Abstract
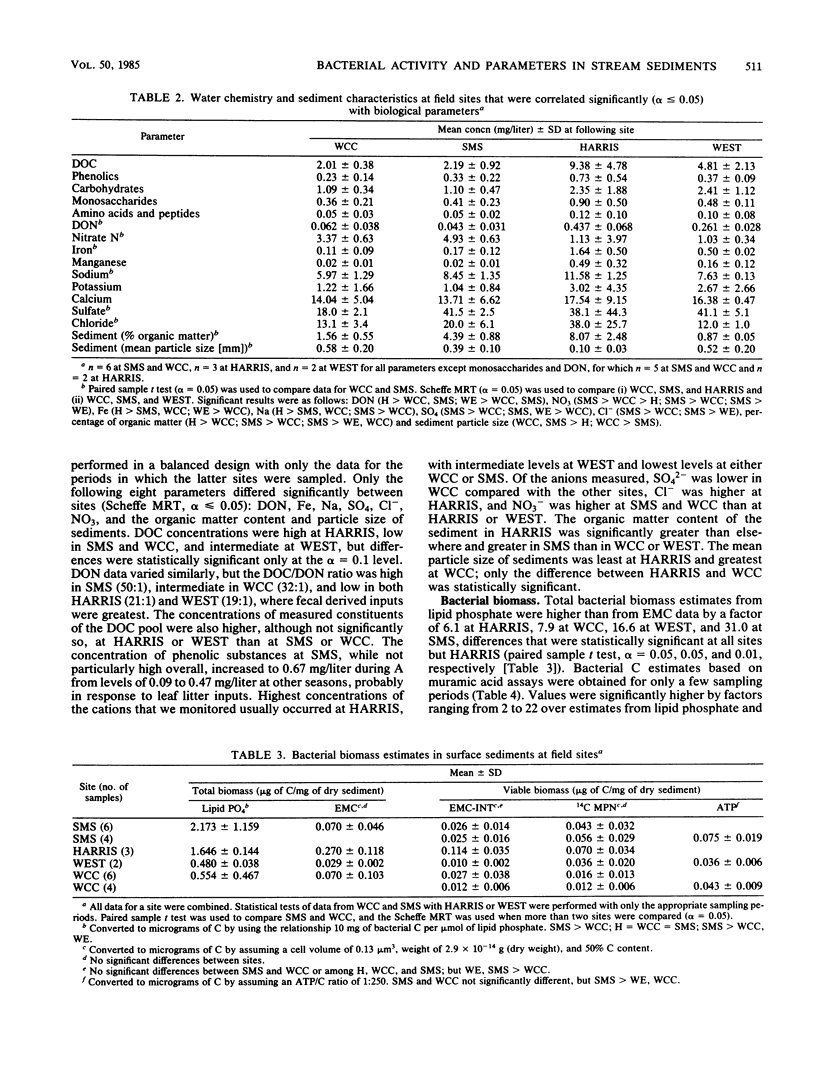
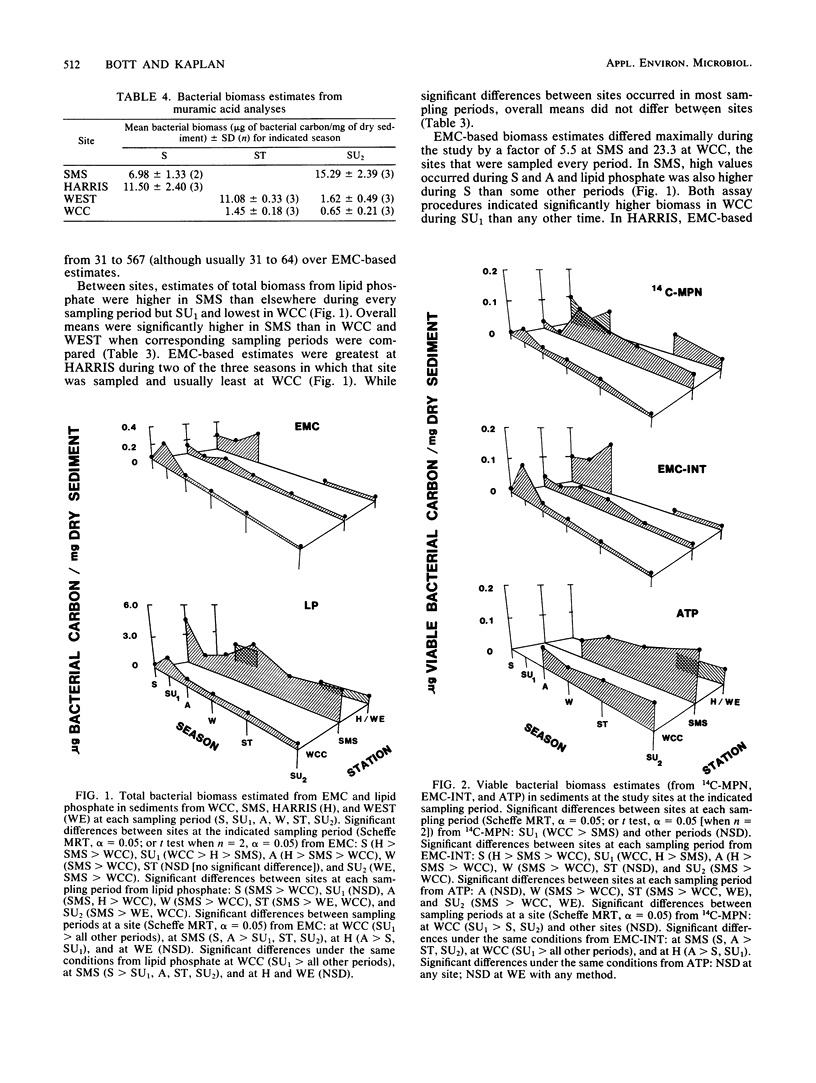
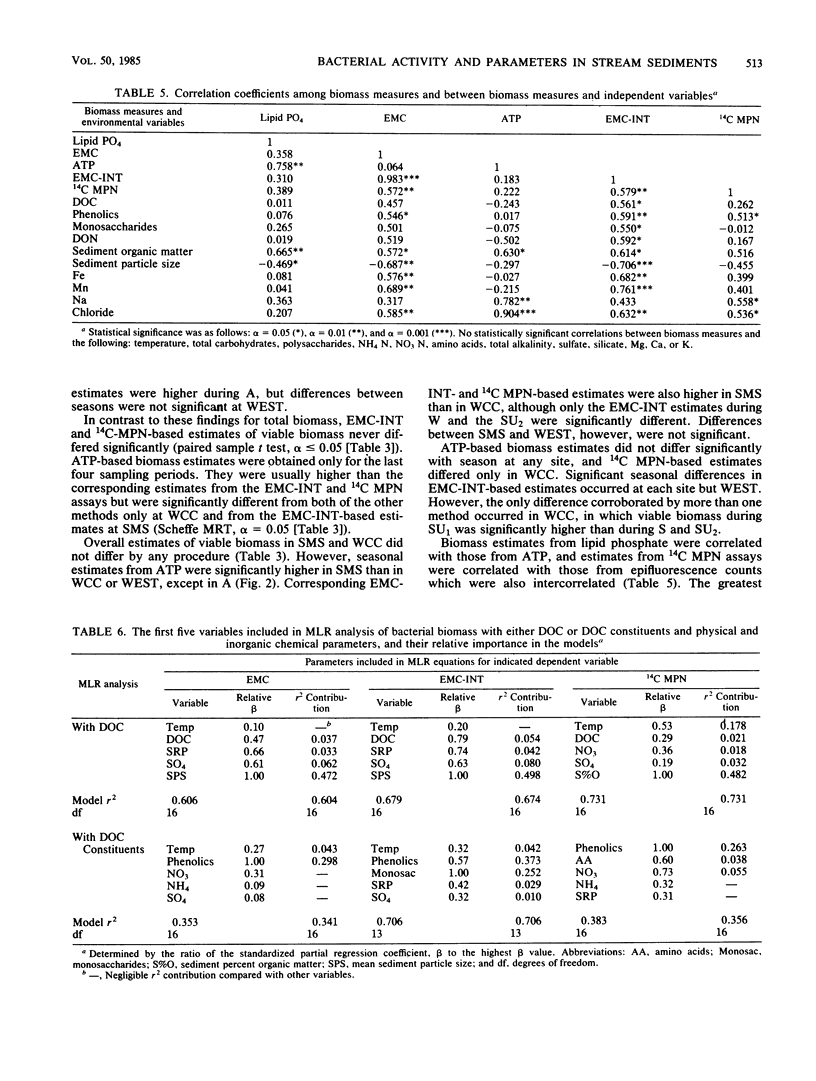
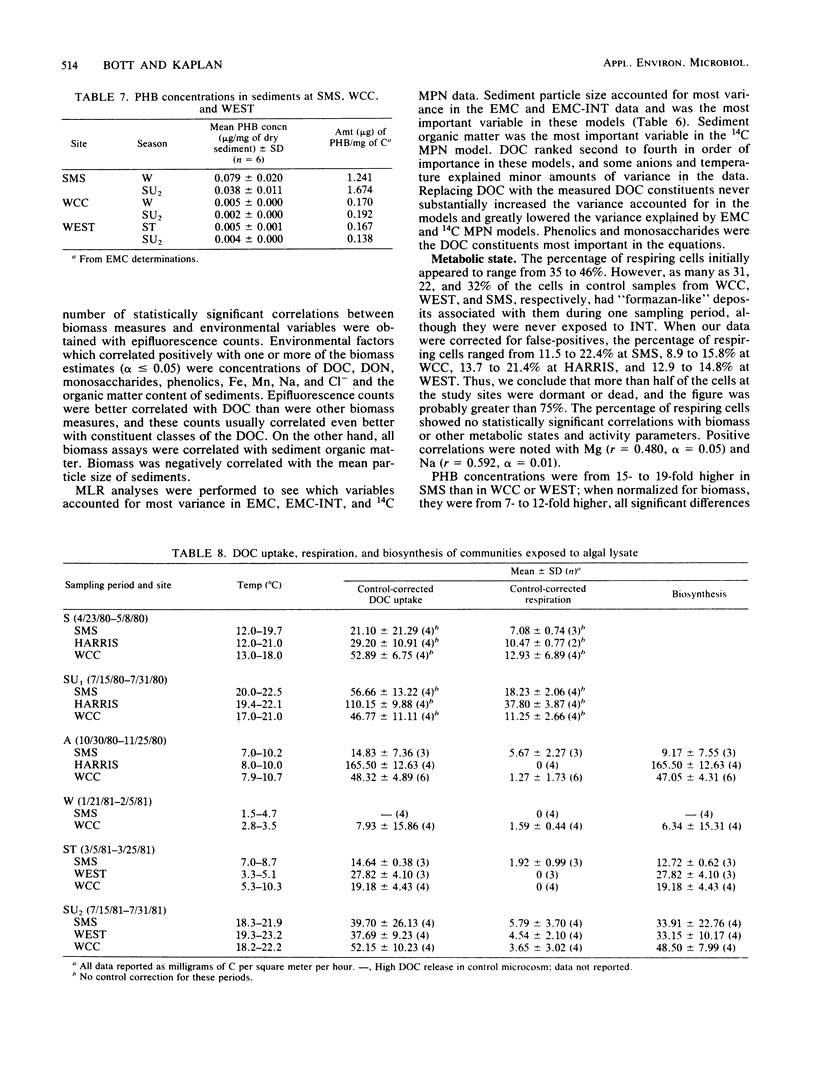
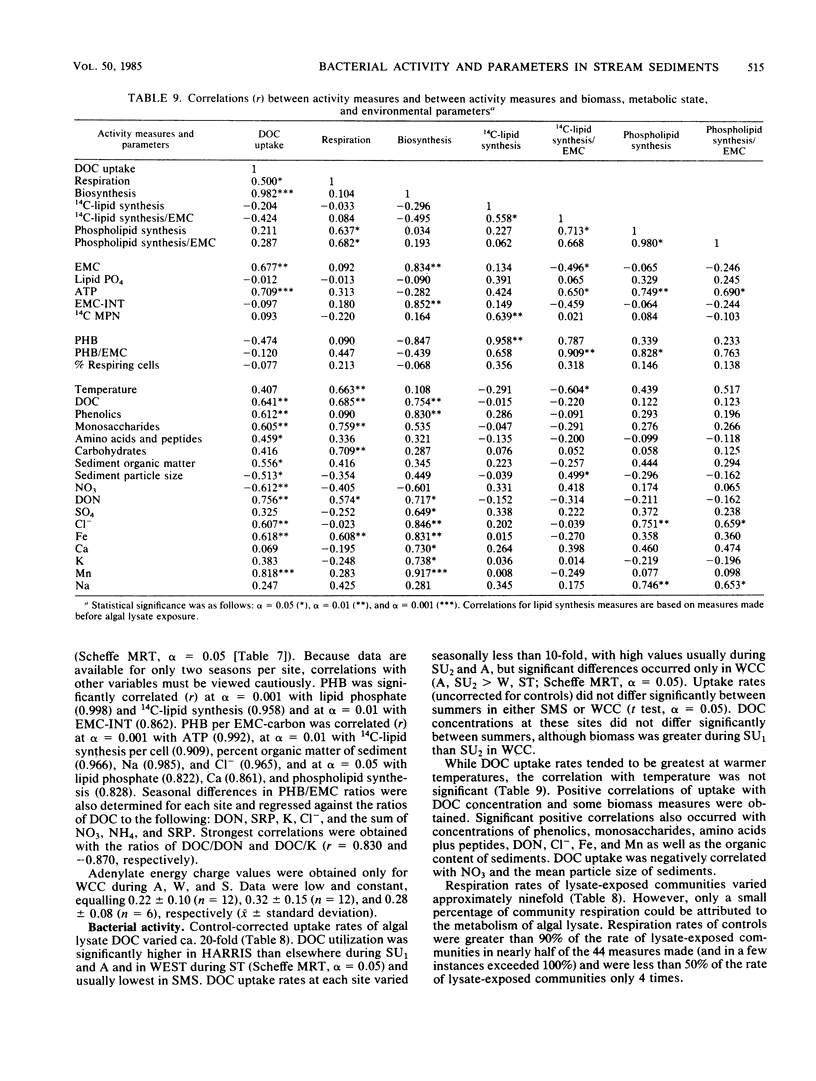
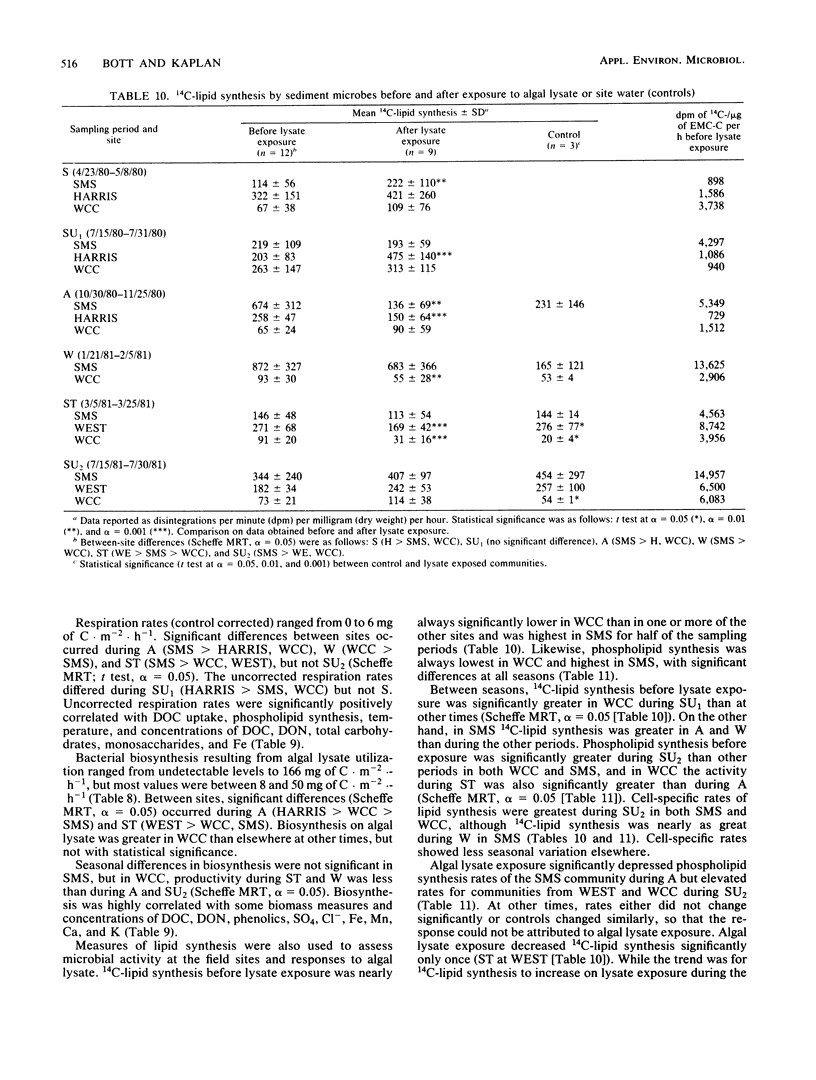
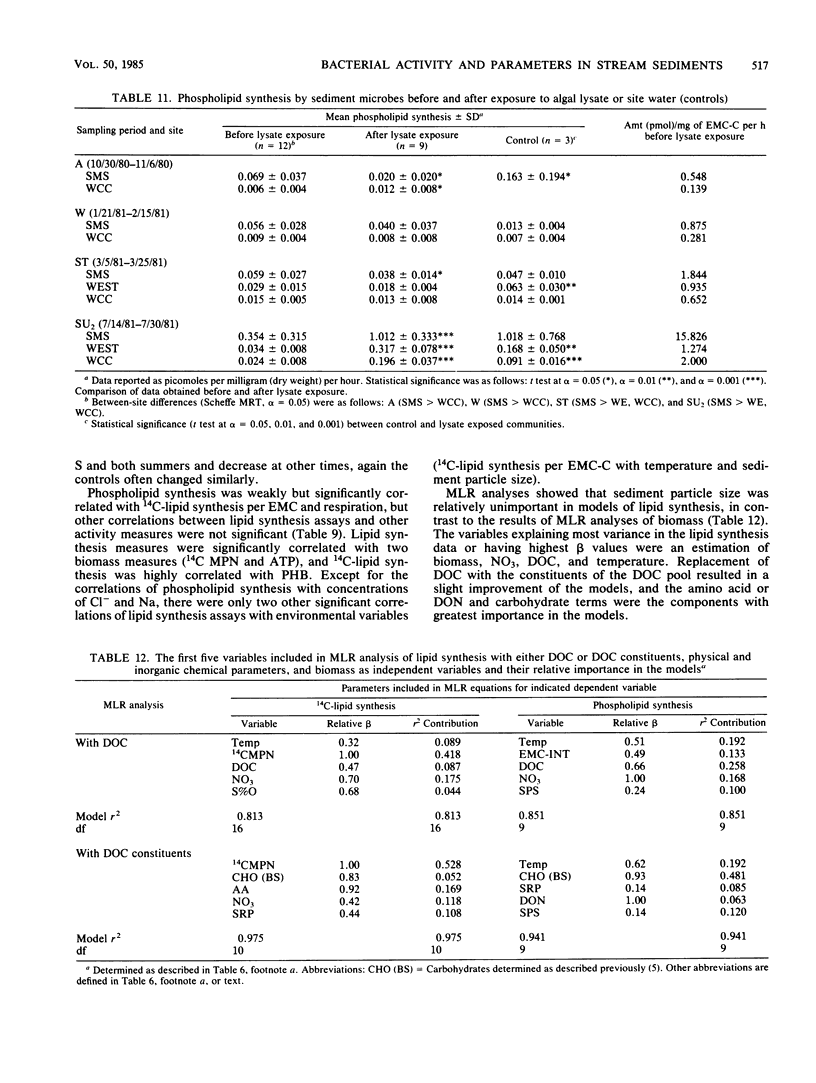
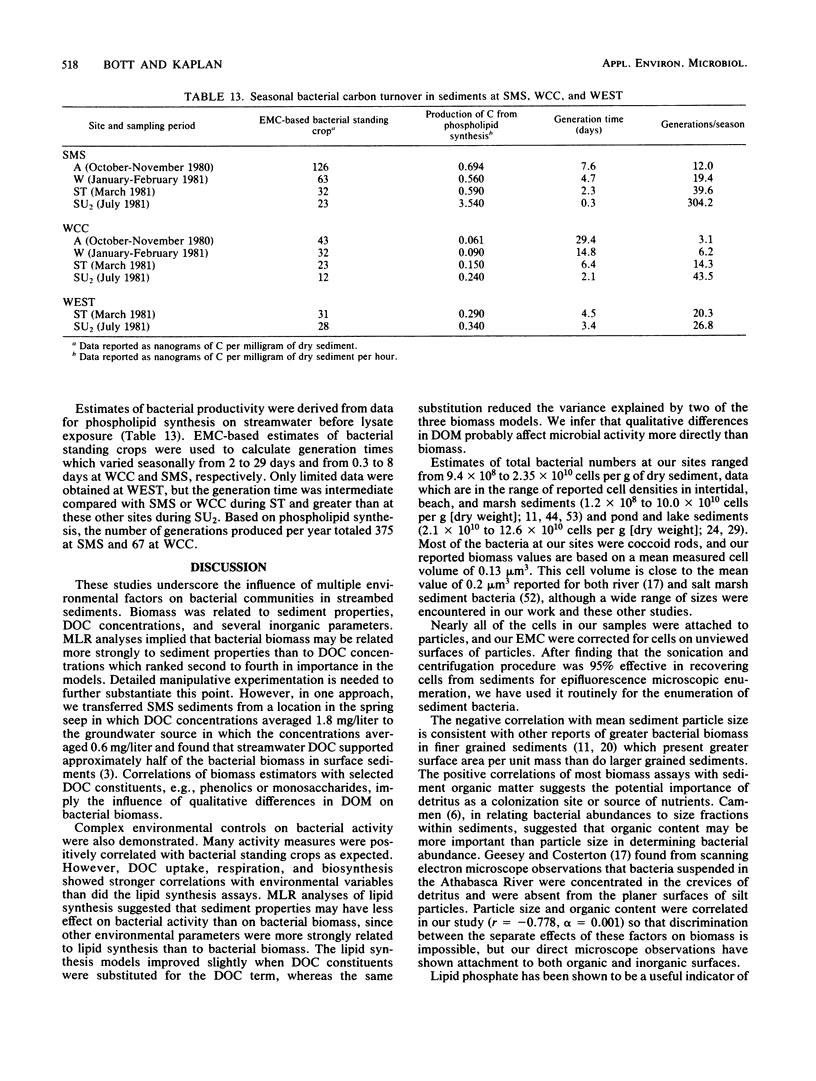
Bacterial biomass, metabolic condition, and activity were measured over a 16-month period in the surface sediments of the following four field sites with differing dissolved organic matter regimes: a woodlot spring seep, a meadow spring seep, a second-order stream, and a third-order stream. Total bacterial biomass was measured by lipid phosphate and epifluorescence microscopic counts (EMC), and viable biomass was measured by 14C most probable number, EMC with 2-(p-iodophenyl)-3-(p-nitrophenyl)-5-phenyl tetrazolium chloride reduction, and ATP. Bacterial metabolic condition was determined from the percentage of respiring cells, poly-β-hydroxybutyrate concentrations, and adenylate energy charge. Activity measures included 14C-lipid synthesis, 32P-phospholipid synthesis, the rate of uptake of algal lysate dissolved organic carbon, and respiration, from which biosynthesis was calculated (dissolved organic carbon uptake corrected for respiration). Total bacterial biomass (from EMC) ranged from 0.012 to 0.354 μg of C/mg of dry sediment and was usually lowest in the third-order stream. The percentage of cells respiring was less than 25% at all sites, indicating that most bacteria were dormant or dead. Adenylate energy charge was measured only in the third-order stream and was uniformly low. Poly-β-hydroxybutyrate concentrations were greater in the woodlot spring seep than in the second- and third-order streams. Uptake of algal lysate dissolved organic carbon ranged from undetectable levels to 166 mg of C · m−2 · h−1. Little community respiration could be attributed to algal lysate metabolism. Phospholipid synthesis ranged from 0.006 to 0.354 pmol · mg of dry sediment−1 · h−1. Phospholipid synthesis rates were used to estimate bacterial turnover at the study sites. An estimated 375 bacterial generations per year were produced in the woodlot spring seep, and 67 per year were produced in the third-order stream.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chapman A. G., Fall L., Atkinson D. E. Adenylate energy charge in Escherichia coli during growth and starvation. J Bacteriol. 1971 Dec;108(3):1072–1086. doi: 10.1128/jb.108.3.1072-1086.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durska G., Kaszubiak H. Occurrence of bound muramic acid and alpha, epsilon-diaminopimelic acid in soil and comparison of their contents with bacterial biomass. Acta Microbiol Pol. 1983;32(3):257–263. [PubMed] [Google Scholar]
- Findlay R. H., White D. C. In situ determination of metabolic activity in aquatic environments. Microbiol Sci. 1984 Jul;1(4):90-2,95. [PubMed] [Google Scholar]
- Findlay R. H., White D. C. Polymeric Beta-Hydroxyalkanoates from Environmental Samples and Bacillus megaterium. Appl Environ Microbiol. 1983 Jan;45(1):71–78. doi: 10.1128/aem.45.1.71-78.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geesey G. G., Costerton J. W. Microbiology of a northern river: bacterial distribution and relationship to suspended sediment and organic carbon. Can J Microbiol. 1979 Sep;25(9):1058–1062. doi: 10.1139/m79-162. [DOI] [PubMed] [Google Scholar]
- Herron J. S., King J. D., White D. C. Recovery of Poly-beta-Hydroxybutyrate from Estuarine Microflora. Appl Environ Microbiol. 1978 Feb;35(2):251–257. doi: 10.1128/aem.35.2.251-257.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höfle M. G. Transient Responses of Glucose-Limited Cultures of Cytophaga johnsonae to Nutrient Excess and Starvation. Appl Environ Microbiol. 1984 Feb;47(2):356–362. doi: 10.1128/aem.47.2.356-362.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl D. M., Craven D. B. Effects of alkaline phosphatase activity on nucleotide measurements in aquatic microbial communities. Appl Environ Microbiol. 1980 Sep;40(3):549–561. doi: 10.1128/aem.40.3.549-561.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchman D., Mitchell R. Contribution of particle-bound bacteria to total microheterotrophic activity in five ponds and two marshes. Appl Environ Microbiol. 1982 Jan;43(1):200–209. doi: 10.1128/aem.43.1.200-209.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAW J. H., SLEPECKY R. A. Assay of poly-beta-hydroxybutyric acid. J Bacteriol. 1961 Jul;82:33–36. doi: 10.1128/jb.82.1.33-36.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmicke L. G., Williams R. T., Crawford R. L. 14C-most-probable-number method for enumeration of active heterotrophic microorganisms in natural waters. Appl Environ Microbiol. 1979 Oct;38(4):644–649. doi: 10.1128/aem.38.4.644-649.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki J. S., Remsen C. C. Comparison of two direct-count methods for determining metabolizing bacteria in freshwater. Appl Environ Microbiol. 1981 May;41(5):1132–1138. doi: 10.1128/aem.41.5.1132-1138.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell S. Y. Modification of the gelatin-matrix method for enumeration of respiring bacterial cells for use with salt-marsh water samples. Appl Environ Microbiol. 1984 Apr;47(4):873–875. doi: 10.1128/aem.47.4.873-875.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickels J. S., King J. D., White D. C. Poly-beta-Hydroxybutyrate Accumulation as a Measure of Unbalanced Growth of the Estuarine Detrital Microbiota. Appl Environ Microbiol. 1979 Mar;37(3):459–465. doi: 10.1128/aem.37.3.459-465.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samejima K., Dairman W., Stone J., Udenfriend S. Condensation of ninhydrin with aldehydes and primary amines to yield highly fluorescent ternary products. II. Application to the detection and assay of peptides, amino acids, amines, and amino sugars. Anal Biochem. 1971 Jul;42(1):237–247. doi: 10.1016/0003-2697(71)90031-5. [DOI] [PubMed] [Google Scholar]
- Senior P. J., Beech G. A., Ritchie G. A., Dawes E. A. The role of oxygen limitation in the formation of poly- -hydroxybutyrate during batch and continuous culture of Azotobacter beijerinckii. Biochem J. 1972 Aug;128(5):1193–1201. doi: 10.1042/bj1281193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley P. E., Williams S. G. Use of the liquid scintillation spectrometer for determining adenosine triphosphate by the luciferase enzyme. Anal Biochem. 1969 Jun;29(3):381–392. doi: 10.1016/0003-2697(69)90323-6. [DOI] [PubMed] [Google Scholar]
- Tabor P. S., Neihof R. A. Improved method for determination of respiring individual microorganisms in natural waters. Appl Environ Microbiol. 1982 Jun;43(6):1249–1255. doi: 10.1128/aem.43.6.1249-1255.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakisaka Y., Masaki E., Nishimoto Y. Formation of Crystalline delta-Endotoxin or Poly-beta-Hydroxybutyric Acid Granules by Asporogenous Mutants of Bacillus thuringiensis. Appl Environ Microbiol. 1982 Jun;43(6):1473–1480. doi: 10.1128/aem.43.6.1473-1480.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. C., Tucker A. N. Phospholipid metabolism during bacterial growth. J Lipid Res. 1969 Mar;10(2):220–233. [PubMed] [Google Scholar]
- Zimmermann R., Iturriaga R., Becker-Birck J. Simultaneous determination of the total number of aquatic bacteria and the number thereof involved in respiration. Appl Environ Microbiol. 1978 Dec;36(6):926–935. doi: 10.1128/aem.36.6.926-935.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


