://www.cancer.org/docroot/STT/STT_0.asp. [DOI] [PMC free article] [PubMed]
- Arnold L, Lu Y, Crofford L, et al. A double-blind, multicenter trial comparing duloxetine with placebo in the treatment of fibromyalgia patients with or without major depressive disorder. Arthritis Rheum. 2004;50:2974–84. doi: 10.1002/art.20485. [DOI] [PubMed] [Google Scholar]
- Backonja MM. Anticonvulsants (antineuropathics) for neuropathic pain syndromes. Clin J Pain. 2000;16(Suppl 6):67–72. doi: 10.1097/00002508-200006001-00012. [DOI] [PubMed] [Google Scholar]
- Balducci L. Management of cancer pain in geriatric patients. J Support Oncol. 2003;1:175–91. [PubMed] [Google Scholar]
- Ballantyne JC, Mao J. Opioid therapy for chronic pain. N Engl J Med. 2003;349:1943–53. doi: 10.1056/NEJMra025411. [DOI] [PubMed] [Google Scholar]
- Becker R, Jakob D, Uhle E, et al. The significance of intrathecal opioid therapy for the treatment of neuropathic cancer pain conditions. Stereotact Funct Neurosurg. 2000;75:16–26. doi: 10.1159/000048379. [DOI] [PubMed] [Google Scholar]
- Bedder MD, Burchiel K, Larson A. Cost analysis of two implantable narcotic delivery systems. J Pain Symptom Manage. 1991;6:368–73. doi: 10.1016/0885-3924(91)90028-3. [DOI] [PubMed] [Google Scholar]
- Benedetti C, Brock C, Cleeland C, et al. NCCN practice guidelines for cancer pain. Oncology. 2000;14:135–50. [PubMed] [Google Scholar]
- Berland D. Pain management in patients with advanced cancer. Ann Intern Med. 2000;132:593. doi: 10.7326/0003-4819-132-7-200004040-00019. [DOI] [PubMed] [Google Scholar]
- Bloodworth D. Issues in opioid management. Am J Phys Med Rehabil. 2005;84(Suppl 3):S42–55. [PubMed] [Google Scholar]
- Bombardier C, Laine L, Reicin A, et al. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. New Eng J Med. 2000;343:1520–28. doi: 10.1056/NEJM200011233432103. [DOI] [PubMed] [Google Scholar]
- Bruera E, Macmillan K, Hanson J, et al. The cognitive effects of the administration of narcotic analgesics in patients with cancer pain. Pain. 1989;39:13–16. doi: 10.1016/0304-3959(89)90169-3. [DOI] [PubMed] [Google Scholar]
- Budd K. Buprenorphine and the transdermal system: the ideal match in pain management. Int J Clin Pract Suppl. 2003;133:9–14. [PubMed] [Google Scholar]
- Burton AW, Driver LC, Mendoza TR, et al. Oral transmucosal fentanyl citrate in the outpatient management of severe cancer pain crises. Clin J Pain. 2004a;20:195–97. doi: 10.1097/00002508-200405000-00011. [DOI] [PubMed] [Google Scholar]
- Burton AW, Rajagopal A, Shah HN, et al. Epidural and intrathecal analgesia is effective in treating refractory cancer pain. Pain Med. 2004b;5:239–47. doi: 10.1111/j.1526-4637.2004.04037.x. [DOI] [PubMed] [Google Scholar]
- Caraceni A, Zecca E, Bonezzi C, et al. Gabapentin for neuropathic cancer pain: a randomized controlled trial from the Gabapentin Cancer Pain Study Group. J Clin Oncol. 2004;22:2909–17. doi: 10.1200/JCO.2004.08.141. [DOI] [PubMed] [Google Scholar]
- Caraceni A, Zecca E, Martini C, et al. Gabapentin as an adjuvant to opioid analgesia for neuropathic cancer pain. J Pain Symptom Manage. 1999;17:441–45. doi: 10.1016/s0885-3924(99)00033-0. [DOI] [PubMed] [Google Scholar]
- Carr DB, Goudas LC, Balk EM, et al. Evidence report on the treatment of pain in cancer patients. J Nat Cancer Inst Monogri. 2004;32:23–31. doi: 10.1093/jncimonographs/lgh012. [DOI] [PubMed] [Google Scholar]
- Chapman SL, Byas-Smith MG, Reed BA. Effects of intermediate- and long-term use of opioids on cognition in patients with chronic pain. Clin J Pain. 2002;18(Suppl 4):S83–90. doi: 10.1097/00002508-200207001-00010. [DOI] [PubMed] [Google Scholar]
- Cherny NJ. The management of cancer pain. CA Cancer J Clin. 2000;50:70–116. doi: 10.3322/canjclin.50.2.70. [DOI] [PubMed] [Google Scholar]
- Collins SL, Moore RA, McQuay HJ, et al. Antidepressants and anticonvulsants for diabetic neuropathy and postherpetic neuralgia: a quantitative systematic review. J Pain Symptom Manage. 2000;20:449–58. doi: 10.1016/s0885-3924(00)00218-9. [DOI] [PubMed] [Google Scholar]
- Coluzzi PH, Schwartzberg L, Conroy JD, et al. Breakthrough cancer pain: a randomized trial comparing oral transmucosal fentanyl citrate (OTFC) and morphine sulfate immediate release (MSIR) Pain. 2001;91:123–30. doi: 10.1016/s0304-3959(00)00427-9. [DOI] [PubMed] [Google Scholar]
- Dalal S, Melzack R. Potentiation of opioid analgesia by psychostimulant drugs: a review. J Pain Symptom Manage. 1998;16:245–53. doi: 10.1016/s0885-3924(98)00084-0. [DOI] [PubMed] [Google Scholar]
- Davis MP, Walsh D. Methadone for relief of cancer pain: a review of pharmacokinetics, pharmacodynamics, drug interactions and protocols of administration. Support Care Cancer. 2001;9:73–83. doi: 10.1007/s005200000180. [DOI] [PubMed] [Google Scholar]
- Desmeules JA, Piguet V, Collart L, et al. Contribution of monoaminergic modulation to the analgesic effect of tramadol. Br J Clin Pharmacol. 1996;41:7–12. doi: 10.1111/j.1365-2125.1996.tb00152.x. [DOI] [PubMed] [Google Scholar]
- Dews TE, Mekhail N. Safe use of opioids in chronic noncancer pain. Cleve Clin J Med. 2004;71:897–904. doi: 10.3949/ccjm.71.11.897. [DOI] [PubMed] [Google Scholar]
- Doggrell SA. Intrathecal ziconotide for refractory pain. Expert Opin Investig Drugs. 2004;13:875–77. doi: 10.1517/13543784.13.7.875. [DOI] [PubMed] [Google Scholar]
- Donnely S, Davis MP, Walsh D, et al. Morphine in cancer pain management: a practical guide. Support Care Cancer. 2002;10:13–35. doi: 10.1007/s005200100274. [DOI] [PubMed] [Google Scholar]
- Donner B, Zenz M, Strumpf M, et al. Long-term treatment of cancer pain with transdermal fentanyl. J Pain Symptom Manage. 1998;15:168–75. doi: 10.1016/s0885-3924(97)00361-8. [DOI] [PubMed] [Google Scholar]
- Dunn MJ. Nonsteroidal anti-inflammatory drugs and renal function. Ann Rev Med. 1984;35:411–28. doi: 10.1146/annurev.me.35.020184.002211. [DOI] [PubMed] [Google Scholar]
- Dunteman ED. Levetiracetam as an adjunctive analgesic in neoplastic plexopathies: case series and commentary. J Pain Palliat Care Pharmacother. 2005;19:35–43. [PubMed] [Google Scholar]
- Dworkin RH, Backonja M, Rowbotham MC, et al. Advances in neuropathic pain: diagnosis mechanisms and treatment recommendations. Arch Neurol. 2003a;60:1524–34. doi: 10.1001/archneur.60.11.1524. [DOI] [PubMed] [Google Scholar]
- Dworkin RH, Corbin AE, Young JP, et al. Pregabalin for the treatment of postherpetic neuralgia: a randomized, placebo-controlled trial. Neurology. 2003b;60:1274–83. doi: 10.1212/01.wnl.0000055433.55136.55. [DOI] [PubMed] [Google Scholar]
- Eisenach JC, Du Pen S, Dubois M, et al. Epidural clonidine analgesia for intractable cancer pain. The Epidural Clonidine Study Group. Pain. 1995;61:391–99. doi: 10.1016/0304-3959(94)00209-W. [DOI] [PubMed] [Google Scholar]
- Eisenberg E, Carr DB, Chalmers TC. Neurolytic celiac plexus block for treatment of cancer pain: a meta-analysis. Anesth Analg. 1995;80:290–95. doi: 10.1097/00000539-199502000-00015. [DOI] [PubMed] [Google Scholar]
- Erdine S, Talu GK. Cost effectiveness of implantable devices versus tunneled catheters. Curr Rev Pain. 1998;2:157–62. [Google Scholar]
- Ettinger AB, Portenoy RK. The use of corticosteroids in the treatment of symptoms associated with cancer. J Pain Symptom Manage. 1988;3:99–103. doi: 10.1016/0885-3924(88)90167-4. [DOI] [PubMed] [Google Scholar]
- Evans HC, Easthope SE. Transdermal buprenotphine. Drugs. 2003;63:1999–2010. doi: 10.2165/00003495-200363190-00003. [DOI] [PubMed] [Google Scholar]
- Farr WC. The use of corticosteroids for symptom management in terminally ill patients. Am J Hosp Care. 1990;7:41–46. doi: 10.1177/104990919000700107. [DOI] [PubMed] [Google Scholar]
- Farrar JT, Cleary J, Rauck R, et al. Oral transmucosal fentanyl citrate: randomized, double blind placebo controlled trial for treatment of breakthrough pain in cancer patients. Anesth Analg. 1998;89:732–38. doi: 10.1093/jnci/90.8.611. [DOI] [PubMed] [Google Scholar]
- Fenstermaker RA. Neurosurgical invasive techniques for cancer pain: a pain specialist’s view. Curr Pain Headache Rep. 1999;6:190–97. doi: 10.1007/s11916-999-0013-1. [DOI] [PubMed] [Google Scholar]
- Finch PM, Roberts LJ, Price L, et al. Hypogonadism in patients treated with intrathecal morphine. Clin J Pain. 2000;16:251–54. doi: 10.1097/00002508-200009000-00011. [DOI] [PubMed] [Google Scholar]
- Fine PG, Busch MA. Characterization of breakthrough pain by hospice patients and their caregivers. J Pain Symptom Manage. 1998;16:179–83. doi: 10.1016/s0885-3924(98)00045-1. [DOI] [PubMed] [Google Scholar]
- Fourney DR, Schomer DF, Nader R, et al. Percutaneous vertebroplasty and kyphoplasty for painful vertebral body fractures in cancer patients. J Neurosurg. 2003;98(Suppl 1):21–30. doi: 10.3171/spi.2003.98.1.0021. [DOI] [PubMed] [Google Scholar]
- Friedman LL, Rodgers PE. Pain management in palliative care. Clin Fam Pract. 2004;6:371. [Google Scholar]
- Fukshansky M, Are M, Burton AW. The role of opioids in cancer pain management. Pain Practice. 2005;5:43–54. doi: 10.1111/j.1533-2500.2005.05106.x. [DOI] [PubMed] [Google Scholar]
- Gatscher S, Becker R, Uhle E, et al. Combined intrathecal baclofen and morphine infusion for the treatment of spasticity related pain and central deafferentation pain. Acta Neurochir Suppl (Wien) 2002;79:75–76. doi: 10.1007/978-3-7091-6105-0_16. [DOI] [PubMed] [Google Scholar]
- Gilmer-Hill HS, Boggan JE, Smith KA, et al. Intrathecal morphine delivered via subcutaneous pump for intractable cancer pain: a review of the literature. Surg Neurol. 1999;51:12–15. doi: 10.1016/s0090-3019(98)00080-9. [DOI] [PubMed] [Google Scholar]
- Glassman AH, Bigger JT. Cardiovascular effects of therapeutic doses of tricyclic antidepressants. A review. Arch Gen Psychiatr. 1981;38:815–20. doi: 10.1001/archpsyc.1981.01780320095011. [DOI] [PubMed] [Google Scholar]
- Gordon DH. Efficacy and safety of intravenous bisphosphonates for patients with breast cancer metastatic to bone: a review of randomized, double-blinded, phase III trials. Clin Breast Cancer. 2005;6:125–31. doi: 10.3816/CBC.2005.n.014. [DOI] [PubMed] [Google Scholar]
- Greenberg HS, Kim JH, Posner JB. Epidural spinal cord compression from metastatic tumor: results with a new treatment protocol. Ann Neurology. 1980;8:361–66. doi: 10.1002/ana.410080404. [DOI] [PubMed] [Google Scholar]
- Grond S, Radbruch L, Meuser T, et al. High-dose tramadol in comparison to low-dose morphine for cancer pain relief. J Pain Symptom Manage. 1999;18:174–79. doi: 10.1016/s0885-3924(99)00060-3. [DOI] [PubMed] [Google Scholar]
- Grond S, Zech D, Lehmann K, et al. Transdermal fentanyl in the long-term treatment of cancer pain: a prospective study of 50 patients with advanced cancer of the gastrointestinal tract or the head and neck region. Pain. 1997;69:191–98. doi: 10.1016/s0304-3959(96)03254-x. [DOI] [PubMed] [Google Scholar]
- Gutstein HB, Akil H. Opioid analgesics. In: Hardman JG, Limbrid E, editors. Goodman and Gilman’s the Pharmacological Basis of Therapeutics. 10. New York: McGraw-Hill Professional; 2001. pp. 569–619. [Google Scholar]
- Haegerstam GAT. Pathophysiology of bone pain: A review. Acta Orthop Scand. 2001;72:308–17. doi: 10.1080/00016470152846682. [DOI] [PubMed] [Google Scholar]
- Hagen NA, Thirlwell M, Eisenhoffer J, et al. Efficacy, safety, and steady-state pharmacokinetics of once-a-day controlled-release morphine (MSContinXL) in cancer pain. J Pain Symptom Manage. 2005;29:80–90. doi: 10.1016/j.jpainsymman.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Harati Y, Gooch C, Swenson M, et al. Double-blind randomized trial of tramadol for the treatment of the pain of diabetic neuropathy. Neurology. 1998;50:1842–46. doi: 10.1212/wnl.50.6.1842. [DOI] [PubMed] [Google Scholar]
- Hassenbusch SJ, Gunes S, Wachsman S, et al. Intrathecal clonidine in the treatment of intractable pain: a phase I/II study. Pain Med. 2002;3:85–91. doi: 10.1046/j.1526-4637.2002.02014.x. [DOI] [PubMed] [Google Scholar]
- Heiskanen TE, Ruismaki PM, Seppala TA, et al. Morphine or oxycodone in cancer pain? Acta Oncologica. 2000;39:941–47. doi: 10.1080/02841860050215927. [DOI] [PubMed] [Google Scholar]
- van Hilten BJ, van de Beek WJ, Hoff JI, et al. Intrathecal baclofen for the treatment of dystonia in patients with reflex sympathetic dystrophy. N Engl J Med. 2000;343:625–30. doi: 10.1056/NEJM200008313430905. [DOI] [PubMed] [Google Scholar]
- Hocking G, Cousins MJ. Ketamine in chronic pain management: an evidence-based review. Anesth Analg. 2003;97:1730–39. doi: 10.1213/01.ANE.0000086618.28845.9B. [DOI] [PubMed] [Google Scholar]
- Jacox A, Carr DB, Payne R, et al. (Publication #94-0592). AHCPR (Agency for Health Care Policy and Research) Washington DC: Dept of Health and Human Services; 1994. Clinical practice guideline #9: Management of cancer pain [online] Accessed 2 April 2006. URL: http://www.ahrq.gov/clinic/cpgarchv.htm. [Google Scholar]
- Jemal A, Tiwari RC, Murray T, et al. Cancer Statistics, 2004. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- Jensen TS. Anticonvulsants in neuropathic pain: rationale and clinical evidence. Eur J Pain. 2002;6A:61–8. doi: 10.1053/eujp.2001.0324. [DOI] [PubMed] [Google Scholar]
- Jones B, Finlay I, Ray A, et al. Is there still a role for open cordotomy in cancer pain management? J Pain Symptom Manage. 2003;25:179–84. doi: 10.1016/s0885-3924(02)00689-9. [DOI] [PubMed] [Google Scholar]
- Kannan TR, Saxena A, Bhatnagar S, et al. Oral ketamine as an adjuvant to oral morphine for neuropathic pain in cancer patients. J Pain Symptom Manage. 2002;23:60–5. doi: 10.1016/s0885-3924(01)00373-6. [DOI] [PubMed] [Google Scholar]
- Kanpolat Y, Caglar S, Akyar S, et al. CT-guided pain procedures for intractable pain in malignancy. Acta Neurochir Suppl (Wien) 1995;64:88–91. doi: 10.1007/978-3-7091-9419-5_19. [DOI] [PubMed] [Google Scholar]
- Kao LW, Amin Y, Kirk MA, et al. Intrathecal baclofen withdrawal mimicking sepsis. J Emerg Med. 2003;24:423–27. doi: 10.1016/s0736-4679(03)00039-8. [DOI] [PubMed] [Google Scholar]
- Kedlaya D, Reynolds L, Waldman S. Epidural and intrathecal analgesia for cancer pain. Best Pract Res Clin Anaesthesiol. 2002;16:651–65. doi: 10.1053/bean.2002.0253. [DOI] [PubMed] [Google Scholar]
- Khojainova N, Santiago-Palma J, Kornick CA, et al. Olanzapine in the management of cancer pain. J Pain Symptom Manage. 2002;23:346–50. doi: 10.1016/s0885-3924(02)00378-0. [DOI] [PubMed] [Google Scholar]
- Kornick CA, Santiago-Palma J, Moryl N, et al. Benefit-risk assessment of transdermal fentanyl for the treatment of chronic pain. Drug Safety. 2003;26:951–73. doi: 10.2165/00002018-200326130-00004. [DOI] [PubMed] [Google Scholar]
- Korte W, Stoutz N, Morant R. Day-to-day titration to initiate transdermal fentanyl in cancer patients: short- and long-term experience in a prospective study of 39 patients. J Pain Symptom Manage. 1996;11:139–46. doi: 10.1016/0885-3924(95)00162-x. [DOI] [PubMed] [Google Scholar]
- Lawlor PG, Turner KS, Hanson J, et al. Dose ratio between morphine and methadone in patients with cancer pain: a retrospective study. Cancer. 1998;82:1167–73. [PubMed] [Google Scholar]
- de Leon-Casasola OA, Kent E, Lema MJ. Neurolytic superior hypogastric plexus block for chronic pelvic pain associated with cancer pain. Pain. 1993;54:145–51. doi: 10.1016/0304-3959(93)90202-Z. [DOI] [PubMed] [Google Scholar]
- Lordon SP. Interventional approach to cancer pain. Curr Pain Headache Rep. 2002;6:202–6. doi: 10.1007/s11916-002-0036-3. [DOI] [PubMed] [Google Scholar]
- Lussier D, Huskey AG, Portenoy RK. Adjuvant analgesics in cancer pain management. The Oncologist. 2004;9:571–91. doi: 10.1634/theoncologist.9-5-571. [DOI] [PubMed] [Google Scholar]
- Lussier D, Pappagallo M. 10 most commonly asked questions about the use of opioids for chronic pain. The Neurologist. 2004;10:221–24. doi: 10.1097/01.nrl.0000131922.29715.62. [DOI] [PubMed] [Google Scholar]
- Lynch M. Antidepressants as analgesics: a review of randomized controlled trials. J Psychiatry Neurosci. 2001;26:30–36. [PMC free article] [PubMed] [Google Scholar]
- Magni G, Arsie D, De Leo D. Antidepressants in the treatment of cancer pain: a survey in Italy. Pain. 1987;29:347–53. doi: 10.1016/0304-3959(87)90049-2. [DOI] [PubMed] [Google Scholar]
- Maizels M, McCarberg B. Antidepressants and antiepileptic drugs for chronic non-cancer pain. Am Fam Physician. 2005;71:483–90. [PubMed] [Google Scholar]
- Mao J, Price DD, Mayer DJ. Thermal hyperalgesia in association with the developemnt of morphine tolerance in rats: roles of excitatory amino acid receptors and protein kinase C. J Neurosci. 1994;56:2301–12. doi: 10.1523/JNEUROSCI.14-04-02301.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J, Price DD, Mayer DJ. Mehcnisms of hyperalgesia and opiate tolerance: a current view of their possible interactions. Pain. 1995;62:259–74. doi: 10.1016/0304-3959(95)00073-2. [DOI] [PubMed] [Google Scholar]
- Masand PS, Gupta S. Selective serotonin-reuptake inhibitors: an update. Harvard Rev Psychiatry. 1999;52:547–52. [PubMed] [Google Scholar]
- Mathur V. Ziconotide: a new pharmacological class of drug for the management of pain. Semin Anesth Periop Med Pain. 2000;19:67–75. [Google Scholar]
- McCarberg BH, Barkin RL. Long-acting opioids for chronic pain: pharmacotherapeutic opportunities to enhance compliance, quality of life, and analgesia. Am J Ther. 2001;8:181–86. doi: 10.1097/00045391-200105000-00006. [DOI] [PubMed] [Google Scholar]
- McGivern JG, McDonough SI. Voltage-gated calcium channels as targets for the treatment of chronic pain. Curr Drug Targets CNS Neurol Disord. 2004;3:457–78. doi: 10.2174/1568007043336743. [DOI] [PubMed] [Google Scholar]
- Meldrum M. The ladder and the clock: cancer pain and public policy at the end of the twentieth century. J Pain Symptom Manage. 2005;29:41–54. doi: 10.1016/j.jpainsymman.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Mercandante S, Casuccio A, Genovese G. Ineffectiveness of dextromethorphan in cancer pain. J Pain Symptom Manage. 1998;16:317–22. [PubMed] [Google Scholar]
- Mercadante S. Opioid rotation for cancer pain: rationale and clinical aspects. Cancer. 1999a;86:1856–66. doi: 10.1002/(sici)1097-0142(19991101)86:9<1856::aid-cncr30>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Mercadante S. Neuraxial techniques for cancer pain: an opinion about unresolved therapeutic dilemmas. Reg Anesth Pain Med. 1999b;24:74–83. doi: 10.1016/s1098-7339(99)90169-4. [DOI] [PubMed] [Google Scholar]
- Mercadante S, Arcuri E, Tirelli W, et al. Analgesic effects of intravenous ketamine in cancer patients on morphine therapy: a randomized controlled, double-blind, crossover, double-dose study. J Pain Symptom Manage. 2000;20:246–52. doi: 10.1016/s0885-3924(00)00194-9. [DOI] [PubMed] [Google Scholar]
- Mercadante S, Fulfaro F, Casuccio A. The use of corticosteroids in home palliative care. Support Care Cancer. 2001;9:386–89. doi: 10.1007/s005200000218. [DOI] [PubMed] [Google Scholar]
- Merck. Merck announces voluntary worldwide withdrawal of VIOXX®. News release [online] 2004 Accessed 2 April 2006. URL: http://www.vioxx.com/vioxx/documents/english/vioxx_press_release.pdf.
- Miguel R. Interventional treatment of cancer pain: the fourth step in the World Health Organization analgesic ladder? Cancer Control. 2000;7:149–56. doi: 10.1177/107327480000700205. [DOI] [PubMed] [Google Scholar]
- Miljanich GP. Ziconotide: neuronal calcium channel blocker for treating severe chronic pain. Curr Med Chem. 2004;11:3029–40. doi: 10.2174/0929867043363884. [DOI] [PubMed] [Google Scholar]
- Miljanich GP, Ramachandran J. Antagonists of neuronal calcium channels: structure, function, and therapeutic implications. Annu Rev Pharmacol Toxicol. 1995;35:707–34. doi: 10.1146/annurev.pa.35.040195.003423. [DOI] [PubMed] [Google Scholar]
- Mohammed I, Hussain A. Intrathecal baclofen withdrawal syndrome- a life-threatening complication of baclofen pump: A case report. BMC Clin Pharmacol. 2004;4:1–5. doi: 10.1186/1472-6904-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales E, Mucksavage JJ. Cyclooxygenase-2 inhibitor-associated acute renal failure: case report with rofecoxib and review of the literature. Pharmacotherapy. 2002;22:1317–21. doi: 10.1592/phco.22.15.1317.33472. [DOI] [PubMed] [Google Scholar]
- Mukherjee D, Nissen SE, Topol EJ. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA. 2001;286:954–59. doi: 10.1001/jama.286.8.954. [DOI] [PubMed] [Google Scholar]
- Nauta HJ, Soukup VM, Fabian RH, et al. Punctate midline myelotomy for the relief of visceral cancer pain. J Neurosurg. 2000;92(Suppl 2):125–30. doi: 10.3171/spi.2000.92.2.0125. [DOI] [PubMed] [Google Scholar]
- [NCCN] National Comprehensive Cancer Network. Cancer pain treatment guidelines for patients (version II) [online] 2005 Accessed 4 April 2006. URL: http://www.nccn.org/patients/patient_gls.asp.
- Nedeljkovic SS, Wasan A, Jamison RN. Assessment of efficacy of long-term opioid therapy in pain patients with substance abuse potential. Clin J Pain. 2002;18(Suppl 4):S39–51. doi: 10.1097/00002508-200207001-00005. [DOI] [PubMed] [Google Scholar]
- Noble B, Clark D, Meldrum M, et al. The measurement of pain, 1945–2000. J Pain Symptom Manage. 2005;29:14–21. doi: 10.1016/j.jpainsymman.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Oneschuk D, al-Shahri MZ. The pattern of gabapentin use in a tertiary palliative care unit. J Palliat Care. 2003;195:185–87. [PubMed] [Google Scholar]
- Onghena P, van Houdenhove B. Antidepressant-induced analgesia in chronic non-malignant pain: a meta-analysis of 39 placebo-controlled studies. Pain. 1992;49:205–19. doi: 10.1016/0304-3959(92)90144-Z. [DOI] [PubMed] [Google Scholar]
- Panchal SJ, Anghelescu DL, Benedetti C, et al. Adult cancer pain. Clinical Practice Guidelines in Oncology (version 2.2005) [online] National Comprehensive Cancer Network. 2005 Accessed 6 April 2006. URL: http://www.nccn.org/professionals/cms/pdf/pain.pdf.
- Payne R, Mathias SD, Pasta DJ, et al. Quality of life and cancer pain: satisfaction and side effects with transdermal fentanyl versus oral morphine. J Clin Oncol. 1998;16:1588–93. doi: 10.1200/JCO.1998.16.4.1588. [DOI] [PubMed] [Google Scholar]
- Pereira J, Lawlor P, Vigano A, et al. Equianalgesic dose ratios for opioids: a critical review and proposals for long-term dosing. J Pain Symptom Manage. 2001;22:672–87. doi: 10.1016/s0885-3924(01)00294-9. [DOI] [PubMed] [Google Scholar]
- Pharo GH, Zhou L. Pharmacologic Management of Cancer Pain. JAOA. 2005;105:S21–28. [PubMed] [Google Scholar]
- Plancarte R, de Leon-Casasola OA, El-Helay M, et al. Neurolytic superior hypogastric plexus block for chronic pelvic pain associated with cancer. Reg Anesth. 1997;22:562–68. [PubMed] [Google Scholar]
- Portenoy RK. Opioid therapy for chronic nonmalignant pain: A review of the critical issues. J Pain Symptom Manage. 1996;11:203–17. doi: 10.1016/0885-3924(95)00187-5. [DOI] [PubMed] [Google Scholar]
- Portenoy RK, Hagen NA. Breakthrough pain: definition, prevalence and characteristics. Pain. 1990;41:273–82. doi: 10.1016/0304-3959(90)90004-W. [DOI] [PubMed] [Google Scholar]
- Price MJ. Levetiracetam in the treatment of neuropathic pain: three case studies. Clin J Pain. 2004;20:33–36. doi: 10.1097/00002508-200401000-00007. [DOI] [PubMed] [Google Scholar]
- Pud D, Eisenberg E, Spitzer A, et al. The NMDA receptor antagonist amantadine reduces surgical neuropathic pain in cancer patients: a double blind, randomized, placebo-controlled trial. Pain. 1998;75:349–54. doi: 10.1016/s0304-3959(98)00014-1. [DOI] [PubMed] [Google Scholar]
- Radbruch L, Sabatowski R, Petzke F, et al. Transdermal fentanyl for the management of cancer pain: a survey of 1005 patients. Palliat Med. 2001;15:309–21. doi: 10.1191/026921601678320296. [DOI] [PubMed] [Google Scholar]
- Raffa RB. Pharmacology of oral combination analgesics: rational therapy for pain. J Clin Pharm Ther. 2001;26:257–64. doi: 10.1046/j.1365-2710.2001.00355.x. [DOI] [PubMed] [Google Scholar]
- Raffa RB, Friderichs E, Reimann W, et al. Opioid and nonopioid components independently contribute to the mechanism of action of tramadol, an ‘atypical’ opioid analgesic. J Pharmacol Exp Ther. 1992;260:275–85. [PubMed] [Google Scholar]
- Rauck RL, Cherry D, Boyer MF, et al. Long-term intrathecal opioid therapy with a patient-activated implanted delivery system for the treatment of refractory cancer pain. J Pain. 2003;4:441–47. doi: 10.1067/s1526-5900(03)00730-2. [DOI] [PubMed] [Google Scholar]
- Rice AS, Maton S. Gabapentin in postherpetic neuralgia: a randomized, double blind, placebo-controlled study. Pain. 2001;94:215–24. doi: 10.1016/S0304-3959(01)00407-9. [DOI] [PubMed] [Google Scholar]
- Rigoli G, Terrini G, Cordioli Z. Intrathecal baclofen withdrawal syndrome caused by low residual volume in the pump reservoir: A report of 2 cases. Arch Phys Med Rehabil. 85:2064–66. doi: 10.1016/j.apmr.2004.02.020. [DOI] [PubMed] [Google Scholar]
- Ripamonti C, Groff L, Brunelli C, et al. Switching from morphine to oral methadone in treating cancer pain: what is the equianalgesic dose ratio? J Clin Oncol. 1998;16:3216–21. doi: 10.1200/JCO.1998.16.10.3216. [DOI] [PubMed] [Google Scholar]
- Rosen LS, Gordon D, Kaminski M, et al. Zoledronic acid versus pamidronate in the treatment of skeletal metastases in patients with breast cancer or osteolytic lesions of multiple myeloma: a phase III, double-blind, comparative trial. Cancer J. 2001;7:377–87. [PubMed] [Google Scholar]
- Roth A, Kolaric K. Analgesic activity of calcitonin in patients with painful osteolytic metastases of breast cancer: results of a controlled randomized study. Oncology. 1986;43:283–87. doi: 10.1159/000226383. [DOI] [PubMed] [Google Scholar]
- Rouff G, Lema M. Strategies in pain management: new and potential indications for COX-2 specific inhibitors. J Pain Symptom Manage. 2003;25(Suppl 2):S21–31. doi: 10.1016/s0885-3924(02)00628-0. [DOI] [PubMed] [Google Scholar]
- Rowbotham M, Harden N, Stacey B, et al. Gabapentin for the treatment of postherpetic neuralgia: a randomized controlled trial. JAMA. 1998;280:1837–42. doi: 10.1001/jama.280.21.1837. [DOI] [PubMed] [Google Scholar]
- Rozans M, Dreisbach A, Lertora JJ, et al. Palliative uses of methylphenidate in patients with cancer: a review. J Clin Oncol. 2002;20:335–39. doi: 10.1200/JCO.2002.20.1.335. [DOI] [PubMed] [Google Scholar]
- Russell IJ, Kamin M, Bennett RM, et al. Eficacy of tramadol in treatment of pain in fbromyalgia. J Clin Rheumatol. 2000;6:250–57. doi: 10.1097/00124743-200010000-00004. [DOI] [PubMed] [Google Scholar]
- Saarto T, Wiffen PJ. Antidepressants for neuropathic pain. Cochrane Database Syst Rev. 2005;3:CD005454. doi: 10.1002/14651858.CD005454. [DOI] [PubMed] [Google Scholar]
- Sarhill N, Walsh D, Nelson KA. Hydromorphone: pharmacology and clinical applications in cancer patients. Support Care Cancer. 2001;9:84–96. doi: 10.1007/s005200000183. [DOI] [PubMed] [Google Scholar]
- Schug SA, Zech D, Dorr U. Cancer pain management according to WHO analgesic guidelines. J Pain Symptom Manage. 1990;5:27–32. doi: 10.1016/s0885-3924(05)80006-5. [DOI] [PubMed] [Google Scholar]
- Serafini AN. Therapy of metastatic bone pain. J Nucl Med. 2001;42:895–906. [PubMed] [Google Scholar]
- Shah HN, Burton AW, Mendoza T, et al. Neurolytic blockade of the splanchnic nerves block relieves refractory pain associated with upper abdominal malignancy. Annual Meeting of the American Society of Anesthesiologists; October 11–15; San Francisco California. 2003. Abstract A1024. [Google Scholar]
- Sindrup SH, Bach FW, Madsen C, et al. Venlafaxine versus imipramine in painful polyneuropathy: a randomized, controlled trial. Neurology. 2003;60:1284–89. doi: 10.1212/01.wnl.0000058749.49264.bd. [DOI] [PubMed] [Google Scholar]
- Sindrup SH, Bjerre U, Dejgaard A, et al. The selective serotonin reuptake inhibitor citalopram relieves the symptoms of diabetic neuropathy. Clin Pharmacol Ther. 1992;52:547–52. doi: 10.1038/clpt.1992.183. [DOI] [PubMed] [Google Scholar]
- Sindrup SH, Gram LF, Brosen K, et al. The selective serotonin reuptake inhibitor paroxetine is effective in the treatment of diabetic neuropathy symptoms. Pain. 1990;42:135–44. doi: 10.1016/0304-3959(90)91157-E. [DOI] [PubMed] [Google Scholar]
- Singh D. Merck withdraws arthritis drug worldwide. BMJ. 2004;329:816. doi: 10.1136/bmj.329.7470.816-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaer TL. Practice guidelines for transdermal opioids in malignant pain. Drugs. 2004;64:2629–38. doi: 10.2165/00003495-200464230-00002. [DOI] [PubMed] [Google Scholar]
- Slavin KV, Hsu FPK, Fessler RG. Intrathecal opioids: intrathecal drug-delivery systems. In: Burchiel KJ, editor. Surgical management of pain. New York: Thieme; 2002. pp. 603–13. [Google Scholar]
- Slavin KV, Solko AM. Intrathecal narcotics: spinal and intraventricular. In: Schulder, editor. Handbook of stereotactic and functional neurosurgery. New York: Marcel Dekker; 2003. pp. 443–57. [Google Scholar]
- Slavin KV, Tesoro EP, Mucksavage JJ. The treatment of cancer pain. Drugs Today (Barc.) 2004;40:235–45. doi: 10.1358/dot.2004.40.3.820087. [DOI] [PubMed] [Google Scholar]
- Smith TJ, Staats PS, Deer T, et al. Randomized clinical trial of an implantable drug delivery system compared with comprehensive medical management for refractory cancer pain: impact on pain, drug-related toxicity, and survival. J Clin Oncol. 2002;20:4040–49. doi: 10.1200/JCO.2002.02.118. [DOI] [PubMed] [Google Scholar]
- Staats PS, Yearwood T, Charapata S, et al. Intrathecal ziconotide in the treatment of refractory pain in patients with cancer or AIDS. JAMA. 2004;291:63–70. doi: 10.1001/jama.291.1.63. [DOI] [PubMed] [Google Scholar]
- Szanto J, Ady N, Jozsef S. Pain killing with calcitonin nasal spray in patients with malignant tumors. Oncology. 1992;49:180–82. doi: 10.1159/000227035. [DOI] [PubMed] [Google Scholar]
- Taub E. Spinal cord stimulation for cancer-related pain: a neglected indication?. Quadrennial Meeting Am Soc Stereotactic Funct Neurosurg; May 18–21; New York. 2003. Abstract 52. [Google Scholar]
- Tremont-Lukats IW, Megeff C, Backonja MM. Anticonvulsants for neuropathic pain syndromes: mechanism of action and place in therapy. Drugs. 2000;60:1029–52. doi: 10.2165/00003495-200060050-00005. [DOI] [PubMed] [Google Scholar]
- Trujillo KA, Akil H. Inhibition of morphine tolerance and dependence by the NMDA receptor antagonist MK-801. Science. 1991;251:85–7. doi: 10.1126/science.1824728. [DOI] [PubMed] [Google Scholar]
- Turk DC, Monarch ES, Williams AD. Cancer patients in pain: considerations for assessing the whole person. Hematol Oncol Clin North Am. 2002;16:511–25. doi: 10.1016/s0889-8588(02)00015-1. [DOI] [PubMed] [Google Scholar]
- Valdimarsdottir U, Helgason AR, Furst CJ, et al. The unrecognized cost of cancer patients’ unrelieved symptoms: a nationwide follow-up of their surviving partners. Br J Cancer. 2002;86:1540–45. doi: 10.1038/sj.bjc.6600271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varvel JR, Shafer SL, Hwang SS, et al. Absorption characteristics of transdermally administered fentanyl. Anesthesiology. 1989;70:928–34. doi: 10.1097/00000542-198906000-00008. [DOI] [PubMed] [Google Scholar]
- Vestergaard K, Andersen G, Gottrup H, et al. Lamotrigine for central poststroke pain: a randomized controlled trial. Neurology. 2001;56:184–90. doi: 10.1212/wnl.56.2.184. [DOI] [PubMed] [Google Scholar]
- Vielhaber A, Portenoy RK. Advances in cancer pain management. Hematol Oncol Clin North Am. 2002;16:527–41. doi: 10.1016/s0889-8588(02)00016-3. [DOI] [PubMed] [Google Scholar]
- Walsh TD. Controlled study of imipramine and morphine in chronic pain due to advanced cancer. Proc Am Soc Clin Oncol. 1986;5:237. [Google Scholar]
- Watanabe S, Bruera E. Corticosteroids as adjuvant analgesics. J Pain Symptom Manage. 1994;9:442–45. doi: 10.1016/0885-3924(94)90200-3. [DOI] [PubMed] [Google Scholar]
- Watson CP. The treatment of neuropathic pain: antidepressants and opioids. Clin J Pain. 2000;16(Suppl 2):49–55. doi: 10.1097/00002508-200006001-00009. [DOI] [PubMed] [Google Scholar]
- Webster L, Henderson R, Katz N, et al. Characterization of confusion, an adverse event associated with intrathecal ziconotide infusion in chronic pain patients. Pain Med. 2001;2:253–54. [Google Scholar]
- Weinbroum AA, Bender B, Nirkin A, et al. Dextromethorphan-associated epidural patient-controlled analgesia provides better pain- and analgesics-sparing effects than dextromethorphan-associated intravenous patient-controlled analgesia after bone-malignancy resection: a randomized, placebo-controlled, double-blinded study. Anesth Analg. 2004;98:714–22. doi: 10.1213/01.ane.0000100151.56901.eb. [DOI] [PubMed] [Google Scholar]
- Wermeling D, Drass M, Ellis D, et al. Pharmacokinetics and pharmacodynamics of intrathecal ziconotide in chronic pain patients. J Clin Pharmacol. 2003;43:624–36. [PubMed] [Google Scholar]
- Whittle IR, Jenkinson JL. CT-guided stereotactic antero-medial pulvinotomy and centromedian-parafascicular thalamotomy for intractable malignant pain. Br J Neurosurg. 1995;9:195–200. doi: 10.1080/02688699550041548. [DOI] [PubMed] [Google Scholar]
- Wilson PR, Caplan RA, Connis RT, et al. Practice guidelines for chronic pain management: a report by the American Society of Anesthesiologists Task Force on Pain Management, Chronic Pain Section. Anesthesiology. 1997;86:995–1004. [PubMed] [Google Scholar]
- Winslow M, Seymour J, Clark D. Stories of cancer pain: a historical perspective. J Pain Symptom Manage. 2005;29:22–31. doi: 10.1016/j.jpainsymman.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Wong DL, Baker CM. Pain in children: comparison of assessment scales. Pediatr Nurs. 1988;14:9–17. [PubMed] [Google Scholar]
- Wong ET, Gunes S, Guaghan E, et al. Palliation of intractable cancer pain by MRI-guided cingulotomy. Clin J Pain. 1997;13:260–63. doi: 10.1097/00002508-199709000-00013. [DOI] [PubMed] [Google Scholar]
- Yajnik S, Singh GP, Singh G, et al. Phenytoin as a coanalgesic in cancer pain. J Pain Symptom Manage. 1992;7:209–30. doi: 10.1016/0885-3924(92)90077-u. [DOI] [PubMed] [Google Scholar]
- Zakrzewska JM, Chaudhry Z, Nurmikko TJ, et al. Lamotrigine (lamictal) in refractory trigeminal neuralgia: results from a double-blind placebo controlled crossover trial. Pain. 1997;73:223–30. doi: 10.1016/S0304-3959(97)00104-8. [DOI] [PubMed] [Google Scholar]
- Zech DF, Grond S, Lynch J, et al. Validation of World Health Organization guidelines for cancer pain relief: a 10-year prospective study. Pain. 1995;63:65–76. doi: 10.1016/0304-3959(95)00017-M. [DOI] [PubMed] [Google Scholar]


