Abstract
The partial molecular characterization of multiple sclerosis (MS)-associated retrovirus (MSRV), a novel retrovirus previously called LM7, is reported. MSRV has been isolated repeatedly from leptomeningeal, choroid plexus and from Epstein–Barr virus-immortalized B cells of MS patients. A strategy based on reverse transcriptase PCR with RNA-purified extracellular virions yielded an initial pol fragment from which other regions of the retroviral genome were subsequently obtained by sequence extension. MSRV-specific PCR primers amplified a pol region from RNA present at the peak of reverse transcriptase activity, coinciding with extracellular viral particles in sucrose density gradients. The same sequence was detected in noncellular RNA from MS patient plasma and in cerebrospinal fluid from untreated MS patients. MSRV is related to, but distinct from, the endogenous retroviral sequence ERV9. Whether MSRV represents an exogenous retrovirus with closely related endogenous elements or a replication-competent, virion-producing, endogenous provirus is as yet unknown. Further molecular epidemiological studies are required to determine precisely the apparent association of virions containing MSRV RNA with MS.
Multiple sclerosis (MS) is the most common neurological disease of young adults, with a prevalence in Europe and North America of between 20 and 200 per 100,000. It is characterized clinically by a relapsing/remitting or chronic progressive course, frequently leading to severe disability. Current knowledge suggests that MS is associated with autoimmunity (1), that genetic background has an important influence (2), and that “infectious” agent(s) may be involved (3). Indeed, many viruses have been proposed as possible candidates, but, as yet, none of them has been shown to play an etiological role.
The discovery of pathogenic retroviruses in humans (HTLVs and HIVs) was followed by great interest in their ability to impair the immune system and to provoke central nervous system inflammation and/or degeneration. In the case of HTLV-1, its association with a chronic inflammatory demyelinating disease (4) led to extensive investigations to search for an HTLV-1-like retrovirus in MS patients (5, 6). However, despite initial claims, the presence of HTLV-1 or HTLV-like retroviruses has not been confirmed (7, 8).
In 1989, we described the production of extracellular virions associated with reverse transcriptase (RT) activity by a culture of leptomeningeal cells (LM7) obtained from the cerebrospinal fluid (CSF) of a patient with MS (9). This was followed by similar findings in monocyte cultures from a series of MS patients (10). Neither viral particles nor viral RT activity was found in control individuals. Furthermore, we were able to transfer the LM7 virus to noninfected leptomeningeal cells in vitro (11). The molecular characterization of the “LM7” retrovirus was a prerequisite for further evaluation of its possible role in MS. Considerable difficulties arose from the absence of continuously productive retroviral cultures and from the low levels of expression in the few transient cultures (12). The strategy described here focused on RNA from extracellular virions, to avoid nonspecific detection of cellular RNA and of endogenous elements from contaminating human DNA. A specific retroviral sequence associated with virions produced by cell cultures from several MS patients has been identified. The entire sequence of this novel retroviral genome is currently being obtained using RT-PCR on RNA from extracellular virions. The retrovirus previously called “LM7 virus” is an oncovirus and is now designated MSRV (MS-associated retrovirus). In this report, we present the partial molecular characterization of MSRV together with preliminary results on its detection in MS patients’ sera and CSF.
MATERIALS AND METHODS
Patients and Clinical Samples.
Choroid plexus cells from MS patients and controls were obtained from the brain cell library, Laboratoire R. Escourolles, Hôpital de la Salpêtriére, Paris. Nontumoral leptomeningeal cells from controls were obtained as described (11). Peripheral blood and CSF from MS and control patients were obtained from the Neurological Departments, Centre Hospitalier Universitaire, Grenoble, France, from Institut National de la Santé et de la Recherche Médicale U134, Paris, and from Centro Universitario Sclerosi Multiple, Milan. Clinical details of the patients who provided CSF samples are given in Table 2.
Table 2.
Detection of MSRV in the CSF of patients with MS and patients with other neurological diseases
| Patient | Age, yr/sex | Diagnosis | MS type | MS activity | MS duration, yr | MS treatment | MSRV ELOSA |
|---|---|---|---|---|---|---|---|
| ITMS1 | 27/M | MS | 2° progressive | Slow progression | 5 | Corticosteroids | Negative |
| ITMS2 | 55/M | MS | 1° progressive | Slow progression | 9 | None | Positive |
| ITMS3 | 51/F | MS | 1° progressive | Slow progression | 2 | None | Negative |
| ITMS4 | 22/F | MS | Relapsing/remitting | In remission | 8 | None | Positive |
| ITMS5 | 27/F | MS | 1° progressive | Slow progression | 8 | Cyclophosphamide | Negative |
| ITMS6 | 33/M | MS | 2° progressive | Slow progression | 16 | PRE | Negative |
| ITMS7 | 33/F | MS | 2° progressive | Slow progression | 9 | None | Positive |
| ITMS8 | 25/F | MS | Relapsing/remitting | Stable | 3 | None | Positive |
| ITMS9 | 36/F | MS | 2° progressive | Slow progression | 3 | None | Positive |
| ITMS10 | 38/M | MS | 2° progressive | Slow progression | 7 | Corticosteroids | Negative |
| OND1 | 37/F | Cerebellar atrophy | NA | NA | NA | NA | Negative |
| OND2 | 26/F | Viral myelitis | NA | NA | NA | NA | Negative |
| OND3 | 38/F | Viral encephalitis | NA | NA | NA | NA | Negative |
| OND4 | 28/F | Viral encephalitis | NA | NA | NA | NA | Negative |
| OND5 | 54/M | Viral encephalitis | NA | NA | NA | NA | Negative |
| OND6 | 32/M | Guillain–Barré | NA | NA | NA | NA | Negative |
| OND7 | 54/F | Cerebrovascular | NA | NA | NA | NA | Negative |
| OND8 | 52/F | Hydrocephalus | NA | NA | NA | NA | Negative |
| OND9 | 25/F | 1° cerebral tumor | NA | NA | NA | NA | Negative |
| OND10 | 21/M | Epilepsy | NA | NA | NA | NA | Negative |
NA, not applicable; PRE, previous immunosuppressive treatments.
Cell Cultures, Virus Isolation, and Purification.
All cell types were cultured as described (9–12). All cultures were regularly screened for mycoplasma contamination with an ELISA mycoplasma detection kit (Boehringer Mannheim). No cell extract or supernatant used in this study contained detectable mycoplasma.
Extracellular virion purification and sucrose density gradients were performed as described (9–11). From each sucrose gradient, 0.5- to 1-ml fractions were collected; 60 μl was used for RT activity assay, and the rest was mixed with 1 volume of buffer containing 4 M guanidinium thiocyanate, 0.5% N-lauroyl sarcosin, 25 mM EDTA, and 0.2% 2-mercaptoethanol adjusted to pH 5.5 with acetic acid. These mixtures were frozen at −80°C for further RNA extraction or were directly processed according to Chomczynski (13). RNA was dissolved in 20–50 μl of diethyl-pyrocarbonate-treated water in the presence of 1–2 μl of recombinant RNase-inhibitor (Promega) and 0.1 mM DTT. Aliquots (10 μl) were used for each RT-PCR.
RT Activity.
cDNA Synthesis and “Pan-Retro” RT-PCR with Degenerate Primers.
A total RT activity between 106 and 107 dpm was required in fractions with purified virions. The “Pan-retro” RT-PCR technique was performed on virion or CSF RNA according to Tuke et al. (14), with DNase increased from 0.3 to 3 units.
Cloning and Sequencing.
PCR products were cloned using the TA-cloning kit (British Biotechnology, Oxford) according to the manufacturer’s recommendations. Sequencing reactions were performed using the “Prism ready reaction kit dye deoxyterminator cycle sequencing kit” (Applied Biosystems). Automatic sequence analysis was performed on an automatic sequencer (Applied Biosystems, node 373 A).
RT-PCR with ST1 Primer Sets.
The first PCR round was performed directly from the cDNA reaction mixture according to a one-step RT-PCR technique (15). RNA was extracted as previously from 2 ml of plasma (snap-frozen in liquid nitrogen and stored at −80°C) or from a 500-μl sucrose fraction with a total RT activity above 106 dpm and resuspended in 50 μl of RNase-free water. For each RT-PCR, 10 μl of RNA solution was incubated in a Perkin–Elmer 480 thermocycler for 15 min at 20°C with 1 unit of RNase-free DNASE 1 and 1.2 μl of 10× DNase buffer (50 mM Tris/10 mM MgCl2/0.1 mM DTT) containing 1 unit/μl of RNase-inhibitor (Promega) and heated at 70°C for 10 min for DNase inactivation. The solution was placed on ice and mixed (in conditions preventing airborne dust/DNA contamination) with 88 μl of PCR mix containing: 1× Taq buffer, 25 nM/tube 2′-deoxynucleoside 5′-triphosphates, 40 pM/tube of each first round primer (ST1.1 upstream primer: 5′-AGGAGTAAGGAAACCCAACGGAC-3′; ST1.1 downstream primer: 5′-TAAGAGTTGCACAAGTGCG-3′), 2.5 units/tube of Taq (Appligene, Strasbourg, France), and 10 units/tube of avian myeloblastosis virus–RT (Boehringer Mannheim). Each tube was further incubated in a Perkin–Elmer 480 thermocycler for 10 min at 65°C, followed by 2 h at 42°C for cDNA synthesis and 5 min at 95°C for inactivation of avian myeloblastosis virus–RT and DNA denaturation. First round parameters were 40 cycles of 95°C for 1 min, 53°C for 2.5 min., 72°C for 1 min, and a final extension of 10 min at 72°C; 10 μl of the first round was transferred to the second round PCR mix previously treated at 20°C for 15 min with RNase-free DNase 1 (0.02 units/μl) followed by DNase inactivation at 70°C for 10 min. This mix contained 1× Taq buffer, 25 nM/tube 2′-deoxynucleoside 5′-triphosphates, 40 pM/tube of each second round primer (ST1.2 upstream primer: 5′-TCAGGGATAGCCCCCATCTAT-3′; ST1.2 downstream primer: 5′-AACCCTTTGCCACTACATCAATTT-3′), and 2.5 units/tube of Taq (Appligene). Second round parameters were 30 cycles of 95°C for 1 min, 53°C for 1.5 min, 72°C for 1 min, and a final extension of 8 min at 72°C. This nested RT-PCR product (20 μl) was deposited on a 0.7% agarose gel containing ethidium bromide and was exposed to UV light for the visualization of amplified products.
Hybridization Analysis of PCR Products: MSRV-pol Detection by ELOSA.
The protocol was essentially as previously described (16) but with the following modifications: Nunc Maxisorb microtiter plates were coated with 100 ng per well capture probe CpV1b (Fig. 2) either by passive adsorption or alternatively by using streptavidin coated plates and biotinylated CpV1b. Peroxidase-labeled detector probe DpV1 (Fig. 2) was used and the assay cut-off was defined as the mean of 4 negative controls plus 0.2 OD492 units.
Figure 2.

MSRV-cpol sequence amplified between the conserved motifs in the pol gene of retroviruses: alignment with other retroviruses. “Deletions” are represented by dashes; i, inosine. The most highly conserved VLPQG and YXDD regions are shown as separate blocks in bold type at the end of each sequence. Amino acids that are present in all or in all but one of the sequences are underlined. PCR primers (modified from ref. 20; see also ref. 14) PAN-UO and PAN-UI are orientated 5′ to 3′ (sense) whereas primer PAN-DI is 3′ to 5′ (antisense). Degeneracies are shown above (PAN-UO and PAN-DI) or below (PAN-UI) the PCR primer sequences. ⊥, the nine-base 5′ extension cttggatcc; ⌉, the nine-base 5′ extension ctcaagctt. The capture and detector probes DpV1 and CpV1b used in the ELOSA assay are shown below a representative MSRV-cpol sequence. At three positions below the translated MSRV-cpol sequence, alternative amino acids (representing “nonsilent” nucleic acid variations) are shown in italics; K and Y substitutions only were observed in PLI-1-derived clones whereas R and W were encoded by a significant proportion of the clones irrespective of derivation. Note that DpV1 is peroxidase-labeled and that CpV1b may be biotinylated at the 5′ end if streptavidin-coated plates are used. The name of each sequence is indicated on the Left. HTLV1, human leukemia virus type 1; MoMLV, Moloney murine leukemia virus; MPMV, Mason–Pfizer monkey virus; ERV9, endogenous retrovirus 9; MSRV-cpol, MS-associated retrovirus conserved pol region.
RNA Extraction, cDNA Synthesis, and PCR Amplification from MS Plasma Samples.
Total RNA was extracted from human MS plasma by a guanidium method as described elsewhere (17). Total RNA extracted from 100 μl of plasma, was treated with RNase-free DNase I (0.1 unit/μl; Boehringer Mannheim) and reverse transcribed under the conditions recommended by the manufacturer using superscript RT (GIBCO/BRL). The resulting cDNAs were amplified by semi-nested PCR through 35 cycles (94°C for 1 min, 55°C for 1 min, 72°C for 1.5 min) and incubated at 72°C for 8 min for a final extension.
Three different fragments in the pol region were amplified: (i) in the protease (PRT; Fig. 3) region, for the first and second round of PCR, respectively, sense primer (5′-TCC AGC AGC AGG ACT GAG GGT-3′) and antisense primers (5′-CTG TCC GTT GGG TTT CCT TAC TCC T-3′/5′-GAC AGC AAA TGG GTA TTC CTT TCC-3′); (ii) in the fragment A of the RT region (Fig. 3) for the first and second round of PCR, respectively, sense primer (5′-AGG AGT AAG GAA ACC CAA CGG ACA G-3′) and antisense primers (5′-TGT ATA TAA TGG TCT GGC TAT TGG G 3′/5′ TTC GGC AGA AAC CTG TTA TGC CAA GG-3′); and (iii) in the fragment B of the RT region (Fig. 3) for the first and second round of PCR, respectively, sense primers (5′-GGC TCT GCT CAC AGG AGA TTA GAT AC-3′/5′-AAA GGC ACC AGG GCC CTC AGT GAG GA-3′) and antisense primer (5′-GGT TTA AGA GTT GCA CAA GTG CGC AGT C-3′). The amplified fragments were analyzed on gel, cloned, and sequenced.
Figure 3.
Consensus sequence with putative ORF from MSRV pol clones. The region corresponding to the PRT ORF cloned in a recombinant vector and expressed in Escherichia coli is boxed. The regions corresponding to the A and B fragments amplified from plasma samples from MS patients are indicated by brackets. The RT and RNase H (RNH) region is boxed with a dotted line. The highly conserved amino acids and/or active sites of enzyme activities of both PRT and RT (including RNH) are underlined.
RESULTS
Specific Retroviral RNA Is Found in Extracellular Virions from MS Patient-Derived Cell Cultures and in MS Patients’ CSF.
Choroid plexus cells (12) (obtained postmortem) and Epstein–Barr virus-immortalized peripheral blood B lymphocytes (18–19) from MS patients gave rise to cultures expressing 100-nm viral particles associated with RT activity similar to that of the original LM7 isolate (9). Similar cell types from non-MS donors produced neither this RT activity nor virions. All of the “infected” cultures were poorly and/or transiently productive and/or had a limited life span. Therefore, to analyze the genomic RNA present in the very limited quantity of extracellular virions, we used an RT-PCR approach to amplify, with degenerate primers, a conserved region of the pol gene present in all known retroviruses (20); the techniques based on this approach will be called “Pan-retro” RT-PCR. Extensive DNase treatment of samples and reagents was essential because human DNA contains many endogenous retroviral elements amplifiable by this technique.
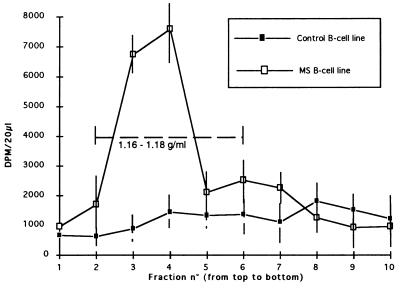
Pan-retro RT-PCR experiments were performed on sucrose density gradient-purified virions from supernatants of different types of cell cultures and their noninfected controls: (i) choroid plexus cells sampled postmortem from MS brain (PLI-1), (ii) choroid plexus cells from non-MS brain autopsy infected by coculture with irradiated LM7 cells (LM7P), and (iii) identical noninfected choroid plexus cells. “Early” B cell lines obtained by spontaneous in vitro transformation of two Epstein–Barr virus-seropositive individuals, one MS patient, and one non-MS control also were analyzed. Fig. 1 illustrates the RT activity in sucrose gradient fractions obtained from the B cell cultures. It should be noted that the background activity observed in control samples is relatively high, due to use of H3--H3-guanosine with very high specific activity. The technique described by Shih et al. (20) was modified in a semi-nested RT-PCR protocol (14) using degenerate primers (Fig. 2) and extensive DNase treatment. PCR amplifications were performed in London (Department of Virology, University College London Medical School) on coded aliquots of the density gradient fractions. Blind and systematic cloning and sequencing of the PCR products were undertaken in an independent laboratory (bioMérieux, Lyon, France). After complete sequencing of 20–30 clones per sucrose gradient fraction, the codes were broken and the results were analyzed in parallel with the RT activity data.
Figure 1.
RT activity profile of two sucrose density gradients. Control B cell line was obtained from the relative of a patient with mitochondriopathy. MS B cell line was obtained from a patient with definite MS.
Table 1 presents the distribution of sequences obtained from sucrose gradient fractions containing the peak of viral RT activity in MS-derived cultures and also the sequences amplified from the corresponding RT activity-negative fractions of uninfected cultures. The predominant sequence detected in bands of the expected size (≈140 bp) amplified in all of the RT activity-positive fractions (but not in the RT activity-negative fractions) was different from known retroviruses and was designated MSRV-cpol. MSRV-cpol sequences exhibited partial homology (70–75%) with ERV9, a previously described endogenous retroviral sequence (21). A few ERV9 sequences (>90% homology with ERV9) also were present but clearly represented a minority of clones. In addition to typical pol sequences, numerous PCR artifacts (primer multimers, concatemers, or single-primer amplifications) related to the use of degenerate primers and low temperature annealing, were found in all samples (Table 1).
Table 1.
Sequences generated by Pan-retrovirus RT-PCR of density gradient fractions containing the peak of RT activity or the corresponding control fraction
| Culture | MSRV-cpol | ERV9 (v) | Artefacts (vi) | Total clones |
|---|---|---|---|---|
| LM7P (i) | 16 | 4 | 6 | 26 |
| PLI-1 (ii) | 9 | 1 | 13 | 23 |
| MS B cell line (iii) | 9 | 2 | 8 | 19 |
| Control B cell line (iv) | 0 | 0 | 26 | 26 |
i, LM7-infected choroid plexus cell culture; ii, MS patient-derived choroid plexus cell culture (PLI-1); iii, MS patient-derived spontaneous B cell line (immortalized by endogenous Epstein–Barr virus strain); iv, non-MS control B cell line; v, clones with >90% homology with the GenBank sequence HSERV9 are designated ERV9 in this study; vi, PCR artefacts including primer multimers, concatemers, single primer amplifications, etc.
Fig. 2 shows an alignment of a consensus sequence of MSRV-cpol with the corresponding VLPQG/YMDD region of diverse retroviruses. An analysis based on the evolutionarily conserved amino acid sequences of retroviruses in this region indicates that MSRV is phylogenically related to the C-type group of oncovirinae (not shown).
A small scale study was performed to determine the prevalence of MSRV-cpol sequences in the CSF of patients with MS. We therefore devised an enzyme-linked oligosorbent assay (ELOSA) using a capture probe (CpV1B) and a peroxidase-labeled detector probe (DpV1) for the rapid identification of MSRV-cpol sequences in Pan-retrovirus PCR products (Fig. 2). The specificity of this sandwich hybridization assay for MSRV-cpol was tested with both distantly related (HIV and MoMLV) and closely related (ERV9) pol sequences. No significant cross-reactivity with such targets was observed despite the ability of the ELOSA to detect as little as 0.01 ng of MSRV-cpol DNA.
CSF samples were available from 10 patients with MS and from 10 patients with other neurological disorders. Total RNA was extracted from CSF cell pellets, reverse transcribed, and amplified as above. ELOSA analysis (Table 2) of the PCR products revealed MSRV-cpol sequences in 5 of 10 MS patient samples but in none of the 10 samples from patients with other neurological diseases (P < 0.05). The presence of MSRV-cpol did not appear to be correlated with age, sex, or type of MS but was seen in untreated patients only (five of seven). No patient on immunosuppressive therapy was found positive. In addition, among the seven patients who were untreated at the time of CSF puncture, one had been heavily treated for many years with various immunosuppressive drugs (Table 1, ITMS6). No correlation between MSRV-cpol detection and CSF cell count was observed.
Cloning and Sequencing a Larger Region of the pol Gene.
An independent identification of the MSRV genomic sequence was obtained by a non-PCR approach using RNA extracted from concentrated virions derived from 2.5 liters of LM7-infected subcultures of choroid plexus cells. A limited number of clones was obtained by direct cloning of the cDNA, one of which (PSJ17) showed partial homology with ERV9 pol. Specific primers based on the MSRV-cpol region and on the PSJ17 clone amplified a 740-bp fragment linking the two independent sequences in RNA extracted from purified virions. PSJ17 was localized on the 3′ side of MSRV-cpol. Further sequence extension on the 5′ side of MSRV-cpol and on the 3′ side of PSJ17 was obtained using RT-PCR approaches on RNA from purified LM7-like virions produced in MS choroid plexus cultures (12).
In Fig. 3, the nucleotide sequence corresponding to overlapping clones obtained by sequence extension in the pol gene is represented with the amino acid translation corresponding to the putative ORFs of the PRT and of the RT. The active site motifs of the PRT and of the RT are underlined. In the C-terminal region of the RT sequence, the dispersed amino acid residues regularly present in retroviral RNase H domains are also underlined.
Nondegenerate Primers Detect MSRV-Specific RNA in Virions Associated with the Peak of RT Activity and in MS Patients’ Plasma.
PCR primers (ST1.1 primer set; positions 603–625/1732–1714; Fig. 3) based on overlapping clones in the pol gene amplified a 1.13-kb segment of the RT region from several different isolates obtained from different MS patients. Nested primers (ST1.2; positions 869–889/1513–1490; Fig. 3) generated a 644-bp fragment. The specificity of PCR products was confirmed by stringent hybridization with a peroxidase-labeled MSRV-cpol probe (Fig. 2) using the ELOSA technique (16).
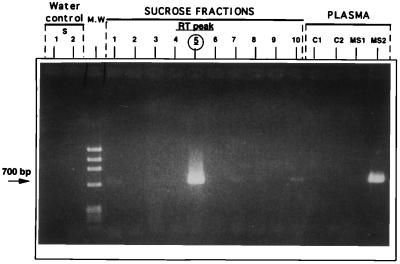
The ST1.1 + ST1.2 primer set was used to detect extracellular MSRV RNA in virion purified from cell culture isolates and in human plasma although suboptimal for this latter application. Fig. 4 illustrates the results of PCR amplification of cDNA from the sucrose density gradient fractions of an MS choroid plexus isolate tested in parallel with cDNA derived from two MS patient and two control plasma samples. ELOSA analysis of PCR products from sucrose fractions with MSRV-cpol was positive in fraction 5 where a strong band coinciding with the peak of RT activity (not shown) was seen and in fraction 10 (a faint 644-bp band is also visible on gel), which corresponds to the bottom of the tube where aggregated particles usually sediment. It should also be mentioned that, unlike Fig. 1, the RT peak was collected in a single fraction of this new gradient. Similar ELOSA and gel positivity was obtained with plasma RNA from the patient with an acute relapse of MS and not with plasma from the MS patient in long term remission and from the two non-MS controls. Taq sequencing of the 700-bp bands confirmed the presence of MSRV sequence. Control RT-PCR for cellular aldolase transcripts on plasma-derived RNA was negative, indicating that the results were not due to cellular RNA released by cell lysis during plasma separation. It should be noted that this PCR technique was not designed for epidemiological studies because its sensitivity is impaired by the length of the cDNA required (1.13 kb).
Figure 4.
Specific detection of MSRV-pol RNA sequence by RT-PCR in gradient-purified virion and in MS plasma. PCR products amplified from round 1 (ST1.1) with the ST1.2 primer set. From left to right: water control 1 from RT-PCR step with ST1.1 set; water control 2 amplified from water control 1 with ST1.2 nested primers; molecular weight markers; fractions 1–10 from sucrose gradient with MSRV virion; plasma samples C1 and C2 from healthy blood donors; and plasma samples MS1 and MS2 from two MS patients.
The same pol region could also be amplified in virion preparations made from MS B cell line supernatants with significant RT activity, which had been treated with DNase and RNase before virion lysis and RNA extraction. These conditions avoided detection of nonencapsidated RNA and further confirmed that MSRV sequence detection was consistent with virion-packaged RNA.
Nondegenerate primers amplifying three fragments of the pol gene (the whole PRT region and regions A and B of the RT; Fig. 3) also were used to confirm the presence of MSRV sequences in DNase-treated RNA from MS plasma. These fragments were amplified from the plasma of a further four MS patients with active disease. Sequence analysis confirmed that the PRT and RT regions were homologous (>95% and >90%, respectively) to MSRV sequences previously obtained from cultured virions. No such sequences were detected in plasma from healthy controls (n = 4) tested in parallel with MS plasma.
DISCUSSION
Phylogeny of MSRV.
From the results of this study, we conclude that the virus previously referred to as “LM7” (9–12) possesses an RNA genome containing the MSRV pol sequence described here.
The conserved RT motif of both MSRV and ERV9 is two amino acids shorter than that of other retroviruses, apart from human foamy viruses that nonetheless have a functional RT. The potential ORF encompassing the entire PRT–RT region is consistent with the virion-associated RT activity detected in sucrose density gradients from infected culture supernatants. Moreover, because we recently have succeeded in expressing a recombinant protein from the sequence of MSRV PRT cloned from MS plasma, we can confirm the reality of the potential PRT ORF. Similar cloning and expression of other sequences containing potential ORFs for MSRV proteins is being undertaken to confirm their ability to encode enzymes and structural proteins of MSRV virions.
Phylogenic analysis based on the most conserved amino acid sequence in retroviruses (VLPQG … YXDD) reveals that the MSRV pol gene is related to the C-type oncoviruses. Apart from ERV9, the closest known retroviral element is RTLV-H, a human endogenous sequence known to have a subtype with a functional pol gene (22). In the pol region, this phylogenic affiliation to C-type oncoviruses apparently contradicts our previous assumptions based on the general morphology of the particles observed by electron microscopy, which were compatible with a B- or D-type oncovirus (9, 11). However, preliminary data on env sequences detected in MSRV virions suggest a greater phylogenic proximity to D-type. Such difference in phylogenies of the pol and env genes have been described in MPMV and suggest a recombinatorial origin in D-type retroviruses (23). D- to C-type morphological conversion is also possible because it has been reported that a single amino acid substitution in the gag protein can convert retrovirus morphology to that of a different type (24).
Is MSRV an Exogenous Retrovirus Sharing Extensive Homology with a Related Endogenous Retrovirus Family or an Endogenous Retrovirus Producing Extracellular Virions?
Southern blot analysis with an MSRV pol probe under stringent conditions showed hybridization with a multicopy endogenous family (data not presented), indicating the existence of endogenous elements more closely related to MSRV than ERV9 itself. Consequently, we were unable to look for a virion-specific provirus in MSRV-producing cells. In agreement with Southern blot findings, PCR studies on genomic DNA showed multiple band amplification of MSRV-related endogenous sequences. pol is the most conserved retroviral gene, so the sequence described here is the least suitable region to discriminate between exogenous and endogenous sequences. It is hoped that sequence information from other parts of the genome may permit such a discrimination, as has recently been demonstrated for the Jaagsiekte retrovirus (JSRV) of sheep (25). With such sequence data, it would then become possible to identify the MSRV-specific provirus in the genome of virion-producing cell cultures.
MSRV could represent a virion-producing exogenous member of an ERV9-like endogenous family, just as exogenous strains exist in the well studied mouse mammary tumor virus (MMTV) and murine leukemia virus (MuLV) retroviral families of mice and in the JSRV retroviral family of sheep (26). Alternatively, it is also conceivable that the extracellular MSRV virions may be produced by a replication-competent endogenous provirus. Whether MSRV is exogenous or endogenous, conceptual similarities exist with the category of retroviruses represented by MuLV, MMTV, and JSRV. Unlike defective endogenous elements, this category of agents are known to produce infectious and pathogenic virions, to cause neurological disease (27) and solid tumors/leukemias (26, 28) and to express “endogenous superantigens” (29, 30). Furthermore, in MuLV infections, the genetic endogenous retroviral background of the mouse strain can determine susceptibility or resistance to disease (30, 31). Indeed, such interactions between an infectious retrovirus and its endogenous counterpart may be relevant in the pathogenesis of MS because endogenous retroviral genotypes are not identical in all individuals. A genetic control due to related endogenous retroviral genotypes could therefore contribute to the known hereditary susceptibility to MS (2), if MSRV does indeed play an active role in this disease.
The data in Table 1 suggest that ERV9 elements may be coexpressed, possibly via transactivation in infected cells, and give rise to heterologous RNA packaging in MSRV virions. Such heterologous packaging is known to occur in other retroviral systems (32).
A Role for the Numerous Common Viruses Previously Evoked in MS?
Among the numerous reports of viruses putatively involved in the etiopathogenesis of MS, a significant proportion focuses on two viral families, the paramyxoviridae and the herpesviridae. Regarding the paramyxoviridae, the key observation is of a frequently increased antibody titer to measles virus in MS patients essentially directed, in CSF, against measles fusion protein (33). The existence of amino acid similarities between conserved domains of the fusion proteins of paramyxoviridae and the transmembrane protein of retroviruses (34) may explain this observation if antigenic cross-reactivity between these two proteins occurred.
With regard to the herpesvirus family, the involvement of Epstein–Barr virus, herpes simplex virus type 1, and, most recently, human herpes virus 6 has been proposed (19, 35, 36). From our previous studies and from those of other groups, it appears that herpesviruses may play an important role in MSRV expression; we have shown that HSV-1 immediate-early ICP0 and ICP4 proteins can transactivate MSRV/LM7 in vitro (37), and Haahr et al. have proposed an important epidemiological role for Epstein–Barr virus as a cofactor in MS, triggering retrovirus reactivation (19). The recent description by Challoner et al. (36) showing significant expression of HHV6 proteins in MS plaques may also suggest a similar role for HHV6 in the brain.
CONCLUSION
In conclusion, the present study has provided molecular evidence that the production of extracellular virions containing MSRV pol sequence appears associated with MS and that the virus previously referred to as LM7 is indeed a novel retrovirus. Its exact contribution as a causative agent or as a link in the pathogenic process will be determined by future studies. However, our results showing the specific production of a potent gliotoxic protein in some MSRV-producing cells as well as its presence in CSF of MS patients (38) may provide such a link in the pathogenic process of MS.
Nevertheless, the possibility that MSRV expression simply represents an interesting epiphenomenon rather than a causative factor has yet to be formally excluded. If so, MSRV RNA associated with extracellular virion in body fluids would nonetheless represent a useful marker of the disease for diagnosis, prognosis, and/or therapeutic monitoring. To address these questions and for future epidemiological studies, we are currently evaluating PCR primers that amplify other regions of the MSRV genome.
Acknowledgments
This work was supported by BioMérieux S.A. and was generously supported by the Nouvelle Association Française contre la Sclérose en Plaques, the Union Santé et Sclérose en Plaques, the Association SEP Rhône-Alpes, and the MS Society of Great Britain and Northern Ireland.
ABBREVIATIONS
- MS
multiple sclerosis
- RT
reverse transcriptase
- MSRV
multiple sclerosis-associated retrovirus
- CSF
cerebrospinal fluid
- PRT
protease
- ELOSA
enzyme-linked oligosorbent assay
References
- 1.Martin R, McFarland H F, McFarlin D. Annu Rev Immunol. 1992;10:153–187. doi: 10.1146/annurev.iy.10.040192.001101. [DOI] [PubMed] [Google Scholar]
- 2.Bell J I, Lathrop G M. Nat Genet. 1996;13:377–378. doi: 10.1038/ng0896-377. [DOI] [PubMed] [Google Scholar]
- 3.Kurtzke J F. Clin Microbiol Rev. 1993;6:382–427. doi: 10.1128/cmr.6.4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gessain A, Barin F, Vernant J C, Gout O, Maurs L, Calender A, deThe G. Lancet. 1985;ii:407–410. doi: 10.1016/s0140-6736(85)92734-5. [DOI] [PubMed] [Google Scholar]
- 5.Koprowsky H, DeFreitas E C, Harper M E, Sandberg-Wollheim M, Sheremata W A, Robert-Guroff M, Saxinger C W, Feinberg M B, Wong-Staal F, Gallo R C. Nature (London) 1985;318:154–160. doi: 10.1038/318154a0. [DOI] [PubMed] [Google Scholar]
- 6.Greenberg S J, Ehrlich G D, Abbott M A, Hurwitz B J, Waldman T A, Poiesz B J. Proc Natl Acad Sci USA. 1989;86:2878–2882. doi: 10.1073/pnas.86.8.2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fugger L, Morling N, Ryder L P, Sandberg-Wollheim M, Svejgaard A. J Gen Virol. 1990;71:1103–1107. doi: 10.1099/0022-1317-71-5-1103. [DOI] [PubMed] [Google Scholar]
- 8.Ehrlich G D, Glaser J B, Bryz-Gornia V, Maese J, Waldmann T A, Poiesz B J, Greenberg S J. Neurology. 1991;41:335–343. doi: 10.1212/wnl.41.3.335. [DOI] [PubMed] [Google Scholar]
- 9.Perron H, Geny C, Laurent A, Mouriquand C, Pellat J, Perret J, Seigneurin J M. Res Virol. 1989;140:551–561. doi: 10.1016/s0923-2516(89)80141-4. [DOI] [PubMed] [Google Scholar]
- 10.Perron H, Lalande B, Gratacap B, Laurent A, Genoulaz O, Geny C, Mallaret M, Schuller E, Stoebner P, Seigneurin J M. Lancet. 1991;337:862–863. doi: 10.1016/0140-6736(91)92579-q. [DOI] [PubMed] [Google Scholar]
- 11.Perron H, Gratacap B, Lalande B, Genoulaz O, Laurent A, Geny C, Mallaret M, Innocenti P, Schuller E, Stoebner P, Seigneurin J M. Res Virol. 1992;143:337–350. doi: 10.1016/s0923-2516(06)80122-6. [DOI] [PubMed] [Google Scholar]
- 12.Perron H, Firouzi R, Tuke P, Garson J A, Michel M, Beseme F, Bedin F, Mallet F, Garcia E, Marcel F, Seigneurin J M, Mandrand B. Acta Neurol Scand. 1997;95:22–31. doi: 10.1111/j.1600-0404.1997.tb08146.x. [DOI] [PubMed] [Google Scholar]
- 13.Chomczynski P, Sacchi N. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 14.Tuke P W, Perron H, Bedin F, Beseme F, Garson J A. Acta Neurol Scand. 1997;95:16–21. doi: 10.1111/j.1600-0404.1997.tb08145.x. [DOI] [PubMed] [Google Scholar]
- 15.Mallet F, Oriol G, Mary C, Verrier B, Mandrand B. Biotechniques. 1995;18:678–687. [PubMed] [Google Scholar]
- 16.Mallet F, Hebrard C, Brand D, Chapuis E, Cros P, Allibert P, Besnier J M, Barin F, Mandrand B. J Clin Microbiol. 1993;31:1444–1449. doi: 10.1128/jcm.31.6.1444-1449.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baccala R, Kono D H, Walker S, Balderas R S, Theophilopoulos A N. Proc Natl Acad Sci USA. 1991;88:2908–2912. doi: 10.1073/pnas.88.7.2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haahr S, Koch-Henriksen N, Møller-Larsen A, Eriksen L S, Andersen H M K. Multiple Sclerosis. 1995;1:73–77. doi: 10.1177/135245859500100203. [DOI] [PubMed] [Google Scholar]
- 19.Haahr S, Sommerlund M, Christensen T, Jensen A W, Hansen H J, Møller-Larsen A. Ann NY Acad Sci. 1994;724:148–156. doi: 10.1111/j.1749-6632.1994.tb38903.x. [DOI] [PubMed] [Google Scholar]
- 20.Shih A, Misra R, Rush M G. J Virol. 1989;63:64–75. doi: 10.1128/jvi.63.1.64-75.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LaMantia G, Maglione D, Pengue G, DiCristofano A, Simeone A, Lanfrancone L, Lania L. Nucleic Acids Res. 1991;19:1513–1520. doi: 10.1093/nar/19.7.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilkinson D A, Goodchild N L, Saxton T M, Wood S, Mager D L. J Virol. 1993;67:2981–2989. doi: 10.1128/jvi.67.6.2981-2989.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sonigo P, Barker C, Hunter E, Wain-Hobson S. Cell. 1986;45:375–385. doi: 10.1016/0092-8674(86)90323-5. [DOI] [PubMed] [Google Scholar]
- 24.Rhee S S, Hunter E. Cell. 1990;63:77–86. doi: 10.1016/0092-8674(90)90289-q. [DOI] [PubMed] [Google Scholar]
- 25.Bai J, Zhu R Y, Stedman K, Cousens C, Carlson J, Sharp J M, DeMartini J C. J Virol. 1996;70:3159–3168. doi: 10.1128/jvi.70.5.3159-3168.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palmarini M, Cousens C, Dalziel R G, Bai J, Stedman K, DeMartini J C, Sharp J M. J Virol. 1996;70:1618–1623. doi: 10.1128/jvi.70.3.1618-1623.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Portis J L. In: Retrovirus Infections of the Nervous System: Current Topics in Microbiology and Immunology. Oldstone M B A, Koprowsky H, editors. Berlin: Springer; 1990. pp. 11–27. [Google Scholar]
- 28.Gardner M B, Chivi A, Dougherty M F, Casagrande J, Estes J D. J Natl Cancer Inst. 1979;62:63–69. [PubMed] [Google Scholar]
- 29.Marrack P, Kushnir E, Kappler J. Nature (London) 1991;349:524–526. doi: 10.1038/349524a0. [DOI] [PubMed] [Google Scholar]
- 30.Hügin A W, Vacchio M S, Morse H C. Science. 1991;252:424–427. doi: 10.1126/science.1850169. [DOI] [PubMed] [Google Scholar]
- 31.Gardner M B. In: Retrovirus Infections of the Nervous System: Current Topics in Microbiology and Immunology. Oldstone M B A, Koprowsky H, editors. Berlin: Springer; 1990. pp. 3–10. [PubMed] [Google Scholar]
- 32.Linial M L, Miller A D. In: Retrovirus Infections of the Nervous System: Current Topics in Microbiology and Immunology. Oldstone M B A, Koprowsky H, editors. Berlin: Springer; 1990. pp. 125–152. [Google Scholar]
- 33.Dhib-Jalbut S, Lewis K, Bradburn E, McFarlin D E, McFarland H F. Neurology. 1990;40:430–435. doi: 10.1212/wnl.40.3_part_1.430. [DOI] [PubMed] [Google Scholar]
- 34.Gonzalez-Scarano F, Waxham M N, Ross A M, Hoxie J A. AIDS Res Hum Retroviruses. 1987;3:245–252. doi: 10.1089/aid.1987.3.245. [DOI] [PubMed] [Google Scholar]
- 35.Bergström T, Andersen O, Vahlne A. Ann Neurol. 1989;26:283–285. doi: 10.1002/ana.410260218. [DOI] [PubMed] [Google Scholar]
- 36.Challoner P B, Smith K T, Parker J D, MacLeod D L, Coulter S N, Rose T M, Schultz E R, Bennett J L, Garber R L, Chang M, Schad P A, Stewart P M, Nowinski R C, Brown J P, Burmer J P. Proc Natl Acad Sci USA. 1995;92:7440–7444. doi: 10.1073/pnas.92.16.7440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perron H, Suh M, Lalande B, Gratacap B, Laurent A, Stoener P, Seigneurin J M. J Gen Virol. 1993;74:65–72. doi: 10.1099/0022-1317-74-1-65. [DOI] [PubMed] [Google Scholar]
- 38.Rieger F, Amouri R, Benjelloun N, Cifuentes-Diaz C, Dobransky T, Lyon-Caen O, Hantaz-Ambroise D, Dobransky T, Geny C, Perron H. C R Acad Sci (Paris) 1996;319:343–350. [PubMed] [Google Scholar]