Abstract
A recombinant plasmid carrying the proBA (pro-74) mutant allele which governs osmotic tolerance and proline overproduction was constructed by using the broad-host-range plasmid vector pQSR49. The physiological, biochemical, and genetic properties of strains carrying the pQSR49 derivatives pMJ101 and pMJ1, mutant and wild type, respectively, were investigated. pMJ101 conferred enhanced osmotolerance compared with strains carrying the wild type, pMJ1. These results are in contrast to those obtained previously with strains carrying recombinant plasmids based on pBR322 that failed to confer the osmotic tolerance phenotype. gamma-Glutamyl kinase (first step in proline biosynthesis) from strains carrying pMJ101 was 200-fold less sensitive to feedback inhibition than was the wild-type enzyme. As expected, the intracellular proline levels of strains carrying pMJ101 were more than an order of magnitude higher than those of the wild type. An analysis of copy number revealed that the pQSR49 constructs were present in the cell at a level six- to eightfold lower than those of the pBR322 recombinants, which may account for the difference in phenotype. We found that the genetic stability of the pQSR49 derivative in a variety of gram-negative bacteria was dependent on the insert orientation and the presence of foreign DNA on the plasmid. These factors may be significant in future studies aimed at expanding the osmotolerance phenotype to a broad range of gram-negative bacteria.
Full text
PDF

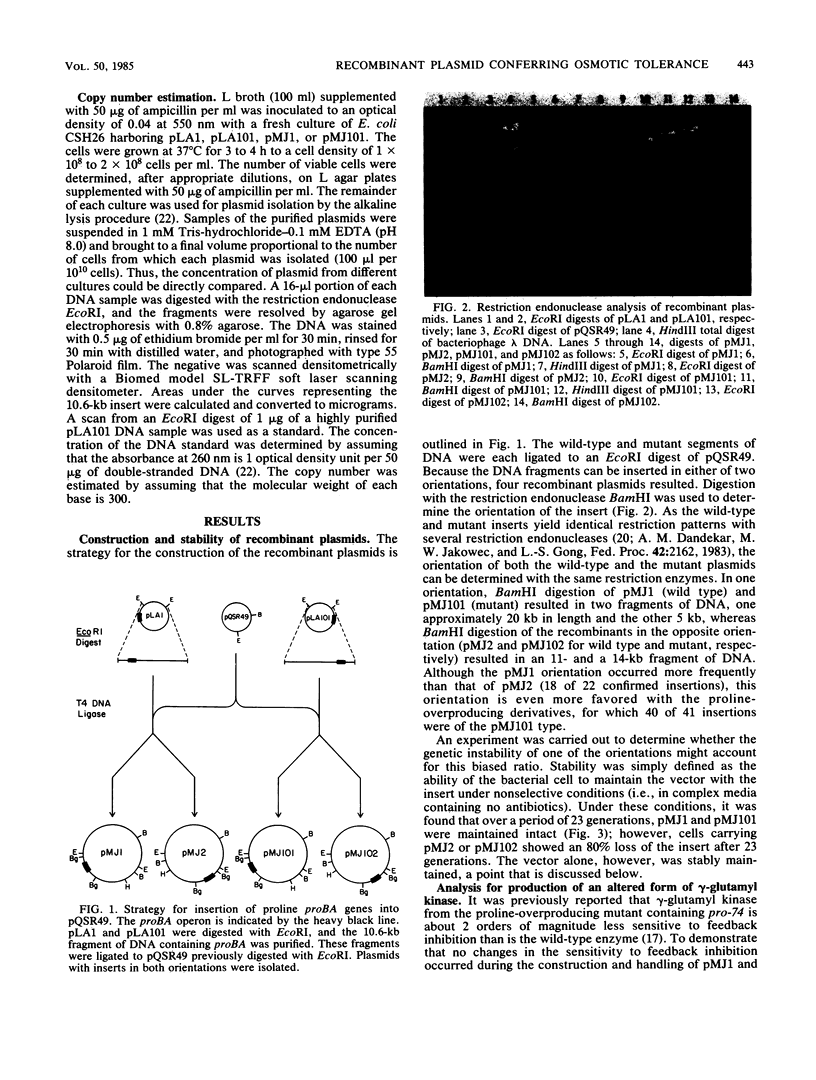
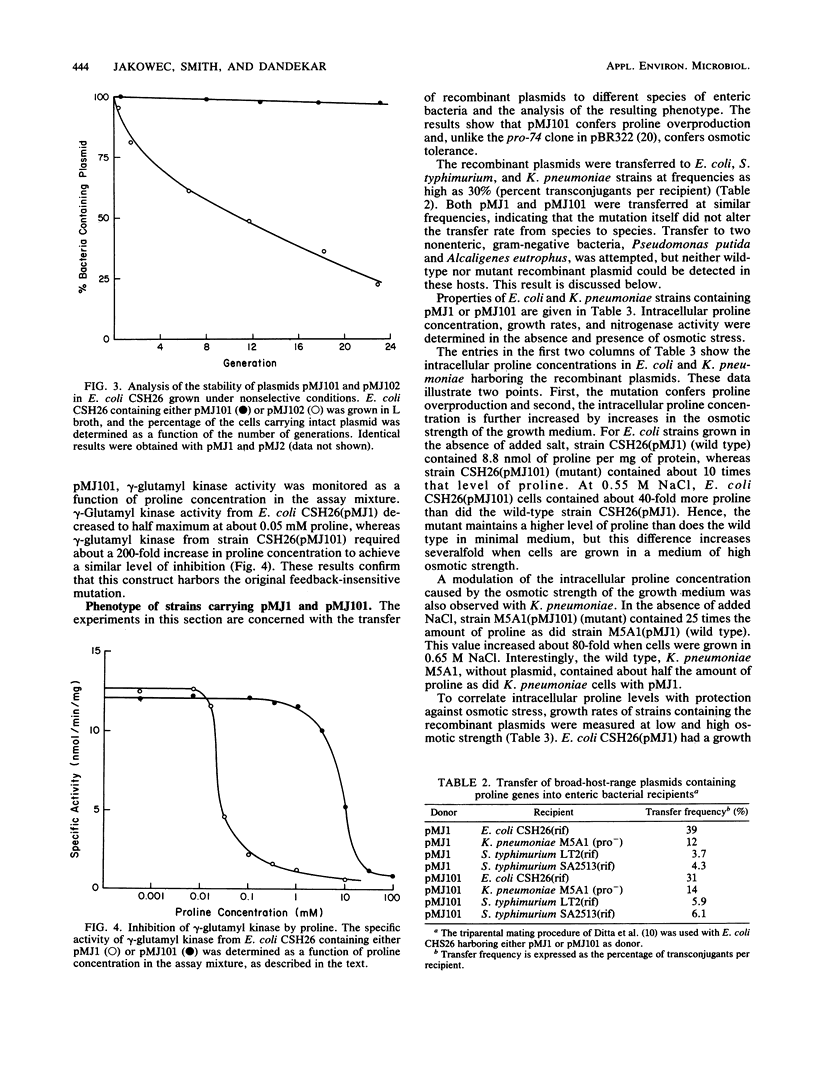
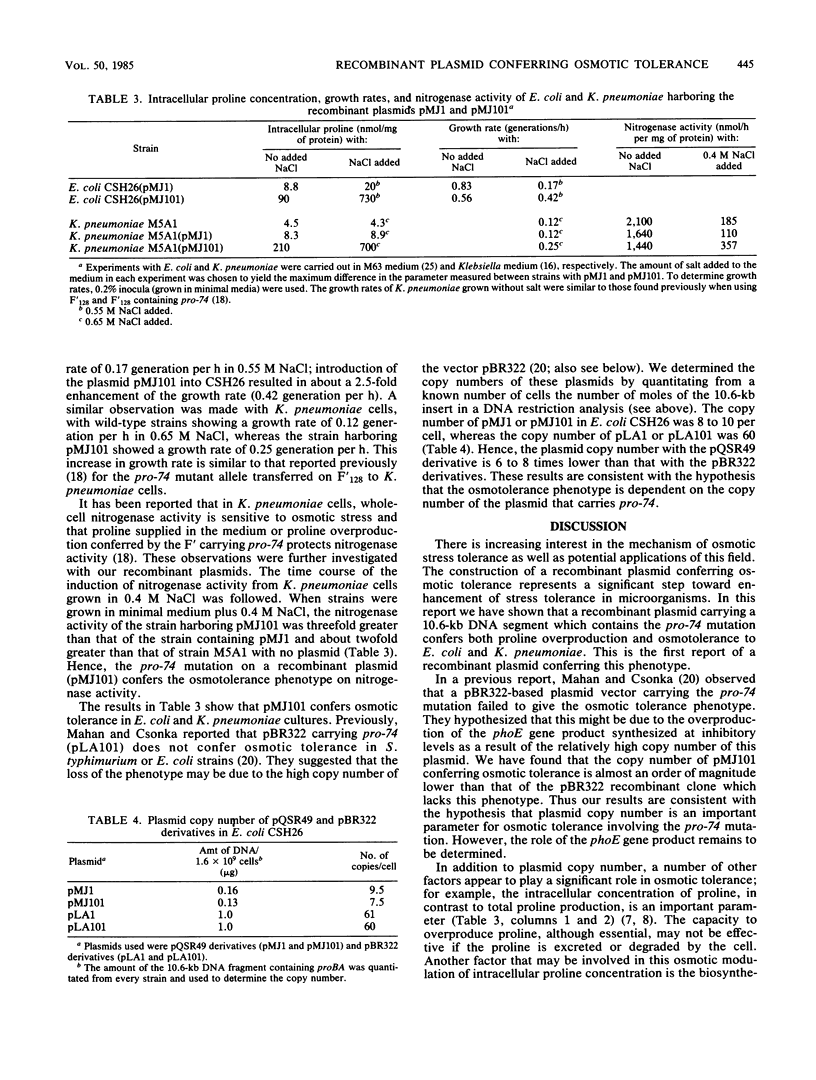

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baich A. Proline synthesis in Escherichia coli. A proline-inhibitable glutamic acid kinase. Biochim Biophys Acta. 1969 Dec 30;192(3):462–467. doi: 10.1016/0304-4165(69)90395-x. [DOI] [PubMed] [Google Scholar]
- Baich A. The biosynthesis of proline in Escherichia coli: phosphate-dependent glutamate -semialdehyde dehydrogenase (NADP), the second enzyme in the pathway. Biochim Biophys Acta. 1971 Jul 20;244(1):129–134. doi: 10.1016/0304-4165(71)90129-2. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csonka L. N. A third L-proline permease in Salmonella typhimurium which functions in media of elevated osmotic strength. J Bacteriol. 1982 Sep;151(3):1433–1443. doi: 10.1128/jb.151.3.1433-1443.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csonka L. N. Proline over-production results in enhanced osmotolerance in Salmonella typhimurium. Mol Gen Genet. 1981;182(1):82–86. doi: 10.1007/BF00422771. [DOI] [PubMed] [Google Scholar]
- Deutch A. H., Rushlow K. E., Smith C. J. Analysis of the Escherichia coli proBA locus by DNA and protein sequencing. Nucleic Acids Res. 1984 Aug 10;12(15):6337–6355. doi: 10.1093/nar/12.15.6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayzer D. J., Leisinger T. Proline biosynthesis in Escherichia coli. Purification and characterisation of glutamate-semialdehyde dehydrogenase. Eur J Biochem. 1982 Jan;121(3):561–565. doi: 10.1111/j.1432-1033.1982.tb05823.x. [DOI] [PubMed] [Google Scholar]
- Hayzer D. J., Leisinger T. The gene-enzyme relationships of proline biosynthesis in Escherichia coli. J Gen Microbiol. 1980 Jun;118(2):287–293. doi: 10.1099/00221287-118-2-287. [DOI] [PubMed] [Google Scholar]
- Hua S. S., Tsai V. Y., Lichens G. M., Noma A. T. Accumulation of Amino Acids in Rhizobium sp. Strain WR1001 in Response to Sodium Chloride Salinity. Appl Environ Microbiol. 1982 Jul;44(1):135–140. doi: 10.1128/aem.44.1.135-140.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K., Meyer R. J. Novel system for recognizing and eliminating foreign DNA in Pseudomonas putida. J Bacteriol. 1984 Aug;159(2):678–682. doi: 10.1128/jb.159.2.678-682.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Le Rudulier D., Bouillard L. Glycine betaine, an osmotic effector in Klebsiella pneumoniae and other members of the Enterobacteriaceae. Appl Environ Microbiol. 1983 Jul;46(1):152–159. doi: 10.1128/aem.46.1.152-159.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Rudulier D., Strom A. R., Dandekar A. M., Smith L. T., Valentine R. C. Molecular biology of osmoregulation. Science. 1984 Jun 8;224(4653):1064–1068. doi: 10.1126/science.224.4653.1064. [DOI] [PubMed] [Google Scholar]
- Le Rudulier D., Yang S. S., Csonka L. N. Nitrogen fixation in Klebsiella pneumoniae during osmotic stress. Effect of exogenous proline or a proline overproducing plasmid. Biochim Biophys Acta. 1982 Nov 24;719(2):273–283. doi: 10.1016/0304-4165(82)90099-x. [DOI] [PubMed] [Google Scholar]
- Mahan M. J., Csonka L. N. Genetic analysis of the proBA genes of Salmonella typhimurium: physical and genetic analyses of the cloned proB+ A+ genes of Escherichia coli and of a mutant allele that confers proline overproduction and enhanced osmotolerance. J Bacteriol. 1983 Dec;156(3):1249–1262. doi: 10.1128/jb.156.3.1249-1262.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Measures J. C. Role of amino acids in osmoregulation of non-halophilic bacteria. Nature. 1975 Oct 2;257(5525):398–400. doi: 10.1038/257398a0. [DOI] [PubMed] [Google Scholar]
- Meyer R., Laux R., Boch G., Hinds M., Bayly R., Shapiro J. A. Broad-host-range IncP-4 plasmid R1162: effects of deletions and insertions on plasmid maintenance and host range. J Bacteriol. 1982 Oct;152(1):140–150. doi: 10.1128/jb.152.1.140-150.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait R. C., Close T. J., Rodriguez R. L., Kado C. I. Isolation of the origin of replication of the IncW-group plasmid pSa. Gene. 1982 Nov;20(1):39–49. doi: 10.1016/0378-1119(82)90085-3. [DOI] [PubMed] [Google Scholar]
- Tempest D. W., Meers J. L., Brown C. M. Influence of environment on the content and composition of microbial free amino acid pools. J Gen Microbiol. 1970 Dec;64(2):171–185. doi: 10.1099/00221287-64-2-171. [DOI] [PubMed] [Google Scholar]