Abstract
Background
To determine whether historic albuminuria measurements provide additional predictive value for diabetic end-stage renal disease (ESRD) and natural mortality over the most recent measurement, ie, whether “regression” from high albuminuria has a different prognosis than stability at the lower level.
Study Design
Observational longitudinal study.
Setting & Participants
Pima Indians 15 years or older with type 2 diabetes and at least 2 consecutive measurements of urinary albumin-creatinine ratio (ACR) within 6 years.
Predictors
Sequential measurements of urinary ACR.
Outcomes & Measurements
Proportional hazards analyses were used to estimate the risk of ESRD and natural death associated with the first and second ACR measurement. The ability of these highly correlated variables to predict outcome was compared with receiver operating characteristic curves calculated by means of the generalized c statistic.
Results
In 983 subjects, 136 developed ESRD and 180 died of natural causes during a maximum follow-up of 12.6 years. Each doubling in the second ACR was associated with a 1.71-fold greater incidence of ESRD (95% confidence interval, 1.54 to 1.89) and 1.16-fold greater natural mortality (95% confidence interval, 1.07 to 1.27) adjusted for age, sex, diabetes duration, and antihypertensive medication. The addition of the first ACR measurement to the model did not add to the predictive value for ESRD or mortality. In pairwise comparisons of c statistics, the second ACR was a significantly better predictor of ESRD than the first ACR.
Limitations
The predictive value of ACR measurements is decreased to the extent that its precision is based on a single measure.
Conclusion
The predictive power of the latest ACR for ESRD and natural mortality in patients with diabetes is not enhanced by knowledge of the preceding ACR. Therefore, ACR changes over time, ie, regression or progression, add minimal predictive value beyond the latest measurement in the series.
INDEX WORDS: Diabetes, albuminuria, end-stage renal disease, mortality, prediction
Increased albuminuria is a marker of glomerular disease and predicts progression to end-stage renal disease (ESRD) and cardiovascular death. In patients with diabetes, increased albuminuria frequently regresses, and regression from a high level predicts a better outcome than no regression.1–6 However, whether patients with diabetes who experience regression to a lower level are at different risk of ESRD and mortality than those who remained at a lower level is unknown.
In the present study, we determined whether historic measurement of albuminuria in Pima Indians with type 2 diabetes added to the ability of the most recent measurement to predict diabetic ESRD and mortality from natural causes.
METHODS
Members of the Gila River Indian Community participate in a longitudinal study of diabetes and its complications. Since 1965, each person 5 years or older is invited to a research examination approximately every 2 years regardless of health. These biennial examinations include measurements of venous plasma glucose obtained 2 hours after a 75-g oral glucose load and assessment for the complications of diabetes. Since July 1, 1982, albumin was measured in urine specimens collected at the end of the 2-hour glucose tolerance test. Albumin is measured by using a nephelometric immunoassay.7 Values less than 6.8 × 10−4 g/dL (6.8 × 10−3 g/L), the threshold below which albuminuria cannot be detected by the assay, were assigned the value of 6.8 × 10−4 g/dL (6.8 × 10−3 g/L). Urine creatinine is measured in the same specimen by using a modification of the Jaffé reaction.8 Albuminuria is defined by urinary albumin-creatinine ratio (ACR). Diabetes is diagnosed by using World Health Organization criteria,9 and the date of diagnosis is determined from these research examinations or review of clinical records if diabetes was diagnosed in the course of routine medical care. Diabetic ESRD is defined as dialysis attributed to diabetic nephropathy or death from diabetic nephropathy if dialysis therapy was unavailable or refused and was ascertained independently of research examinations. Ascertainment of vital status and ESRD was complete through December 31, 2000.
Causes of death were recorded according to terminology and codes of the International Classification of Diseases, Ninth Revision. Accuracy and completeness of the underlying cause of death was determined by review of clinical records, autopsy reports, and death certificates. Deaths were considered natural if they were caused by disease (International Classification of Diseases, Ninth Revision codes 001.0 to 799.9).
The study population included subjects who resided in the community at any time from July 1, 1982, through December 31, 2000; attended research examinations when 15 years or older; and had diabetes. To be eligible, subjects 15 years or older were required to have at least 2 research examinations with ACR measurements at or after the diagnosis of diabetes and within a 6-year period. In all analyses, the second diabetic examination with an ACR measurement was considered the “baseline” examination, and individuals were followed up from this point for the occurrence of death or ESRD.
Statistical Analysis
Characteristics are presented as mean ± SD or median and range. Body mass index was defined as weight divided by the square of height (kilograms per square meter). Mean arterial pressure was calculated as 2/3 diastolic arterial pressure + 1/3 systolic arterial pressure.
The incidence rate of diabetic ESRD was computed as the number of new cases of ESRD per 1,000 person-years at risk. The period of risk began at the second diabetic examination with an ACR measurement and ended at the date of ESRD from any cause, death from causes other than diabetic nephropathy, or December 31, 2000, whichever came first. The date of ESRD was the date of initiation of dialysis therapy or the date of death from diabetic nephropathy if dialysis was refused or not available. Death rates were calculated as the number of subjects who died per 1,000 person-years of follow-up. The period of risk for the mortality analysis began at the second diabetic examination with ACR measurement and ended at the date of death or December 31, 2000, whichever came first. Age- and sex-adjusted incidence rates of ESRD and mortality were standardized to the 1985 Pima Indian population 15 years or older.
Cox regression models were used to estimate risks of diabetic ESRD and mortality associated with the first and second ACR measurements adjusted for age, sex, duration of diabetes, and baseline antihypertensive medication. ACR values are expressed as the logarithm base 2 (log2). The hazard ratio (HR) for the doubling of ACR reflects the risk ratio for the outcome corresponding to a 2-fold difference in ACR. In the mortality analysis, sex violated the proportionality assumptions; therefore, the final model was stratified by this covariate.
ACR measurements were also categorized as normal (ACR < 30 mg/g), microalbuminuria (30 mg/g ≤ACR < 300 mg/g), or macroalbuminuria (ACR ≥ 300 mg/g). Unadjusted rate ratios for diabetic ESRD and mortality relative to persistently normal ACRs were computed from incidence rates. Subjects who experienced progression to a higher ACR category at the second measurement were referred to as progressors; those who experienced regression to a lower ACR category were referred to as regressors. Unadjusted rate ratios and age-, sex-, diabetes duration–, and antihypertensive medication–adjusted HRs for ESRD and mortality were computed for subjects who experienced progression, experienced regression, or remained in the same ACR category. HRs were computed relative to those with normal ACR at both examinations.
Because the relevant predictor variables were highly correlated, the ability of these variables to predict outcome was compared with receiver operating characteristic (ROC) curves. To adjust for potentially confounding variables and account for variable follow-up time, ROC curves predicting the probability that an individual would develop ESRD or die were calculated from Cox regression models that included age, sex, duration of diabetes, antihypertensive medication, and the relevant predictor variable. The area under the ROC curve and its SE were calculated for each model by using the generalized c statistic to account for the variable follow-up time.10 Comparisons between the areas for pairs of variables were assessed according to the formula of Hanley and McNeil.11 This method accounts for the correlation that occurs when ROC curves are derived from correlated variables measured in the same individuals; this correlation was estimated by using a jacknife procedure.12 Calculations were performed using a SAS macro with SAS software, version 8 (SAS Institute, Cary, NC).
RESULTS
Characteristics of the study population corresponding to the second ACR measurement are listed in Table 1. Of 1,622 subjects with diabetes examined during the study period, 983 (331 men, 652 women) met inclusion criteria for this study. During a median follow-up of 8.4 years (range, 4.3 to 12.6 years), 136 developed diabetic ESRD with an age- and sex-adjusted incidence rate of 10.80 cases/1,000 person-years (95% confidence interval [CI], 7.42 to 14.08) and 180 subjects died of natural causes, with an age- and sex-adjusted death rate of 11.76 deaths/1,000 person-years (95% CI, 8.57 to 14.96). At the first ACR measurement, 601 subjects had normal values, 270 had microalbuminuria, and 112 had macroalbuminuria (20 with ACR ≥ 3,000 mg/g; Table 2). Of those with normal ACRs, 76% remained stable and 24% experienced progression to microalbuminuria or macroalbuminuria by the second ACR measurement. Of those with microalbuminuria, 57% remained so, 24% experienced regression to a normal ACR, and 19% experienced progression to macroalbuminuria. The majority of subjects with macroalbuminuria remained in this category (85%) at the second ACR measurement, whereas 15% regressed to microalbuminuria or normoalbuminuria. Of those with macroalbuminuria of 3,000 mg/g or greater, only 1 person experienced regression to microalbuminuria. Overall, 23% experienced progression and 8% experienced regression between the 2 consecutive ACR measurements.
Table 1.
Clinical and Demographic Characteristics of Subjects With Type 2 Diabetes Who Had at Least 2 ACR Measurements Within 6 Years
| Baseline Characteristic | |
|---|---|
| No. of patients | 983 |
| Men | 331 |
| Women | 652 |
| Age (y) | 46.2 ± 13.5 |
| Duration of diabetes (y) | 9.1 ± 7.4 |
| Mean arterial pressure (mm Hg) | 93.4 ± 13.1 |
| Hemoglobin A1c (%) | 8.5 ± 2.5 |
| ACR−1 (mg/g) | 20.6 (11.0–67.4) |
| ACR0 (mg/g) | 26.5 (12.7–120.9) |
| Time between ACR−1 and ACR0 (y) | 2.4 (2.0–3.3) |
| Body mass index (kg/m2) | 34.6 ± 8.2 |
| Serum cholesterol (mg/dL) | 182.9 ± 40.5 |
| Fasting plasma glucose (mg/dL) | 196.4 ± 78.2 |
| Serum creatinine (mg/dL) | 0.8 ± 0.7 |
| Estimated glomerular filtration rate(mL/min/1.73 m2) | 115.9 ± 35.1 |
| Antihypertensive medicines (%) | 22 |
Note: Values expressed as mean ± SD, median (25th to 75th percentiles), number of patients, or percent. Fasting plasma glucose concentration was missing in 11 subjects, and body mass index was missing in 2 subjects. To convert serum creatinine in mg/dL to μmol/L, multiply by 88.4; serum cholesterol in mg/dL to mmol/L, multiply by 0.02586; plasma glucose in mg/dL to mmol/L, multiply by 0.05551; glomerular filtration rate in mL/min to mL/s, multiply by 0.01667.
Abbreviations: ACR0, second ACR measurement; ACR−1, first ACR measurement; ACR, albumin-creatinine ratio.
Table 2.
Changes in ACR Categories Between 2 ACR Measurements Obtained Within 6 Years in Pima Indians With Type 2 Diabetes
| ACR0 (mg/g)
|
|||||
|---|---|---|---|---|---|
| ACR−1 (mg/g) | <30 | 30–300 | 300–3,000 | ≥3,000 | Total |
| <30 | 460 (76.5) | 129 (21.5) | 11 (1.8) | 1 (0.2) | 601 |
| 30–300 | 65 (24.1) | 155 (57.4) | 45 (16.7) | 5 (1.8) | 270 |
| 300–3,000 | 2 (2.2) | 12 (13.0) | 46 (50.0) | 32 (34.8) | 92 |
| ≥3,000 | 0 (0) | 1 (5.0) | 2 (10.0) | 17 (85.0) | 20 |
| Total | 527 | 297 | 104 | 55 | 983 |
Note: Values expressed as number of subjects in each category (percent of row). Numbers in bold type represent subjects with stable ACR.
Abbreviations: ACR0, second ACR measurement; ACR−1, first ACR measurement; ACR, albumin-creatinine ratio.
The incidence rate of diabetic ESRD, either unadjusted or adjusted for age, sex, duration of diabetes, and antihypertensive medication, was greatest in subjects with macroalbuminuria at the second ACR measurement, intermediate in those with microalbuminuria, and lowest in those with normoalbuminuria regardless of their first ACR level (Fig 1). The modest incremental risk associated with past microalbuminuria in the normoalbuminuric group and past macroalbuminuria in the microalbuminuric group was not statistically significant. Death rates also were related to the second ACR level; the level of the first ACR had little effect on this outcome when the second level was considered (Fig 2). The 58 subjects with ACR of 3,000 mg/g or greater (55 with ACR ≥ 3,000 mg/g at the second ACR measurement plus the 3 subjects with ACR ≥ 3,000 mg/g at the first examination who experienced regression by the time of the second ACR measurement) were not included in these analyses because of their small numbers and the rarity of regression at this stage of kidney disease (as discussed). Of subjects with a first ACR in this range, 16 developed diabetic ESRD during a median follow-up of 1.6 years (range, 0.4 to 3.3 years), and 12 died of natural causes during a median follow-up of 5.1 years (range, 3.4 to 7.8 years).
Figure 1.
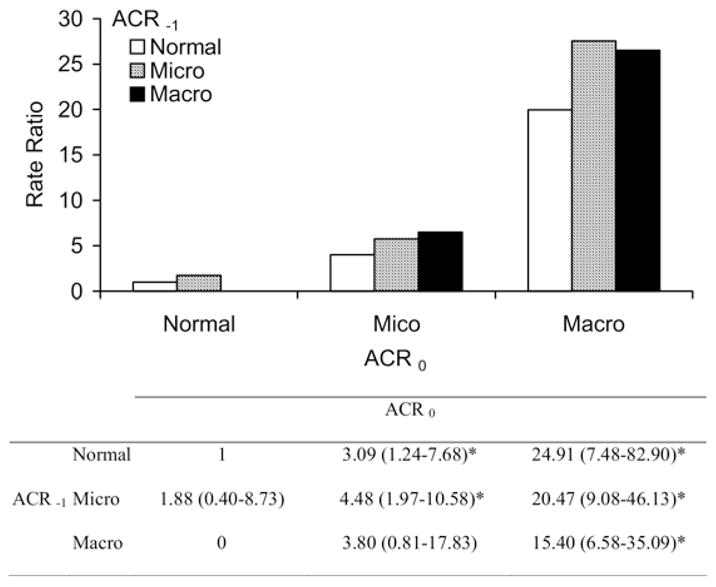
Unadjusted incidence rate ratio for diabetic end-stage renal disease (ESRD; N = 89 cases) relative to persistently normal albumin-creatinine ratio (ACR). The table below the figure comparatively shows hazard ratios for diabetic ESRD according to change in ACR with 95% confidence intervals adjusted for age, sex, diabetes duration, and antihypertensive medication. Macroalbuminuria (Macro) was truncated at less than 3,000 mg/g. Sample size for each category is listed in Table 2. Abbreviations: Normal, normal ACR; Micro, microalbuminuria; ACR−1, first ACR measurement; ACR0, second ACR measurement. *P < 0.05 compared with the group with persistently normal ACR.
Figure 2.
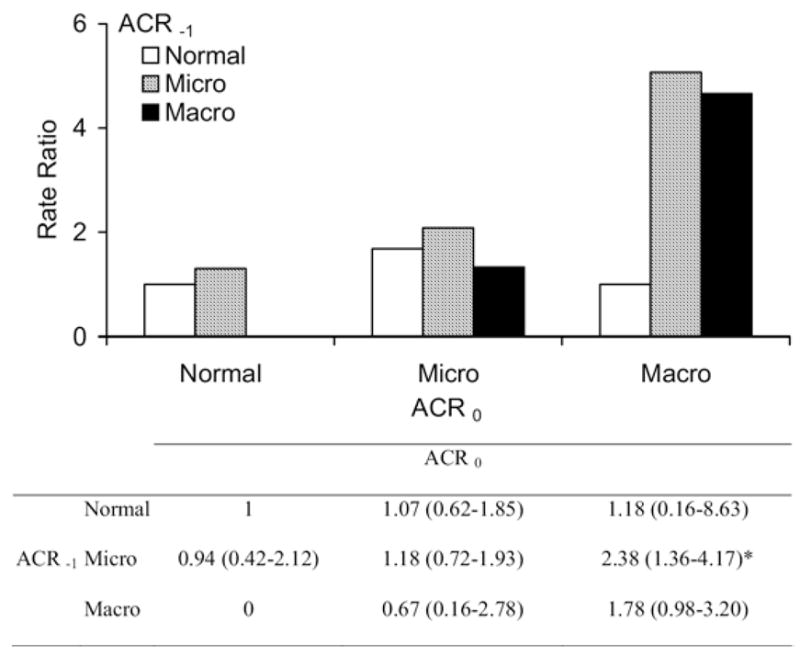
Unadjusted death rate ratio for natural mortality (N = 144 deaths) relative to persistently normal albumin-creatinine ratio (ACR). The table below the figure comparatively shows hazard ratios for natural death according to change in ACR with 95% confidence intervals adjusted for age, sex, diabetes duration, and antihypertensive medication. Macroalbuminuria (Macro) was truncated at less than 3,000 mg/g. Sample size for each category is listed in Table 2. Abbreviations: Normal, normal ACR; Micro, microalbuminuria; ACR−1, first ACR measurement; ACR0, second ACR measurement. *P < 0.05 compared with the group with persistently normal ACR.
Each doubling of the second ACR value was associated with a 1.71-fold increase in incidence of ESRD (95% CI, 1.54 to 1.89) in a Cox regression model adjusted for age, sex, duration of diabetes, and antihypertensive medication. The natural death rate was 1.16 times as high (95% CI, 1.06 to 1.27) when adjusted for the same covariates. After including the first ACR measurement in the models, no additional information about risk of ESRD or natural mortality was obtained: HRs for the earlier ACR were 1.02 (95% CI, 0.92 to 1.13) and 1.01 (95% CI, 0.92 to 1.12), respectively (Table 3). Additional adjustment for baseline body mass index, hemoglobin A1c level, and mean arterial pressure had little effect on HRs; the second ACR remained the better predictor of diabetic ESRD or natural mortality.
Table 3.
HRs for End-Stage Renal Disease (N = 136 cases) and Natural Mortality (N = 179 deaths)
| End-Stage Renal Disease
|
Natural Mortality
|
||||
|---|---|---|---|---|---|
| Model | HR 95% CI | HR 95% CI | |||
| 1 | Doubling of ACR−1 | 1.60 | 1.49–1.72 | 1.19 | 1.12–1.26 |
| 2 | Doubling of ACR0 | 1.75 | 1.62–1.89 | 1.19 | 1.13–1.26 |
| 3 | Doubling of ACR−1 | 1.06 | 0.95–1.17 | 1.05 | 0.95–1.16 |
| Doubling of ACR0 | 1.70 | 1.52–1.87 | 1.15 | 1.06–1.26 | |
| 4 | Baseline antihypertensive medication (0 or 1) | 2.16 | 1.49–3.13 | 1.73 | 1.26–2.39 |
| Doubling of ACR−1 | 1.02 | 0.92–1.13 | 1.01 | 0.92–1.12 | |
| Doubling of ACR0 | 1.71 | 1.54–1.89 | 1.16 | 1.07–1.27 | |
| 5* | Age (/10 y) | 0.90 | 0.79–1.13 | 1.74 | 1.49–2.03 |
| Sex (0 or 1) | 1.15 | 0.78–1.69 | |||
| Diabetes duration (/5 y) | 1.42 | 1.23–1.65 | 1.15 | 1.02–1.29 | |
| Baseline antihypertensive medication (0 or 1) | 2.64 | 1.77–3.93 | 1.96 | 1.41–2.73 | |
| Mean arterial pressure (/1 mm Hg) | 1.00 | 0.99–1.02 | 1.00 | 0.99–1.01 | |
| Body mass index (/1 kg/m2) | 0.97 | 0.94–1.00 | 0.97 | 0.94–1.00 | |
| Doubling of ACR−1 | 1.00 | 0.90–1.13 | 1.00 | 0.91–1.12 | |
| Doubling of ACR0 | 1.70 | 1.50–1.91 | 1.16 | 1.05–1.28 | |
Note: Models 1, 2, and 3 adjusted for sex, baseline age, and diabetes duration. Model 4 adjusted for sex, baseline age, diabetes duration, and antihypertensive medication.
Abbreviations: ACR, albumin-creatinine ratio; HR, hazard ratio; CI, confidence interval; ACR0, second ACR measurement; ACR−1, first ACR measurement.
The full model for end-stage renal disease was stratified by hemoglobin A1c tertiles, and the full model for natural mortality was stratified by sex and hemoglobin A1c tertiles.
Comparing c statistics calculated from the Cox regression models for the first and second ACR values, the second ACR was the stronger predictor of diabetic ESRD (P = 0.006). Although the second ACR was also a stronger predictor of natural death, the difference was not statistically significant (Table 4). The higher correlations for natural mortality probably are caused by the relatively greater contribution of the covariates other than ACR to the mortality outcome than to the ESRD outcome.
Table 4.
C Statistic for Each Predictor Variable
| Variable 1 ESRD | C Statistic 1 | SE 1 | 95% CI | Variable 2 | C Statistic 2 | SE 2 | 95% CI | r | P |
|---|---|---|---|---|---|---|---|---|---|
| ACR0 | 0.920 | 0.011 | 0.898–0.942 | ACR−1 | 0.895 | 0.012 | 0.872–0.919 | 0.81 | <0.001 |
| ACR0 | 0.920 | 0.011 | 0.898–0.942 | Covariates | 0.839 | 0.015 | 0.810–0.868 | 0.51 | <0.001 |
| ACR0 | 0.920 | 0.011 | 0.898–0.942 | ACR−1 + ACR0 | 0.920 | 0.011 | 0.898–0.942 | 0.99 | 0.4 |
| ACR−1 | 0.895 | 0.012 | 0.872–0.919 | Covariates | 0.839 | 0.015 | 0.810–0.868 | 0.70 | <0.001 |
| ACR−1 | 0.895 | 0.012 | 0.872–0.919 | ACR−1 + ACR0 | 0.920 | 0.011 | 0.898–0.942 | 0.81 | <0.001 |
| Covariates | 0.839 | 0.015 | 0.810–0.868 | ACR−1 + ACR0 | 0.920 | 0.011 | 0.898–0.942 | 0.51 | <0.001 |
| Natural mortality | |||||||||
| ACR0 | 0.812 | 0.015 | 0.783–0.841 | ACR−1 | 0.810 | 0.015 | 0.781–0.839 | 0.97 | 0.8 |
| ACR0 | 0.812 | 0.015 | 0.783–0.841 | Covariates | 0.800 | 0.015 | 0.771–0.829 | 0.90 | 0.1 |
| ACR0 | 0.812 | 0.015 | 0.783–0.841 | ACR−1 + ACR0 | 0.812 | 0.015 | 0.783–0.841 | 0.99 | 0.1 |
| ACR−1 | 0.810 | 0.015 | 0.781–0.839 | Covariates | 0.800 | 0.015 | 0.771–0.829 | 0.94 | 0.06 |
| ACR−1 | 0.810 | 0.015 | 0.781–0.839 | ACR−1 + ACR0 | 0.812 | 0.015 | 0.783–0.841 | 0.97 | 0.6 |
| Covariates | 0.800 | 0.015 | 0.771–0.829 | ACR−1 + ACR0 | 0.812 | 0.015 | 0.783–0.841 | 0.90 | 0.08 |
Note: Covariates included sex, baseline age, diabetes duration, and antihypertensive medication. P refers to pairwise comparison between c statistic 1 and c statistic 2, and r is correlation between the 2 statistics introduced by studying the same sample of people.
Abbreviations: ESRD, end-stage renal disease; ACR0, second ACR measurement; ACR−1, first ACR measurement; ACR, albumin-creatinine ratio.
Associations between baseline ACR and diabetic ESRD or natural mortality were similar in subjects included in the study and the 567 subjects with a single ACR measurement who were excluded. In those with a single ACR measure, the HR for diabetic ESRD was 1.7 (95% CI, 1.5 to 1.9) and the HR for natural mortality was 1.1 (95% CI, 1.1 to 1.2), suggesting that exclusion of subjects with only a single ACR had a negligible impact on the outcome.
DISCUSSION
Increased ACR predicts ESRD and natural mortality, and regression of ACR decreases this risk.3–6 The present study indicates that the magnitude of risk reduction associated with regression is not determined by the historic level of ACR, but rather by the most recent ACR, suggesting that subjects with historically high albuminuria who experience regression to lower levels of albuminuria have similar risks for diabetic ESRD and natural mortality as those who remain at the lower levels of albuminuria. Similarly, subjects with an ACR that increases between the first and second examinations have the same risks of ESRD and natural mortality as those with the same ACR level, but for whom ACR has not changed from the previous examination. Although earlier measurements were related to these outcomes, they did not add to the predictive power when the most recent ACR was considered. Accordingly, the latest available ACR is the most informative about a patient’s risk of long-term complications of diabetic nephropathy. Studies of kidney biopsies in Pima Indians with type 2 diabetes showed that albuminuria was associated virtually exclusively with diabetic glomerulosclerosis.13 Hence, the likelihood that other causes of increased albuminuria were operative is remote. A study of persons with nondiabetic kidney disease using different methods came to a similar conclusion, that urine protein excretion during follow-up is a more important predictor of kidney disease progression than baseline level.14 In keeping with this observation, the current level of proteinuria, not the level at onset of treatment, should be the basis of antiproteinuric therapy.
Recent animal studies showed that treatment with renin-angiotensin system inhibitors, in addition to reducing glomerular sclerosis, may also promote glomerular capillary regeneration.15,16 In keeping with these findings, persistent clinical regression of albuminuria may parallel reversing kidney damage,17,18 setting back the clock on long-term outcomes, with no residual risk of a given past albuminuria level. Intensive multifactorial treatment reduces the rate of decline in the glomerular filtration rate in direct proportion to the decrease in proteinuria, suggesting that clinical improvement may be related to reduction in maladaptive glomerular changes, such as glomerular hyperfiltration and increased glomerular capillary pressure.19
Studies estimating the impact of past and recent measurements of blood pressure on risk of cardiovascular disease20,21 indicated that antecedent blood pressure had a better prognostic value than current measurement. This finding suggested that antecedent measures were better at quantifying the long-term exposure to atherosclerosis than recent measurements. However, the same relationship with historic measures may not be true for albuminuria. The regression of increased albuminuria in a substantial number of patients with diabetes1,2 and the lack of significant structural abnormalities in kidneys of many persons with type 2 diabetes and microalbuminuria22–25 suggest that modest increases in albuminuria may occur in response to functional changes in the kidneys, rather than structural disease. The strikingly positive relationship between increasing albuminuria and a sieving defect in the glomerular capillary wall in diabetic Pima Indians occurs only in those with ACR of 3,000 mg/g or greater.26 A decrease in albuminuria before the onset of structural changes may lead to an immediate decrease in risk, whereas after the onset of structural disease, a decrease may be less likely.
Serial ACR measures were highly correlated, with a Spearman correlation coefficient of 0.74. Accordingly, adjusting for the second ACR measure, which can be both a confounder and an intermediate variable, may have decreased the association between the first ACR and ESRD or death.27,28 Nonetheless, the intent of the present study was not to assess causality of these associations, but to show the predictive value of the latest in a series of ACR measurements for the outcomes of interest.
In the present study, subjects were not screened for urinary tract infections and only a single urine collection was obtained at each examination for assessment of ACR; therefore, the predictive value of ACRs decreases to the extent that its precision is based on a single measurement and its accuracy on whether the subject had a urinary tract infection.
Treatment with renin-angiotensin system inhibitors was introduced in the Pima Indian community in November 1986, with only limited use until September 1989. Antihypertensive medicine use related positively to risk of ESRD or death because those using these medicines more likely had advanced disease and greater comorbidity. To examine further how renin-angiotensin system inhibitors might affect the present results, we analyzed separately the subset of people with both ACR measurements before September 1989 (N = 426), those who had their first ACR measurement before September 1989 and the second ACR measurement after this date (N = 161), and the 394 subjects with both ACR measurements after September 1989, with and without adjusting for baseline antihypertensive medication. The predictive value of the latest ACR measurement remained stronger than that of the first ACR measurement in all groups, indicating that use of renin-angiotensin system inhibitors had no discernable impact on conclusions. Antihypertensive medication may also be an intermediate variable on the pathway between increased albuminuria and the outcomes of interest; therefore, inclusion of this variable in the regression model may underestimate the effect of ACR on these outcomes. In the present analysis, adjusting for these medicines had little effect on HRs, in part because of the low prevalence of antihypertensive treatment at both the first and second ACR measurement.
In summary, greater ACR predicted diabetic ESRD and natural mortality in Pima Indians with type 2 diabetes. The incidence of diabetic ESRD was nearly 2 times as high and the death rate was 1.2 times as high with each 2-fold increment in the latest ACR level. Although past measurements were related to these outcomes, they did not add to the predictive power when the current measurement was considered. A low current ACR value was associated with good prognosis regardless of whether earlier values were higher, the same, or lower. Therefore, changes in ACR over time, ie, regression or progression, added minimal predictive value beyond the latest measurement in the series. This finding does not imply that ACR should be measured less frequently. The opposite applies because the relationship between albuminuria and ESRD or mortality is a continuum starting below the currently accepted threshold for microalbuminuria, and treatment should aim to reach and maintain the lowest possible value of albuminuria. Monitoring ACR in high-risk patients certainly is the appropriate way to achieve this goal.
Acknowledgments
The authors thank the members of the Gila River Indian Community for participating in this investigation.
Support: Dr Pavkov was supported by a Mentor-Based Fellowship award from the American Diabetes Association. This research was also supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Financial Disclosure: None.
References
- 1.Perkins BA, Ficociello LH, Silva KH, Finkelstein DM, Warram JH, Krolewski AS. Regression of microalbuminuria in type 1 diabetes. N Engl J Med. 2003;348:2285–2293. doi: 10.1056/NEJMoa021835. [DOI] [PubMed] [Google Scholar]
- 2.Araki S, Haneda M, Sugimoto T, et al. Factors associated with frequent remission of microalbuminuria in patients with type 2 diabetes. Diabetes. 2005;54:2983–2987. doi: 10.2337/diabetes.54.10.2983. [DOI] [PubMed] [Google Scholar]
- 3.Spoelstra-de Man AME, Brouwer CB, Stehouwer CDA, Smulders YM. Rapid progression of albumin excretion is an independent predictor of cardiovascular mortality in patients with type 2 diabetes and microalbuminuria. Diabetes Care. 2001;24:2097–2101. doi: 10.2337/diacare.24.12.2097. [DOI] [PubMed] [Google Scholar]
- 4.Gaede P, Tarnow L, Vedel P, Parving HH, Pedersen O. Remission to normoalbuminuria during multifactorial treatment preserves kidney function in patients with type 2 diabetes and microalbuminuria. Nephrol Dial Transplant. 2004;19:2784–2788. doi: 10.1093/ndt/gfh470. [DOI] [PubMed] [Google Scholar]
- 5.Rossing K, Christensen PK, Hovind P, Parving HH. Remission of nephrotic-range albuminuria reduces risk of end-stage renal disease and improves survival in type 2 diabetic patients. Diabetologia. 2005;48:2241–2247. doi: 10.1007/s00125-005-1937-6. [DOI] [PubMed] [Google Scholar]
- 6.Hovind P, Tarnow L, Parving HH. Remission and regression of diabetic nephropathy. Curr Hypertens Rep. 2004;6:377–382. doi: 10.1007/s11906-004-0057-x. [DOI] [PubMed] [Google Scholar]
- 7.Vasquez B, Flock EV, Savage PJ, et al. Sustained reduction of proteinuria in type 2 (non-insulin-dependent) diabetes following diet-induced reduction of hyperglycemia. Diabetologia. 1984;26:127–133. doi: 10.1007/BF00281119. [DOI] [PubMed] [Google Scholar]
- 8.Chasson AL, Grady HJ, Stanley MA. Determination of creatinine by means of automatic chemical analysis. Tech Bull Regist Med Technol. 1960;30:207–212. [PubMed] [Google Scholar]
- 9.World Health Organization. Technical Report Series No. 727. Geneva, Switzerland: World Health Organization; 1985. Diabetes Mellitus. [Google Scholar]
- 10.Pencina MJ, D’Agostino RB. Overall C as a measure of discrimination in survival analysis: Model specific population value and confidence interval estimation. Stat Med. 2004;23:2109–2123. doi: 10.1002/sim.1802. [DOI] [PubMed] [Google Scholar]
- 11.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148:839–843. doi: 10.1148/radiology.148.3.6878708. [DOI] [PubMed] [Google Scholar]
- 12.Song HH. Analysis of correlated ROC areas in diagnostic testing. Biometrics. 1997;53:370–382. [PubMed] [Google Scholar]
- 13.Pagtalunan ME, Miller PL, Jumping-Eagle S, et al. Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest. 1997;99:342–348. doi: 10.1172/JCI119163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jafar TH, Stark PC, Schmid CH, et al. Proteinuria as a modifiable risk factor for the progression of non-diabetic renal disease. Kidney Int. 2001;60:1131–1140. doi: 10.1046/j.1523-1755.2001.0600031131.x. [DOI] [PubMed] [Google Scholar]
- 15.Remuzzi A, Gagliardini E, Sangalli F, et al. ACE inhibition reduces glomerulosclerosis and regenerates glomerular tissue in a model of progressive renal disease. Kidney Int. 2006;69:1124–1130. doi: 10.1038/sj.ki.5000060. [DOI] [PubMed] [Google Scholar]
- 16.Remuzzi A, Gagliardini E, Donadoni C. Effect of angiotensin II antagonism on the regression of kidney disease in the rat. Kidney Int. 2002;62:885–894. doi: 10.1046/j.1523-1755.2002.00526.x. [DOI] [PubMed] [Google Scholar]
- 17.Nosadini R, Velussi M, Brocco E, et al. Course of renal function in type 2 diabetic patients with abnormalities of albumin excretion rate. Diabetes. 2000;49:476–484. doi: 10.2337/diabetes.49.3.476. [DOI] [PubMed] [Google Scholar]
- 18.Fioretto P, Steffes MW, Sutherland DER, Goetz FC, Mauer M. Reversal of lesions of diabetic nephropathy after pancreas transplantation. N Engl J Med. 1998;339:69–75. doi: 10.1056/NEJM199807093390202. [DOI] [PubMed] [Google Scholar]
- 19.Ruggenenti P, Schieppati A, Remuzzi G. Progression, remission, regression of chronic renal diseases. Lancet. 2001;357:1601–1608. doi: 10.1016/S0140-6736(00)04728-0. [DOI] [PubMed] [Google Scholar]
- 20.Vasan RS, Massaro JM, Wilson PWF, et al. Antecedent blood pressure and risk of cardiovascular disease. The Framingham Heart Study. Circulation. 2002;105:48–53. doi: 10.1161/hc0102.101774. [DOI] [PubMed] [Google Scholar]
- 21.Boshuizen HC, Lanti M, Menotti A, et al. Effects of past and recent blood pressure and cholesterol level on coronary heart disease and stroke mortality, accounting for measurement error. Am J Epidemiol. 2006;165:398–409. doi: 10.1093/aje/kwk021. [DOI] [PubMed] [Google Scholar]
- 22.Caramori ML, Fioretto P, Mauer M. Enhancing the predictive value of urinary albumin for diabetic nephropathy. J Am Soc Nephrol. 2006;17:339–352. doi: 10.1681/ASN.2005101075. [DOI] [PubMed] [Google Scholar]
- 23.Christensen PK, Larsen S, Horn T, Olsen S, Parving HH. Renal function and structure in albuminuric type 2 diabetes without retinopathy. Nephrol Dial Transplant. 2001;16:2337–2347. doi: 10.1093/ndt/16.12.2337. [DOI] [PubMed] [Google Scholar]
- 24.Fioretto P, Mauer SM, Brocco E, et al. Patterns of renal injury in NIDDM patients with microalbuminuria. Diabetologia. 1996;39:1569–1576. doi: 10.1007/s001250050616. [DOI] [PubMed] [Google Scholar]
- 25.Nelson RG, Bennett PH, Beck GJ, et al. for the Diabetic Renal Disease Study Group. Development and progression of renal disease in Pima Indians with non-insulin dependent diabetes mellitus. N Engl J Med. 1996;335:1636–1642. doi: 10.1056/NEJM199611283352203. [DOI] [PubMed] [Google Scholar]
- 26.Lemley KV, Blouch K, Abdullah I, et al. Glomerular permselectivity at the onset of nephropathy in type 2 diabetes mellitus. J Am Soc Nephrol. 2000;11:2095–2105. doi: 10.1681/ASN.V11112095. [DOI] [PubMed] [Google Scholar]
- 27.Tu Y-K, West R, Ellison GTH, Gilthorpe MS. Why evidence for the fetal origins of adult disease might be a statistical artifact: The “reversal paradox” for the relation between birth weight and blood pressure in later life. Am J Epidemiol. 2005;161:27–32. doi: 10.1093/aje/kwi002. [DOI] [PubMed] [Google Scholar]
- 28.Cole TJ. Re: Why evidence for the fetal origins of adult disease might be a statistical artifact: The “reversal paradox” for the relation between birth weight and blood pressure in later life. Am J Epidemiol. 2005;162:394–395. doi: 10.1093/aje/kwi231. letter. [DOI] [PubMed] [Google Scholar]


