Abstract
Direct measurement of neuropeptides in the hypothalamus is essential for neuroendocrine studies. However, the small quantities of peptides released at their neuroterminals and relatively large molecular sizes make these measurements difficult. We have evaluated microdialysis probes with two membrane materials (polycarbonate and polyarylethersulfone, both: molecular cut off 20,000 daltons) in vitro, and adapted the method for in vivo hypothalamic sample collection in non-human primates. The results of in vitro experiments showed that the polyarylethersulfone membrane yielded a several fold higher recovery rate than the polycarbonate membrane. In in vivo experiments, a guide cannula with stylet was inserted into the medial basal hypothalamus through the permanently implanted cranial pedestal under light sedation. The stylet was replaced by a microdialysis probe and artificial CSF was infused. The results indicated that the neuropeptide luteinizing hormone-releasing hormone was readily measurable in dialysates collected at 10 min-intervals, and responded to neuroactive substances applied through the probe. The animals were fully conscious except for the initial hour of sampling. After the experiment the animal was returned to the home cage, and later similarly examined during several additional experiments. Therefore, the microdialysis method described here is a highly useful tool for neuroendocrine studies in non-human primates.
Keywords: LHRH, GnRH, neuropeptides, microdialysis, pulsatility, hypothalamus, primates
Introduction
In vivo studies can provide vital physiological information that cannot be elucidated through in vitro experiments. In particular, direct in vivo measurement of neuropeptide, neurotransmitter, and neuromodulator release in the stalk-median eminence (S-ME) or portal circulation in unanesthetized conscious animals is essential for the study of neuroendocrine function. For direct assessment of in vivo events in the brain, two methods, push-pull perfusion and microdialysis, have been used to monitor the release of a variety of neuropeptidergic regulators. The push-pull perfusion method employs double lumen cannulae allowing collection of tissue “washout” samples from the S-ME, where artificial cerebrospinal fluid (aCSF) is perfused though the push-cannula, while washout samples are collected though the pull cannula, using two respective push and pull pumps. The microdialysis method employs a semi-permeable membrane attached to the end of two cannulae (inlet and outlet) allowing biochemical substances to enter samples by concentration equilibrium across the membrane placed in the base of the hypothalamus, while aCSF is slowly perfused by a pump. Although both approaches are very powerful, each has strengths and weaknesses (Levine et al., 1994; Terasawa, 1994; Robinson, 1995; Myers et al., 1998).
For many years, this lab has used a unique push-pull perfusion method in unaesthetized conscious rhesus monkeys, with which local, real-time changes of neuroendocrine processes in the S-ME have been assessed. This method is a non-terminal procedure and a series of samples can be obtained repeatedly from a single monkey up to a dozen times (Gearing and Terasawa, 1988; Terasawa, 1994). Moreover, with this method, not only can catecholamines and amino acid neurotransmitters be measured, but also various neuropeptides such as luteinizing hormone-releasing hormone (LHRH), neuropeptide Y, growth hormone-releasing hormone, and somatostatin can be measured in the S-ME (Watanabe and Terasawa, 1989; Claypool et al., 1990; Woller et al., 1992; Nakamura et al., 2003). Further, this method allows application of neuroactive substances directly to the perfusion site (Gore et al., 1993; Mitsushima et al., 1994; Terasawa, 1994). However, the push-pull perfusion method has a major drawback, in that prolonged chair-restraint is necessary for experiments in monkeys: Occlusion of the cannula can often occur as a consequence of tissue debris entering through the pull flow, thus the procedure requires a delicate balance between push and pull flow to minimize potential tissue damage. The latter problem requires a 2-day waiting period after cannula insertion, during which tissue debris is absorbed.
The microdialysis method with a permanently implanted cranial guide cannula has been extensively used in many species including rats, sheep, and monkeys (Westerink and Justice, 1991; Kendrick, 1989; 1990, Centeno et al., 2007). However, even though the microdialysis method does not require a prolonged chairing period, the measurement of neuropeptides with this method is challenging and can be problematic for detection of the peptide in an immunoassay, as the recovery rate of neuropeptides through the semi-permeable dialysis membrane is low. Moreover, the chronically implanted guide cannula system does not allow repeated sampling from the brain in the same subject. Nonetheless, significant success in measuring LHRH from rats using this method (Chappell and Levine, 2000, Sisk et al, 2001, Harris and Levine, 2003) encouraged us to adapt the microdialysis method as an alternative tool for in vivo neuropeptide sampling in rhesus monkeys. This paper reports the successful adaptation of the microdialysis method for the measurement of neuropeptide release in the S-ME of non-human primates, with which a series of samples can be obtained several times repeatedly from an individual monkey.
Materials and Methods
Animals
Four long-term ovariectomized adult and two ovarian intact pubertal female rhesus monkeys (Macaca mulatta) were used in this study. The ages of ovariectomized monkeys and intact monkeys were 8–13 years and 2.5–3 years old, respectively. All animals born and raised at the Wisconsin National Primate Research Center (Madison, WI) were housed in pairs (cages 172 × 86 × 86 cm) in rooms with 12 h of light (0600–1800 h), 12 h of dark (1800–0600 h), and controlled temperature (22° C). The animals were fed a standard diet of Harlan 20% Protein Primate Diet once every morning, supplemented with fresh fruit several times per week. Water was available ad libitum. The protocol for this study was reviewed and approved by the Animal Care and Use Committee, University of Wisconsin, and all experiments were conducted under the guidelines established by the NIH and USDA.
Microdialysis system specifications
For in vivo microdialysis in rhesus monkeys we used a custom-made guide cannula and probe set from CMA/Microdialysis (CMA10 and CMA12, Stockholm, Sweden). The guide cannula consisted of a stainless steel shaft (96.0 mm in length, 0.91 mm o.d.) and a removable stainless steel stylet (96.2 mm in length, 0.6 mm o.d.), which allowed insertion of the probe into the hypothalamus before the experiment. The custom-made microdialysis probe (Fig. 1) had a stainless steel shaft (96.2 mm in length, 0.6 mm o.d.), fitted with a membrane (4 mm in length, 0.5 mm o.d.) which was made of either polycarbonate (PC) or polyarylethersulfone (PAES). Both PC and PAES membranes had a molecular cut off 20,000 daltons. The membrane portion of the microdialysis probe was designed to extrude beyond the guide cannula in the brain. Microdialysis probes with both PC and PAES membranes were gas-sterilized with a standard method using anprolene.
Figure 1.
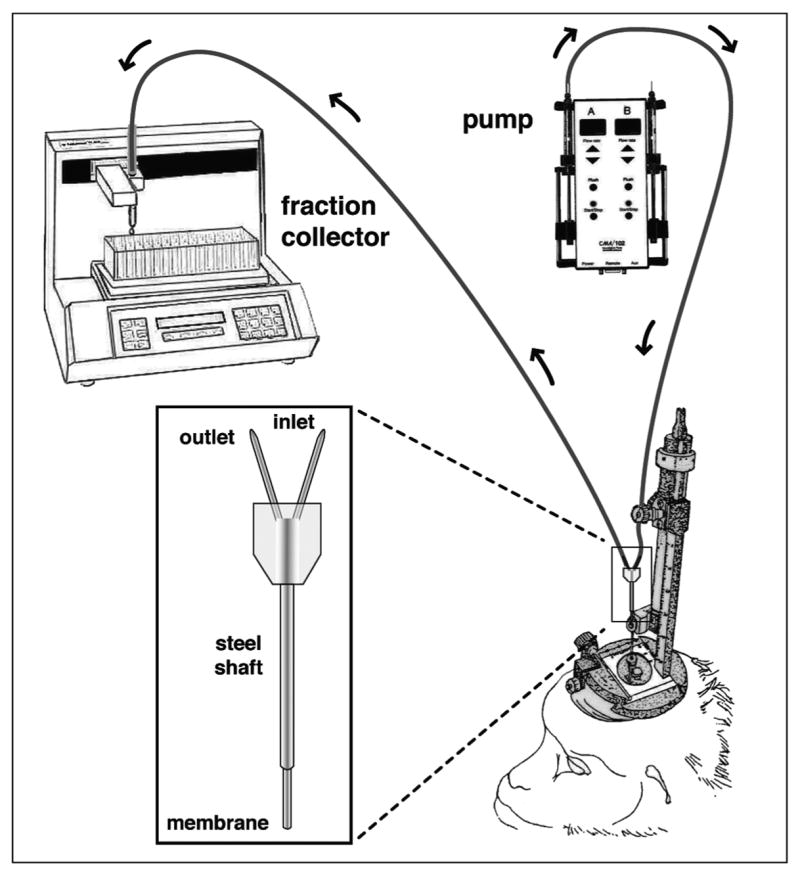
Schematic illustration of the microdialysis system in non-human primates. A custom-made microdialysis probe (insert) for the rhesus monkey was inserted into the medial basal hypothalamus through the guide cannula. The inlet cannula of the microdialysis probe was connected to the infusion pump with a gas tight syringe and the outlet cannula was connected to the fraction collector. Insert: the dialysis membrane made of either polycarbonate or polyarylethersulfone (molecular cut off of the membranes are 20,000 daltons) is glued on the bottom of concentric inlet and outlet cannulae, through which perfusion fluid enters and comes out, respectively. The permeable membrane allows for chemical equilibrium between the fluid inside the probe and the extracellular tissue. Note that prior to the probe insertion, a guide cannula with a stylet was inserted into the medial basal hypothalamus through a permanently implanted cranial pedestal on the monkey skull using a hydraulic microdrive unit and x-ray ventriculographs under light sedation. After insertion the monkey was placed in a chair, to which the monkey was well adapted, the stylet was removed, and the probe was inserted into the aiming point through the guide cannula.
Perfusion of the dialysate solution through the probe was accomplished using CMA/102 pump apparatus (Stockholm, Sweden) outfitted with a 1 or 2.5 ml Hamilton gas tight syringe (Reno, NV) and samples were collected using a fraction collector (Model FC203B, Gilson, Middleton, WI). For the connection between infusion pump and the inlet of the microdialysis probe (inflow) FEP tubing (CMA/Microdialysis, Stockholm, Sweden) was used. Similarly, FEP tubing was used for the connection between the outlet of the microdialysis probe (outflow) and the fraction collector (Fig. 1).
Cranial Chamber Implantation
Before microdialysis perfusion experiments began, 4 long-term ovariectomized adult female monkeys and 2 ovarian intact pubertal monkeys were implanted with a stainless steel cranial pedestal (20 mm o.d.) under isoflurane anesthesia as described previously (Gearing and Terasawa, 1988, Terasawa, 1994). The pedestal was centered stereotaxically straight above the infundibular recess of the third ventricle and fixed to the skull with surgical bolts and dental acrylic. The infundibular recess was visualized by ventriculographs with injections of a radioopaque dye. Following implantation the animal was allowed to recover at least 1 month before the microdialysis perfusion experiments. Each monkey was well adapted to a primate chair as well as the experimental environment before the initiation of experiments as described previously (Gearing and Terasawa, 1988, Terasawa, 1994).
Insertion of guide cannula and microdialysis probe
On the day of the experiment, prior to the insertion in the brain, the microdialysis system (pump-probe-fraction collector) was flushed with aCSF-1 (see below) or aCSF-2 (perfusion fluid CNS by CMA/Microdialysis, see below) at a speed of 10 μl/min for 5 min and then 2 μl/min for 60–90 min. This procedure prevented the potential occlusion of the system prior to introduction of the probe into the monkey brain.
The monkey was placed in the stereotaxic apparatus under ketamine (15mg/kg b.w.) and medetomidine (0.03–0.05 mg/kg b.w.) anesthesia. The guide cannula with stylet, which extruded 0.2 mm from the guide cannula tip, was inserted into the skull 4 mm above the S-ME with a hydraulic microdrive unit (MO95-B, Narshige, Tokyo, Japan). The microdrive unit allowed for accurate three-dimensional adjustment of the tip location. The x, y, and z coordinates for the S-ME were calculated using ventriculographs and the final radiographs taken during chamber implantation surgery. Cannula placement was confirmed with radiographic visualization, as described previously (Gearing and Terasawa, 1988, Terasawa, 1994). To prevent the placement of the cannula tip in the third ventricle, the cannula was inserted 0.3–0.8 mm lateral to the midline.
Following placement of the cannula, the monkey was removed from the stereotaxic apparatus and placed into a primate chair, to which the animal was well adapted before the experiment. Once the monkey was properly placed in the chair, the inner stylet was removed from the guide cannula and quickly (less than 5 s) replaced with the microdialysis probe. To reverse the effects of medetomidine, atipamazole (0.15–0.25mg/kg) was injected to the animal.
In vivo microdialysis perfusion
A modified Krebs-Ringer phosphate buffer solution (aCSF-1, 123 mM NaCl, 4.8 mM KCl, 1.22 mM MgSO4, 13.9 mM NaHPO4, 2.45mM Na2PO4, 1 mM CaCl2, pH 7.4) containing bacitracin (4 U/ml) or perfusion fluid CNS (aCSF-2, NaCl 147mM, KCl 2.7mM, CaCl2 1.2mM, MgCl2 0.85m, purchased from CMA/Microdialysis) containing bacitracin (4 U/ml) was infused through the inflow tubing at 2 μl/min with the microdialysis pump. Perfusates were continuously collected at 10 min intervals for up to 12 hours through the outflow tubing into 12 × 75 mm borosilicate tubes containing 280 μl of RIA buffer (solution containing 0.1% gelatin in 0.01M PO4, 0.15M NaCl, 0.1% NaN3 at pH 7.4) on ice with the fraction collector. The perfusate samples were immediately frozen on dry ice and stored at −80°C.
LHRH agonist challenge
We have previously demonstrated that a LHRH receptor agonist can stimulate LHRH release by a putative paracrine action (Pu and Terasawa, 1994). Therefore, to assess the ability of the microdialysis system to detect secretagogue-induced LHRH release, the effects of an LHRH agonist (Des-Gly10, [D-Ala6]-LHRH Ethylamide, Sigma, St. Louis, MO) at 100 nM or 1 μM in aCSF-1 were examined. After at least a 20 min control sampling period, LHRH agonist was infused through the inflow tubing at 2 μl/min for 20 minutes, while perfusates were continuously collected at 10 min intervals. When two LHRH agonist challenges were given successively, at least a 30-min interval was placed between two challenges.
Membrane recovery analysis in vitro
During the earlier stages of this study, only probes with PC membranes (CMA10) were available for our use. However, during the later stage of this study, probes with PAES membranes (CMA12) became available. Thus, we evaluated the recovery rate of both probes, using three in vitro approaches: In the first method, we infused 125I-LHRH at 200,000 and 1,600,000 cpm/ml concentrations dissolved in aCSF-1 and aCSF-2, respectively, through microdialysis probes at a speed of 2 μl/min, while 2 consecutive samples at 10-min intervals were collected, and cpm/ml in the samples was measured by a γ-counter (Model 1185, Searle, Chicago, IL). In the second method, we perfused aCSF-2 through microdialysis probes placed in a reservoir of 125I-LHRH at a 1,800,000 cpm/ml concentration dissolved in aCSF-2, while 2 consecutive samples at 10-min intervals were collected at a speed of 2 μl/min and cpm/ml in samples was measured by γ-counter. In the first and second experiments, the recovery rate was calculated from cpm/ml counts. In the third method, to mimic in vivo experiments, we infused aCSF-1 or aCSF-2 through the probe in a reservoir of aCSF-1 or aCSF-2 containing 0.1, 1, or 10 nM LHRH at a speed of 2 μl/min, while continuously collecting samples at 10-min intervals for 40 min. LHRH concentrations in dialysate samples and reservoir solutions were assessed with RIA, and subsequently the recovery rate was calculated. In all in vitro experiments we used different probes, except for Experiments I and II with aCSF-2 (Table 1), in which one PC or PAES probe was used for both.
Table 1.
Microdialysis membrane recovery rate assessed with in vitro experiments. Molecular weight of LHRH is 1183. Both polycarbonate and polyarylethersulfone membranes had a molecular cut off of 20,000 daltons.
| Experiments | Membrane composition | Recovery rate |
|---|---|---|
| Experiment I | ||
| 125I-LHRH Infusion (~200,000 cpm/ml in aCSF-1) | Polycarbonate | 1.5% |
| Polyarylethersulfone | 11.6% | |
|
| ||
| 125I-LHRH Infusion (~1,600,000 cpm/ml in aCSF-2) | Polycarbonate# | 2.0% |
| Polyarylethersulfone## | 8.0% | |
|
| ||
| Experiment II | ||
| 125I-LHRH Reservoir (~1,800,000 cpm/ml in aCSF-2) | Polycarbonate# | 4.9% |
| Polyarylethersulfone## | 8.1% | |
|
| ||
| Experiment III (RIA) | ||
| LHRH Reservoir (1 nM in aCSF-1) | Polycarbonate | 4.7% |
| Polyarylethersulfone | 19.2% | |
|
| ||
| LHRH Reservoir (0.1 nM in aCSF-2) | Polycarbonate | 7.3% |
| Polyarylethersulfone | 14.8% | |
|
| ||
| LHRH Reservoir (1 nM in aCSF-2) | Polycarbonate | 4.1% |
| Polyarylethersulfone | 19.6% | |
|
| ||
| Overall recovery: | cv | |
| Polycarbonate | 4.1 ± 0.9 %† | 22.0% |
| Polyarylethersulfone | 13.6 ± 2.1%* | 15.4% |
Mean±SEM,
p<0.001 vs. Polycarbonate,
and
indicate the same probe, respectively.
RIA
LHRH in perfusates collected both in vivo and in vitro (20 μl) were measured by RIA using antisera R1245, provided by Dr. Terry Nett (Colorado State University, Fort Collins, CO), as previously described (Gearing and Terasawa, 1988). Synthetic LHRH (Richelieu Biotechnologies Inc., Montréal Québec, Canada) was used for both radiolabeled antigen and the reference standard. The antigen-antibody complex was precipitated with a sheep anti-rabbit-γ-globulin. Assay sensitivity, defined as the amount of reference standard that reduced binding by 2 × SD from the total bound (B0), ranged from 0.02–0.1 pg/tube, and intra and interassay coefficients of variation were 11.7% and 15.7%, respectively. The crossreactivity of the LHRH agonist at 10–100 nM with our LHRH assay was not detectable, whereas the LHRH agonist at 1 μM was slightly cross reactive (0.5 pg/ml).
Statistical analysis
LHRH peaks in perfusates were identified using the PULSAR algorithm, as described previously (Merriam and Wachter, 1982; Woller et al., 1992). The cut-off criteria for pulse determination, G1, G2, G3, G4, and G5 were 4.4, 2.6, 1.92, 1.46, and 1.13, respectively. Parameters of pulsatile LHRH release were calculated for each experiment as follows. 1) Mean LHRH release was derived from the mean of all LHRH values; 2) basal LHRH release was defined as the mean of all trough LHRH values; the trough was the lowest value between two peaks, 3) LHRH pulse amplitude was defined as the difference between peak and trough; and 4) interpulse interval was defined as the intervals between peaks of LHRH pulses. Subsequently, mean ± SEM for each parameter was calculated from 4 animals. Differences between treatments were evaluated using the t test (unpaired, two-tailed). Significance was attained at p<0.05.
Results
Membrane recovery rate assessed by in vitro experiments (Table 1)
Experiment I, Infusion of 125I-LHRH
We conducted experiments with two different conditions: First, we infused 125I-LHRH at 200,000 cpm/ml dissolved in aCSF-1 while collecting dialysate through either PC membrane (20,000 cut off) or PAES membrane (20,000 cut off), and second we infused 125I-LHRH at 1,600,000 cpm/ml dissolved in aCSF-2. The results indicated that the PAES membrane yielded a several fold higher recovery rate than the PC membrane: With the PC membrane the recovery rate was 1.5% and 2.0%, whereas with the PAES membrane the recovery rate was 11.6% and 8.0% (Table 1).
Experiment II, Perfusion of the CSF-2 through 125I-LHRH reservoir
The results of perfusing aCSF-2 through the membranes also indicated that the recovery rate (8.1%) of the PAES membrane was higher than that (4.9%) of the PC membrane (Table 1).
Experiment III, LHRH reservoir containing LHRH at 0.01–1nM concentrations
We conducted experiments with two different conditions: First, we perfused aCSF-1 in 1 nM LHRH solution while collecting dialysate through either the PAES or PC membrane, and second, we perfused aCSF-2 across the PAES membrane or PC membrane in 0.01, 0.1 or 1 nM LHRH solution, while dialysates were collected. Probes with the PC membrane yielded a recovery rate of 4.1–4.7%, whereas probes with the PAES yielded a recovery rate of 14.8–19.6% (Table 1). Moreover, the peptide clearance through the PAES membrane was much better than that through the PC membrane: While with the PAES membrane the peptide concentration in the third sample after the termination of 0.1 nM LHRH infusion returned to the control level, with the PC membrane residual LHRH peptide was still found in the 4th and 5th samples after the termination of the same 0.1 nM LHRH infusion (Fig. 2). Similarly, with the PAES membrane the peptide level in the 4th sample after the 1 nM LHRH perfusion returned to the control level, with the PC membrane a residual LHRH peptide level was still found in the same 5th sample (Fig. 2).
Figure 2.
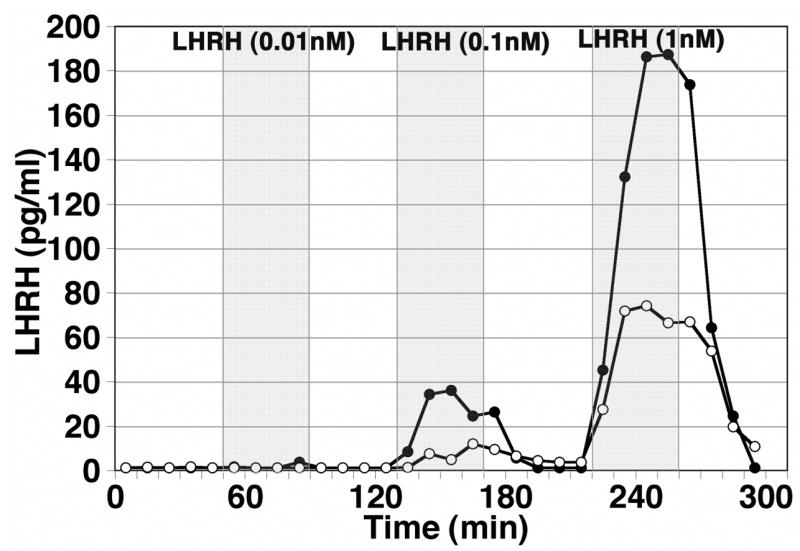
An assessment of the recovery rate in vitro. Artificial CSF was infused through microdialysis probes with either the polycarbonate (PC, open circle) or polyarylethersulfone (PAES, closed circle) membrane, which were placed in a reservoir containing LHRH at 0.01, 0.1 and 1 nM concentrations, while dialysates were continuously collected at 10 min intervals. LHRH concentrations in dialysates were measured by RIA. Note that the recovery rate with the PAES membrane was much higher than that with the PC membrane at both 0.1 and 1 nM reservoir concentrations. Neither membrane yielded detectable levels of LHRH at 0.01 nM reservoir concentration. It is also of interest to note that the PAES membrane allowed quicker clearance than the PC membrane.
The overall recovery rate calculated in three experiments for the PAES membrane (13.6 ± 2.1) was significantly (p<0.001) higher than that (4.1 ± 0.9) of the PC membrane (Table 1). In addition, a slightly lower cv (14.4%) with the PAES membrane than that (19.0%) with the PC (Table 1) suggests that a probe with the PAES membrane would yield more consistent results than a PC probe.
Pulsatile LHRH release
In vivo LHRH release in the S-ME in female ovariectomized monkeys measured by the microdialysis method with the PC membrane was pulsatile (Fig. 3A). Mean LHRH (9.9 ± 3.9 pg/ml), basal release (3.8 ± 2.1 pg/ml), amplitude (10.6 ± 4.3 pg/ml), and interpulse interval (55.8 ± 10.9 min) from 4 ovariectomized adult female monkeys were comparable to those with the push-pull perfusion method in female adult monkeys, as reported previously (Gearing and Terasawa, 1988). In vivo LHRH release in the S-ME in 2 ovarian intact pubertal female monkeys measured by the microdialysis method with the PAES membrane was also pulsatile (Fig. 3B). The profile of LHRH release with both the PC and PAES probes in the examples was very similar.
Figure 3.
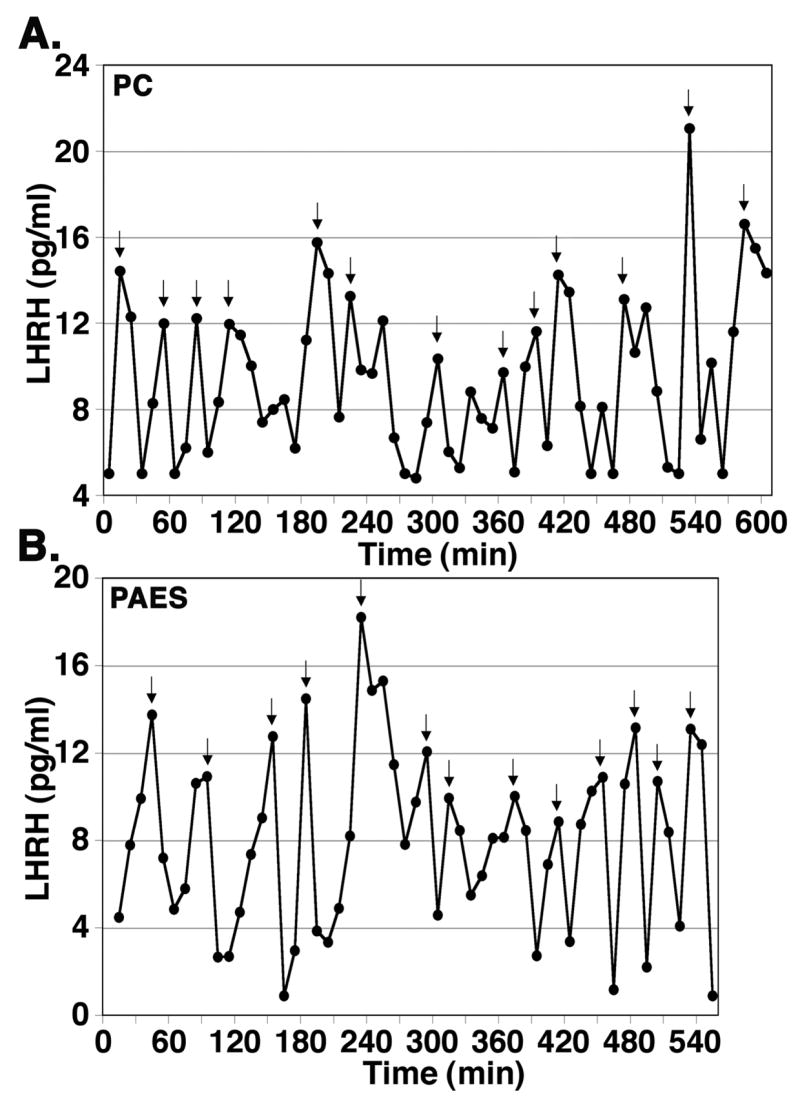
Examples of in vivo LHRH release in the stalk-median eminence measured by the microdialysis method with a PC membrane probe in an adult female ovariectomized rhesus monkey at 9 years of age (A) and with a PAES membrane probe in a pubertal female rhesus monkey at 2.7 years of age (B). Perfusate samples were collected at 10 min intervals for a 9–10 h period, and LHRH concentration in perfusates were measured by RIA. Arrowheads indicate LHRH pulses depicted by the PULSAR algorithm.
LHRH Agonist Challenge
We examined the effects of an LHRH agonist (Des-Gly10, [D-Ala6]-LHRH Ethylamide) on LHRH release. After control sampling with aCSF-1 perfusion, LHRH agonist at doses of 0.01 μM, 0.1 μM, or 1 μM was applied through the inflow tubing at 2 μl/min for 20 min, while perfusates were collected through a probe with the PC membrane. The results showed that LHRH agonist induced a dose dependent increase in LHRH release (Fig 4). Considering the recovery rate across the membrane was only a few percent, local concentrations of an LHRH agonist outside of the membrane would be approximately 100 fold less than the infused amount. Nonetheless, the LHRH response to the LHRH agonist at 0.1 μM was significantly (p<0.05) larger than the response to LHRH agonist at 0.01 μM (Table 2).
Figure 4.
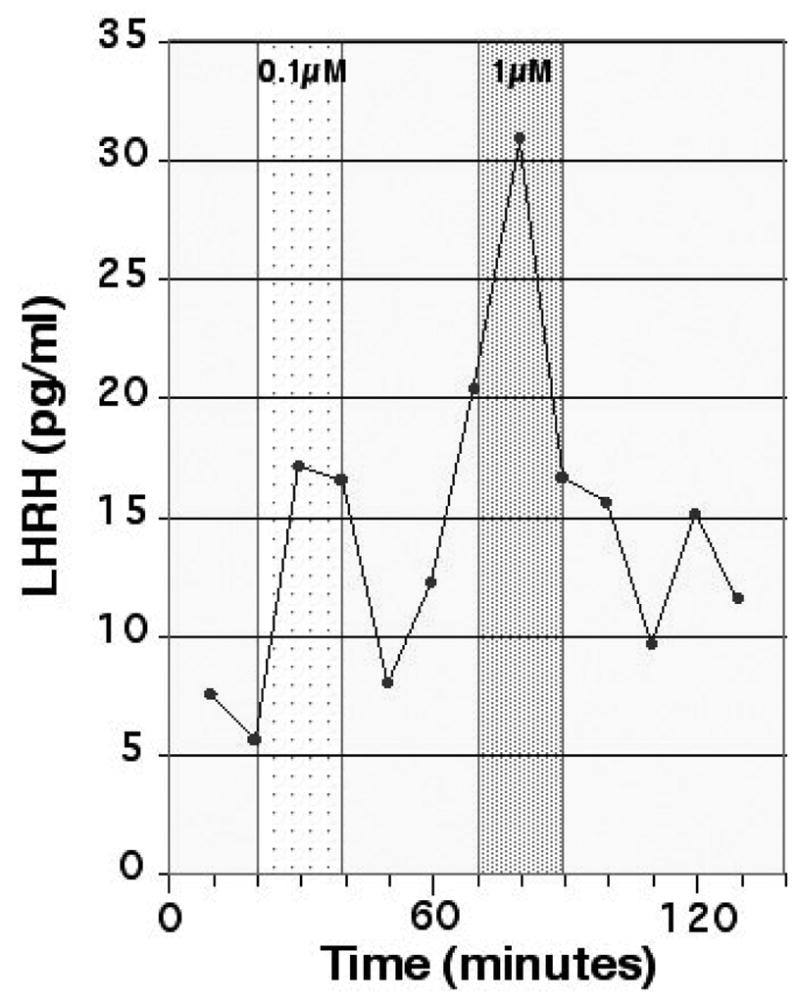
Effects of an LHRH agonist (Des-Gly10, [D-Ala6]-LHRH Ethylamide) at 0.1 μM and 1 μM in an adult female ovariectomized rhesus monkey at 10 years of age. LHRH agonist was infused for a period of 20 min (indicated by shading) while perfusates were continuously collected. Approximately 1 % of the LHRH agonist was diffused through the microdialysis membrane and thus the LHRH agonist at these concentrations was not cross-reactive with our LHRH assay. Note that the infusion of both doses of the LHRH agonist at 0.1 and 1 μM (which was estimated as 1 and 10 nM LHRH agonist concentration, respectively) caused a significant LHRH increase.
Table 2.
Effects of an LHRH agonist (Des-Gly10, [D-Ala6]-LHRH Ethylamide) on in vivo LHRH release in the hypothalamus measured by microdialysis method in 4 adult female ovariectomized rhesus monkeys ranging in age from 8 to 13 years. Note that there is a doses response to the LHRH agonist.
| Dose applied to probe | Estimate dose though dialysis membrane | LHRH response (pg/ml) |
|---|---|---|
| 0.01 μM | 0.1 nM | 4.4 ± 2.2* (n=3) |
| 0.1 μM | 1 nM | 10.9 ± 0.8† (n=4) |
| 1 μM | 10 nM | 12.1 (n=1) |
Mean ± SEM
p<0.05 vs. 0.1 μM
Discussion
In the present study we have shown that microdialysis perfusion with a PAES probe is an excellent method for the assessment of in vivo LHRH release, and potentially for monitoring release of other neuropeptides, in the brain of conscious animals. Although the measurement of circulating anterior pituitary hormones has been widely used as an alternative tool for assessment of stimulatory and inhibitory hypothalamic peptides, the microdialysis method offers direct measurement of neuropeptide release in the medial basal hypothalamus. In fact, the approaches used in this study with a microdialysis guide cannula inserted into the collection site through the cranial pedestal right before probe insertion, are similar to those described for the push-pull perfusion method (Gearing and Terasawa, 1988; Terasawa, 1994), and allows repeated sampling from a single non-human primate, a species which is a highly limited resource. This is a significantly important feature, because it not only yields real-time in vivo data under a variety of physiological conditions without anesthesia but it is also important for longitudinal developmental studies. Methods with a permanently implanted guide cannula (Kendrick, 1990, Camp and Robinson, 1992; Centeno et al., 2007) have a limitation for repeated sampling, and methods with a grid implantation for multiple samplings (Bradberry et al., 2000; Grodin et al., 2003) requires a large uniform brain structure, such as striatum, and is not applicable for the neuroendocrine system.
The microdialysis method described in the present study allows application of neuroactive substances to the sample collection site directly, again similar to that described for the push-pull perfusion method (Kendrick, 1989, Levine et al., 1994; Terasawa, 1994). This is also a significant advantage, because in vivo response to neuroactive substances can be obtained in real-time. The injection of neuroactive substances through the general circulation is not always effective because of the potential elimination by the blood brain barrier, and the application of neuroactive substances into the lateral or third ventricle is problematic as they diffuse widely throughout the brain.
The microdialysis method has strengths and weaknesses, when compared to the push-pull perfusion method. First, a reduction in the chairing period of monkeys is a significant strength with the microdialysis method. While the push-pull perfusion method requires 2-3 days of chairing in order to ensure that the cannula is not clogged by extracellular debris after the cannula insertion, the microdialysis method can be conducted without any waiting period, because the method relies on the principal of membrane diffusion rather than direct extraction. However, this is also a drawback: At the initial phase of microdialysate sampling the animal is not fully conscious, as the guide cannula insertion requires light sedation of the animal. In contrast, with push-pull perfusion the animal is fully conscious, as the cannula insertion takes place a few days before sampling.
Second, because of a low recovery rate of neuropeptides through the dialysis membrane, high local concentrations in the brain and/or a sensitive assay method are essential for the microdialysis method. In this regard, the push-pull perfusion method of collecting extracellular fluid directly with a tip diameter less than 0.3 mm has an advantage over microdialysis. Particularly, to yield a higher recovery rate, the membrane surface area needs to be increased with a lengthening of the membrane portion of the probe (4–5 mm). As a consequence the precision of sample collection site by a microdialysis probe is not as good as the push-pull perfusion method, i.e., with the microdialysis method, the sample is collected from a larger area within the medial basal hypothalamus, rather than a restricted area in the stalk-median eminence. Similarly, the diffusion of an applied neuroactive substance with the dialysis probe would be larger than with the push-pull method. Nonetheless, because non-human primates have a large stalk-median eminence relative to the area of infusion (~700 μm), multiple microdialysis experiments (~8 times) can be performed on a single monkey offering enormous advantages for data collection on such a valuable research model. In addition, in the present study, we found that with the microdialysis method we were able to measure pulsatile LHRH release and to observe an increase in LHRH release in response to LHRH agonist challenge. A preliminary study in this lab also indicated that kisspeptin-54 (molecular weight: 5848) can be measured by the microdialysis method with the PAES membrane (Keen et al., 2007).
There are considerable discussions over lesion size with the microdialysis vs. push-pull perfusion methods (Kendrick, 1990, Robinson, 1995). Lesion size due to the insertion of a microdialysis probe is larger, as the diameter of probe is much larger than the tip of push-pull cannula. In contrast, the size of lesions due to the fluid infusion with microdialysis method is smaller as only 2 μl/min of CSF is perfused with the microdialysis method, whereas 15–22 μl/min of CSF is perfused with the push-pull perfusion method. However, a comprehensive study by Myers and colleagues indicates that sizes of histological lesions by both methods are essentially comparable (Myers et al. 1998).
The advantages of the microdialysis method come at the cost of durability. We found that the microdialysis membrane was fragile and vulnerable to damage, and thus great care had to be taken before and during the experiment. When developing our microdialysis method we found that it was imperative that all equipment be from the same manufacturer, including items such as FEP tubing. If the tubing seals attached to the probe, syringe, or fraction collector were not exactly snug, small leaks would form leading to inaccurate dialysate sample returns. This problem was compounded because of small sample size (20 μl), which leads to errors of 5–10% if a leak in the microdialysis system produced low sample returns.
We found that probes with the PC membrane could be used for a maximum of two times if they were properly flushed and sterilized after the experiment. In order to thoroughly clean the microdialysis probe and FEP tubing a solution such as 1% (v:v) ProClin 150 reagent (Bioanalytical Systems, Inc, West Lafayette, IN) was employed. Because of the delicacy of the microdialysis membrane and the relative small diameter of the FEP tubing, the possibility for occlusions developing in the system was high. Any type of occlusion, whether in the tubing or at the membrane, caused significant back pressure to build up, leading to either low volume returns or development of large leaks in the system. We also noticed that the PAES membrane cannot be resterilized, and thus, a new probe has to be used in each experiment. In addition, we found that aCSF with a phosphate buffer cannot be perfused through the PAES membrane for more than 5–6 hours, as the inlet cannula can be clogged with precipitated salts from the aCSF solution.
Despite the fact that probes with the PAES membrane yielded a higher recovery rate over the PC membrane, in the in vivo study we did not see obvious differences. This does not mean that the efficacy of probes with PC or PAES membrane materials is not important, because 1) animals that were used for PC probe experiments were ovariectomized adult females, whereas the animals that were used for PAES probes were ovarian intact females at the pubertal age, 2) the site of sampling location in the stalk-median eminence influences mean LHRH levels and pulse amplitude, but not pulse frequency (Gearing and Terasawa, 1988), and 3) the number of animals for each probe was too small for comparison (n=4 for PC and n=2 for PAES). It is expected that over a longer period the PAES probe with a higher recovery rate will be more useful, as it provides consistent and reliable results for the examination of neuropeptide release in the brain.
In summary, the microdialysis method remains an efficient tool for in vivo measurement of neuropeptides in the non-human primate. Although in the present study we did not measure small amino acid neurotransmitters, catecholamines and their metabolites, they should be easily assessed, as their molecular size is relatively smaller and unlike neuropeptides they are not sticky. Moreover, the advent of improved microdialysis membrane materials, such as PAES, is increasing the utility of the approach in measuring larger hypothalamic neuropeptides, such as kisspeptin-54 (Keen et al., 2007). While the push-pull perfusion method offers certain advantages, the microdialysis method can be highly desirable because of a significantly shorter time of chairing in non-human primates. With the microdialysis method we can readily assess the events in the neuroendocrine hypothalamus.
Acknowledgments
The authors would like to express their sincere appreciation to our veterinarians Drs. Saverio Capuano and Kevin Brunner for support of this project and to Fritz Wegner for his work on RIAs. This work was supported by NIH grants R01HD15433 and R01HD11355 to ET and P50 HD44405 to JEL, and was possible to perform by NIH supports (P51RR000167, RR15459, and RR020141) to the Wisconsin National Primate Research Center.
Footnotes
Disclosure statement: The authors have nothing to disclose.
References
- Bradberry CW, Barrett-Larimore RL, Jatlow P, Rubino SR. Impact of self-administered cocaine and cocaine cues on extracellular dopamine in mesolimbic and sensorimotor striatum in rhesus monkeys. J Neurosci. 2000;20:3874–3883. doi: 10.1523/JNEUROSCI.20-10-03874.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp DM, Robinson TE. On the use of multiple probe insertions at the same site for repeated intracerebral microdialysis experiments in the nigrostriatal dopamine system of rats. J Neurochem. 1992;58:1706–1715. doi: 10.1111/j.1471-4159.1992.tb10044.x. [DOI] [PubMed] [Google Scholar]
- Centeno ML, Reddy AP, Smith LJ, Sanchez RL, Henderson JA, Salli NC, Hess DJ, Pau FK, Bethea CL. Serotonin in microdialysate from the mediobasal hypothalamus increases after progesterone administration to estrogen primed macaques. Eur J Pharmacol. 2007;555:67–75. doi: 10.1016/j.ejphar.2006.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell PE, Levine JE. Stimulation of gonadotropin-releasing hormone surges by estrogen. I. Role of hypothalamic progesterone receptors. Endocrinology. 2000;141:1477–1485. doi: 10.1210/endo.141.4.7428. [DOI] [PubMed] [Google Scholar]
- Claypool LE, Watanabe G, Terasawa E. Effects of electrical stimulation of the medial basal hypothalamus on the in vivo release of luteinizing hormone-releasing hormone in the prepubertal and peripubertal female monkey. Endocrinology. 1990;127:3014–3022. doi: 10.1210/endo-127-6-3014. [DOI] [PubMed] [Google Scholar]
- Gearing M, Terasawa E. Luteinizing Hormone Releasing Hormone (LHRH) Neuroterminals Mapped Using the Push-Pull Perfusion Method in the Rhesus Monkey. Brain Res Bull. 1988;21:117–121. doi: 10.1016/0361-9230(88)90126-8. [DOI] [PubMed] [Google Scholar]
- Gore AC, Mitsushima D, Terasawa E. A possible role of neuropeptide Y in the control of the onset of puberty in female rhesus monkeys. Neuroendocrinology. 1993;58:23–34. doi: 10.1159/000126508. [DOI] [PubMed] [Google Scholar]
- Grondin R, Cass WA, Zhang Z, Stanford JA, Gash DM, Gerhardt GA. Glial cell line-derived neurotrophic factor increases stimulus-evoked dopamine release and motor speed in aged rhesus monkeys. J Neurosci. 2003;23:1974–1980. doi: 10.1523/JNEUROSCI.23-05-01974.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC, Levine JE. Pubertal acceleration of pulsatile gonadotropin-releasing hormone release in male rats as revealed by microdialysis. Endocrinology. 2003;144:163–171. doi: 10.1210/en.2002-220767. [DOI] [PubMed] [Google Scholar]
- Keen KL, Bloom SR, Ghatei MA, Terasawa E. An increase in kisspeptin-54 release occurs with the pubertal increase in LHRH-1 release in the stalk-median eminence of female rhesus monkeys in vivo. Proceedings of the 89th Annual Meeting of the Endocrine Society; Toronto, Canada. June 2–5, 2007; No.OR8-5. [Google Scholar]
- Kendrick KM. Use of microdialysis in neuroendocrinology. In: Conn PM, editor. Methods in Enzymology. Academic Press; San Diego: 1989. pp. 182–205. [DOI] [PubMed] [Google Scholar]
- Kendrick KM. Microdialysis measurement of in vivo neuropeptide release. J. Neurosci Methods. 1990;34:35–46. doi: 10.1016/0165-0270(90)90040-m. [DOI] [PubMed] [Google Scholar]
- Levine JE, Wolfe AM, Porkka-Heiskanen T, Meredith JM, Norgle JR, Turek FW. In vivo sampling and administration of hormone pulses in rodents. In: Levine JE, editor. Pulsatility in Neuroendocrine Systems. Academic Press; San Diego: 1994. pp. 129–161. [Google Scholar]
- Myers RD, Adell A, Lankford MF. Simultaneous comparison of cerebral dialysis and push-pull perfusion in the brain of rats: a critical review. Neurosci Biobehav Rev. 1998;22:371–387. doi: 10.1016/s0149-7634(97)00025-0. [DOI] [PubMed] [Google Scholar]
- Merriam GR, Wachter KW. Algorithms for the study of episodic hormone secretion. Am J Physiol. 1982;243:E310–E318. doi: 10.1152/ajpendo.1982.243.4.E310. [DOI] [PubMed] [Google Scholar]
- Mitsushima D, Hei DL, Terasawa E. Gamma-Aminobutyric acid is an inhibitory neurotransmitter restricting the release of luteinizing hormone-releasing hormone before the onset of puberty. Proc Natl Acad Sci USA. 1994;91:395–399. doi: 10.1073/pnas.91.1.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S, Mizuno M, Katakami H, Gore AC, Terasawa E. Aging-related changes in in vivo release of growth hormone-releasing hormone and somatostatin from the stalk-median eminence in female rhesus monkeys (Macaca mulatta). J. Clin. Endocrinol. Metab. 2003;88:827–833. doi: 10.1210/jc.2002-021568. [DOI] [PubMed] [Google Scholar]
- Pu S, Terasawa E. Effects of an LHRH agonist analog infusion on the pulsatile release of LHRH and NPY. Proceedings of the 76th Annual Meeting of the Endocrine Society; Anaheim, CA. June 15–18, 1994; No. 1281. [Google Scholar]
- Robinson JE. Microdialysis: a novel tool for research in the reproductive system. Biol Reprod. 1995;52:237–245. doi: 10.1095/biolreprod52.2.237. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Richardson HN, Chappell PE, Levine JE. In vivo gonadotropin-releasing hormone secretion in female rats during peripubertal development and on proestrus. Endocrinology. 2001;142:2929–2936. doi: 10.1210/endo.142.7.8239. [DOI] [PubMed] [Google Scholar]
- Terasawa E. In vivo measurement of pulsatile release of neuropeptides and neurotransmitters in rhesus monkeys using push-pull perfusion. In: Levine JE, editor. Pulsatility in Neuroendocrine Systems. Academic Press; San Diego: 1994. pp. 184–202. [Google Scholar]
- Watanabe G, Terasawa E. In vivo release of luteinizing hormone releasing hormone increases with puberty in the female rhesus monkey. Endocrinology. 1989;125:92–99. doi: 10.1210/endo-125-1-92. [DOI] [PubMed] [Google Scholar]
- Westerink BHC, Justice JB. Microdilaysis compared with other in vivo release models. In: Robinson TE, Justice JB, editors. Micridialysis In the Neurosciences, Techniques in the Behavioral and Neural Sciences. Vol. 7. Elsevier; New York: 1991. pp. 23–46. [Google Scholar]
- Woller MJ, McDonald JK, Reboussin DM, Terasawa E. Neuropeptide Y is a neuromodulator of pulsatile luteinizing hormone-releasing hormone release in the gonadectomized rhesus monkey. Endocrinology. 1992;130:2333–2342. doi: 10.1210/endo.130.4.1547745. [DOI] [PubMed] [Google Scholar]


