Abstract
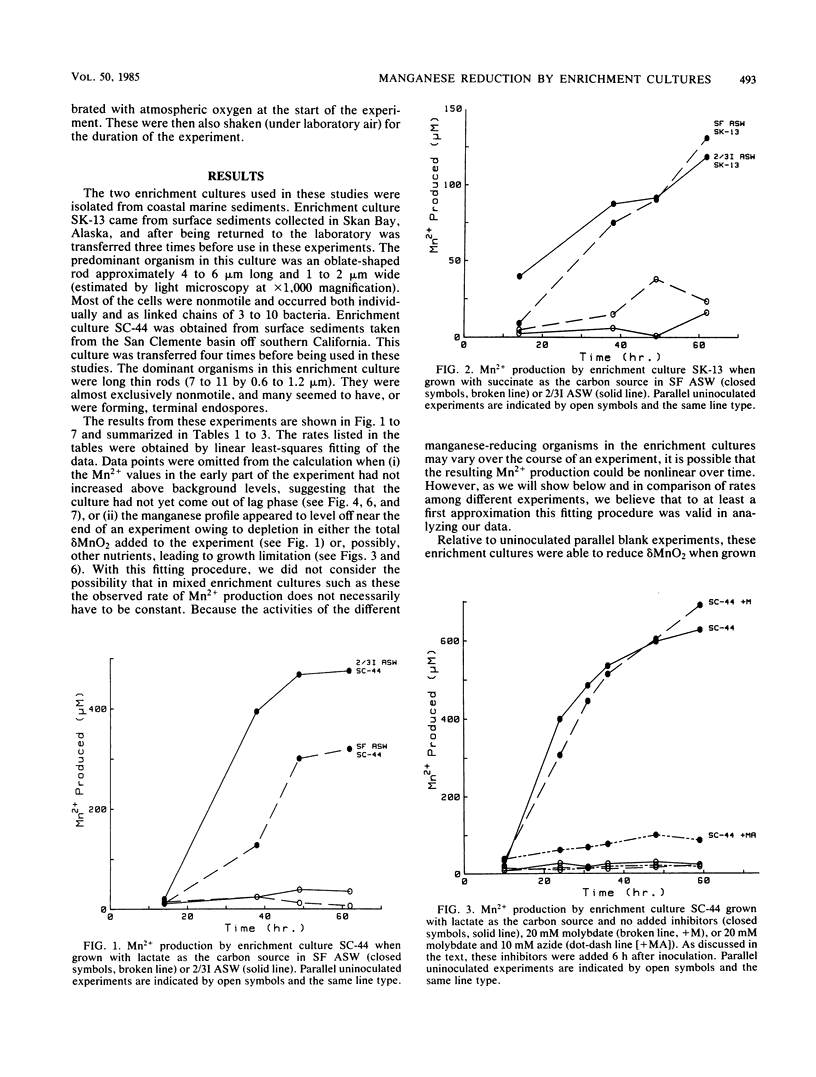
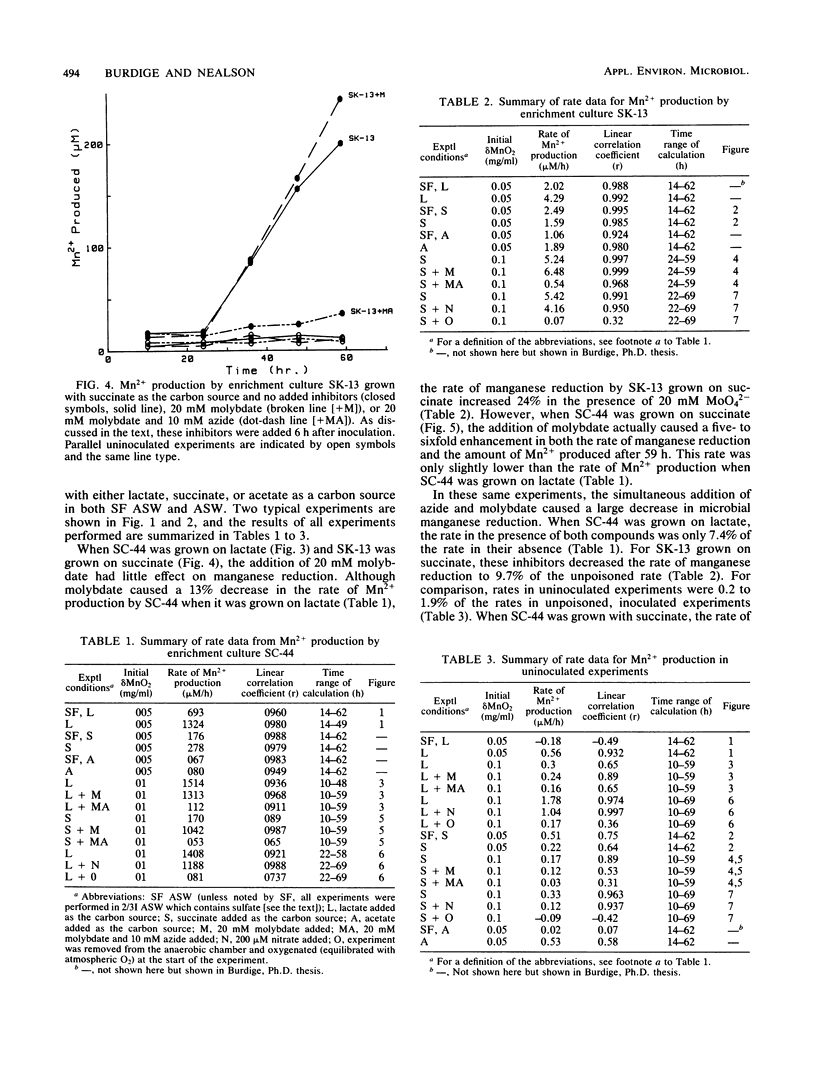
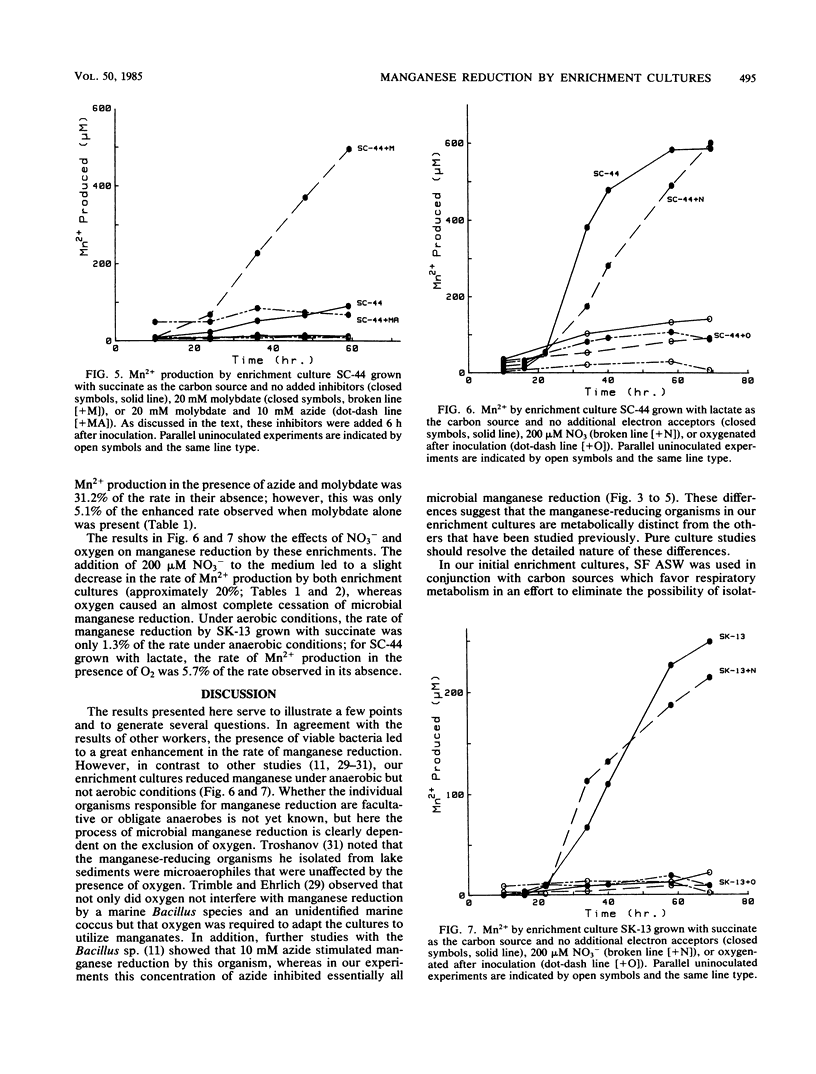
Manganese reduction was catalyzed by enrichment cultures of anaerobic bacteria obtained from coastal marine sediments. In the absence of oxygen, these enrichment cultures reduced manganates when grown on either lactate, succinate, or acetate in both sulfate-free and sulfate-containing artificial seawaters. Sodium azide as well as oxygen completely inhibited microbial manganese reduction by these enrichment cultures, whereas molybdate had no effect on them. The addition of nitrate to the medium slightly decreased the rate of Mn2+ production by these enrichment cultures. These findings are consistent with the hypothesis that the manganese-reducing organisms in these enrichment cultures use manganates as terminal electron acceptors and couple manganese reduction in some way to the oxidation of organic matter.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ghiorse W. C., Ehrlich H. L. Effects of seawater cations and temperature on manganese dioxide-reductase activity in a marine Bacillus. Appl Microbiol. 1974 Nov;28(5):785–792. doi: 10.1128/am.28.5.785-792.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiorse W. C., Ehrlich H. L. Electron transport components of the MnO2 reductase system and the location of the terminal reductase in a marine Bacillus. Appl Environ Microbiol. 1976 Jun;31(6):977–985. doi: 10.1128/aem.31.6.977-985.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOCHSTER R. M., QUASTEL J. H. Manganese dioxide as a terminal hydrogen acceptor in the study of respiratory systems. Arch Biochem Biophys. 1952 Mar;36(1):132–146. doi: 10.1016/0003-9861(52)90385-8. [DOI] [PubMed] [Google Scholar]
- Sørensen J., Christensen D., Jørgensen B. B. Volatile Fatty acids and hydrogen as substrates for sulfate-reducing bacteria in anaerobic marine sediment. Appl Environ Microbiol. 1981 Jul;42(1):5–11. doi: 10.1128/aem.42.1.5-11.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen J. Reduction of ferric iron in anaerobic, marine sediment and interaction with reduction of nitrate and sulfate. Appl Environ Microbiol. 1982 Feb;43(2):319–324. doi: 10.1128/aem.43.2.319-324.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble R. B., Ehrlich H. L. Bacteriology of manganese nodules. IV. Induction of an MnO2-reductase system in a marine bacillus. Appl Microbiol. 1970 Jun;19(6):966–972. doi: 10.1128/am.19.6.966-972.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble R. B., Ehrlich H. L. Bacteriology of manganese nodules: III. Reduction of MnO(2) by two strains of nodule bacteria. Appl Microbiol. 1968 May;16(5):695–702. doi: 10.1128/am.16.5.695-702.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehnder A. J., Brock T. D. Anaerobic methane oxidation: occurrence and ecology. Appl Environ Microbiol. 1980 Jan;39(1):194–204. doi: 10.1128/aem.39.1.194-204.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]



