Abstract
Sepsis, as a severe systemic inflammatory response to bacterial infection, represents a major clinical problem. It is characterized by the excessive production of reactive oxygen species (ROS) both in the circulation and in the affected organs. The excessive generation of ROS inevitably leads to oxidative stress in the microvasculature and has been implicated as a causative event in a number of pathologies including sepsis. In this review, we focus on the role of oxidative and nitrosative stress during the early onset of sepsis. Changes in microvascular endothelial cells, the cell type that occurs in all organs, are discussed. The mechanisms underlying septic induction of oxidative and nitrosative stresses, the functional consequences of these stresses, and potential adjunct therapies for microvascular dysfunction in sepsis are identified.
Keywords: systemic inflammation, oxidant stress, anti-oxidants
Introduction
Sepsis is a complex inflammatory response of the host to bacterial infection. It frequently occurs after trauma, hemorrhage, burn or abdominal surgery, which cause a systemic inflammatory response syndrome (SIRS) [1, 2]. Without timely and aggressive therapeutic intervention, sepsis often leads to multiple organ dysfunction and ultimately to death [3]. SIRS and sepsis remain the prime causes of death in intensive care units worldwide, with mortality rates ranging between 30 and 70% [4, 5].
The initiating event in sepsis may be release of endotoxins (i.e., bacterial cell wall lipopolysaccharides (LPS)) from Gram-negative and Gram-positive pathogenic bacteria [6]. LPS triggers activation of inflammatory cells, such as polymorphonuclear leukocytes (neutrophils; PMN), monocytes/macrophages and lymphocytes. LPS thereby triggers cellular and humoral aspects of the inflammatory immune response. It is generally agreed that it is not the bacterial infection itself, but rather the inflammatory response to infection that is the predominant determinant of outcome in sepsis [7, 8].
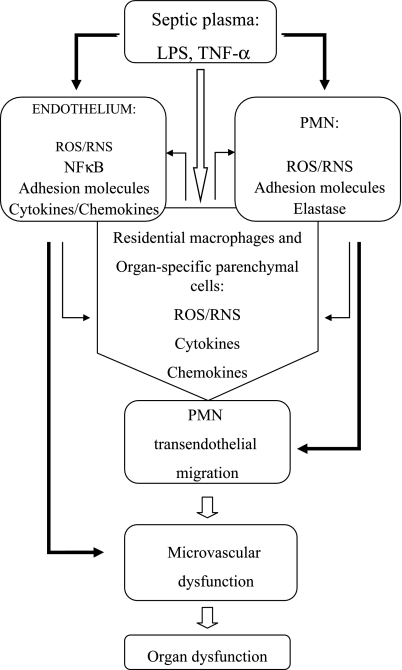
One of the key features of sepsis is tissue infiltration by phagocytic cells [9–13]. In this scenario, PMN and monocytes/macrophages respond to septic stimulation by producing reactive oxygen species (ROS) (e.g., superoxide, hydrogen peroxide) and reactive nitrogen species (RNS) (e.g., nitric oxide) [14]. In addition, PMN release granular enzymes (e.g. elastase, cathepsin) and the myeloperoxidase (MPO)-derived oxidant, hypochlorous acid (HOCl). All these components may contribute to PMN/macrophage-mediated killing of the bacteria. However, if produced in excess during SIRS and sepsis, the ROS, RNS and proteolytic enzymes cause microvascular dysfunction followed by organ dysfunction (Fig. 1).
Fig. 1.
Schematic diagram of cellular responses in sepsis. Sepsis-related inflammatory mediators (e.g. LPS, TNF-α, etc.) present in blood plasma directly activate microvascular endothelial cells and leukocytes with respect to the increased production of oxidants, activation of inflammation-relevant transcription factor, NFκB, and up-regulation of pro-adhesive phenotype, resulting in an increased neutrophilic leukocyte (PMN) migration/accumulation in the tissues and microvascular dysfunction (solid dark arrows). That, in turn, leads to activation of residential macrophages and organ-specific parenchymal cells, which results in feedback activation of the microvascular endothelial cells and leukocytes (dark arrows). Subsequently, these cells further impair microvascular function, allowing pro-inflammatory mediators directly access from the blood to the interstitium (solid white arrow). Because of the systemic nature of the septic response, inflammation in each individual organ may be stimulated repeatedly by pro-inflammatory mediators that are released into the circulation by remote organs. If unchecked, sepsis leads to the multiple organ dysfunction and failure.
An inflammatory response to septic stimuli is crucial for host defense, because it up-regulates anti-inflammatory mediators (e.g., IL-1 receptor antagonist, IL-4, IL-10) and antioxidant enzymes (e.g., catalase, glutathione peroxidase, manganese superoxide dismutase). However, excessive production of pro-inflammatory mediators in sepsis overwhelms the anti-inflammatory signaling. This results in suppression of innate immune functions (especially those of PMN) and leads to immunoparalysis and subsequently to increased susceptibility to infection [15].
It is important to note that, besides immune cells, microvascular endothelial cells also become activated in sepsis and may contribute to amplification of the inflammatory response [6, 11, 16]. In support to this hypothesis, it has been shown that septic stimuli (e.g., LPS, TNF-α) initiate activation of transcription factors such as NFκB and AP-1, resulting in transcriptional activation of multiple genes. This leads to the release of pro-inflammatory cytokines (e.g. TNF-α, IL-1β etc), and increased expression of adhesion molecules (e.g. E-selectin, ICAM-1, VCAM-1) and chemokines by endothelial cells [11, 17–19]. A key role of ROS/RNS in modulation of the endothelial cell pro-inflammatory phenotype has been highlighted previously [20] (Fig. 1).
Given the complexity of SIRS and sepsis, it is perhaps not surprising that little progress has been made in improving outcome [15]. Efforts to block one or other component of the sepsis-associated inflammatory pathways have had little impact on patient survival. Of many drugs tested, few have demonstrated efficacy [21–24]. Future advances in sepsis therapy will depend on improved understanding of the underlying pathophysiology. In this review, we will focus on the early events in the response to septic stimuli of microvascular endothelial cells, the cell type that occurs in all organs.
Microvascular Changes in Sepsis
The endothelium is a continuous single-cell lining of the cardiovascular system. It forms an interface between blood and tissues. In health, the endothelium provides a non-adhesive and anti-thrombotic surface for blood-born components, including circulating leukocytes. However, during sepsis, the microvascular endothelium is activated to a pro-adhesive and pro-thrombotic phenotype. In addition, septic endothelium becomes both a target and a source of ROS and RNS, and it thereby contributes to the onset of organ dysfunction [19, 25].
Clinically, sepsis is characterized by a severe microvascular dysfunction that persists despite fluid resuscitation. It has been suggested that microvascular dysfunction under septic conditions is closely related to: (i) arteriolar hyporesponsiveness to vasoconstrictors and vasodilators, including endothelium-dependent vasodilators; (ii) increased capillary permeability that manifests as a breakdown of the microvascular endothelial barrier; (iii) loss of the anti-adhesive function of endothelial surfaces; (iv) coagulopathy; (v) decreased density of perfused capillaries and elevated proportion of non-perfused capillaries, even in fluid-resuscitated patients with adequate arterial blood oxygenation and cardiac output [6, 26–33].
Microvascular dysfunction is a strong predictor of death in septic patients [29]. Tissue hypoxia develops because the diffusion distance for oxygen increases between blood and tissue cells [27]. This may explain why approximately one-third of patients with severe sepsis (i.e., sepsis with at least one organ failure or dysfunction) die of organ failure even when shock is prevented by fluid resuscitation and administration of vasopressor drugs [29, 34, 35].
Sources and Actions of Reactive Oxygen and Nitrogen Species in Endothelium During Sepsis
Endothelial cells are influenced by ROS that are produced at accelerated rates during sepsis [36]. ROS from activated neutrophils act as paracrine agents to alter endothelial cell function. Additionally, endothelial cells increase intracellular ROS production. For example, superoxide is a ROS that is synthesized in endothelial cells by the mitochondrial electron transport chain, xanthine oxidase, uncoupled nitric oxide synthases (NOS) and NADPH oxidases.
Bacterial bloodstream infection exposes endothelial cells to blood-borne endotoxins (e.g., E. coli lipopolysaccharide, LPS) and inflammatory cytokines (e.g., interferon-γ, IFNγ). The cells respond to this septic insult by activating endothelial NADPH oxidases that synthesize intracellular superoxide. Indeed, NADPH oxidase activity is the principal source for stimulated production of superoxide in microvascular endothelial cells exposed to septic insult [36–38]. For example, in microvascular endothelial cell cultures, septic insult (LPS and IFNγ in combination; LPS + IFNγ) increases superoxide levels within 2 h [37]. NADPH oxidase evidently is the principal source because the rise in superoxide levels is abolished by NADPH oxidase inhibitors (apocynin and diphenylene iodonium) but not by inhibitors of mitochondrial respiration (rotenone), xanthine oxidase (allopurinol) or NOS (L-NAME) [37]. Longer incubation (12 h) with LPS + IFNγ increases NADPH oxidase activity and NADPH oxidase protein expression [37]. Increases in intracellular ROS, whether caused by acute stimulation of NADPH oxidase or by entry of extracellular ROS, induce the expression of NADPH oxidase subunits that subsequently assemble and produce more ROS [39].
Dismutation of superoxide forms hydrogen peroxide and nitration of superoxide forms peroxynitrite. Compared to superoxide, both hydrogen peroxide and peroxynitrite are strong oxidants and the latter is also a nitrosative stressor. Thus, activation of NADPH oxidase during sepsis induces oxidative and nitrosative stress in endothelial cells. The consequences of the increased levels of intracellular oxidants are described next.
Effects of Oxidative and Nitrosative Stress on Microvascular Endothelial Function in Sepsis
Normally endothelial cells regulate vascular smooth muscle tone by basal rates of production of endothelial nitric oxide synthase (eNOS)-derived nitric oxide and prostaglandin endoperoxide H2 synthase-1 (PGHS)-1-derived prostacyclin (PGI2) [40]. Nitric oxide directly enters the smooth muscle cell and activates soluble guanylyl cyclase, thereby raising intracellular cGMP. PGI2 stimulates adenylyl cyclase to raise intracellular cAMP. cGMP and cAMP then mediate smooth muscle relaxation. However, septic insult increases production of superoxide that reacts with nitric oxide to form peroxynitrite that nitrates and inactivates endothelial PGI2 synthase, which can then no longer synthesize PGI2. Superoxide may also decrease the effective cellular level of nitric oxide below that required for guanylyl cyclase activation [40].
NADPH oxidase-derived oxidants (i.e., hydrogen peroxide and peroxynitrite) oxidize tetrahydrobiopterin, which is a cofactor for synthesis of nitric oxide by NOS enzymes [41, 42]. The loss of tetrahydrobiopterin (due to its oxidation) uncouples eNOS, so that the enzyme synthesizes superoxide rather than nitric oxide [41]. This is detrimental to blood flow in capillaries, which depends on nitric oxide synthesized locally by eNOS [43]. Further, ROS activate intracellular redox signaling pathways to increase adhesion of leukocytes, platelets and red blood cells to the endothelium and thereby precipitate capillary blood flow cessation [44–46].
Increased permeability of the endothelium occurs in multiple organs during sepsis, leading to plasma extravasation and edema formation. This causes loss of blood volume and progression of septic shock (i.e., severe sepsis with hypotension unresponsive to fluid resuscitation) [47]. Basal nitric oxide production by eNOS is necessary for maintenance of the endothelial barrier function [48–50]. The protective effect of nitric oxide is diminished during the inflammatory response due to the simultaneous production of superoxide. The source of this superoxide is likely the NADPH oxidase that is co-localized with eNOS in subcellular compartments within endothelial cells [51]. Indeed, there is experimental evidence that NADPH oxidase-derived ROS mediate endothelial barrier failure [52]. Reaction of superoxide with nitric oxide forms peroxynitrite. The latter causes lipid peroxidation, oxidation of sulfhydryl groups and nitration of tyrosine residues. In particular, nitration of cytoskeletal proteins by peroxynitrite appears to be a key step for endothelial barrier dysfunction [53, 54].
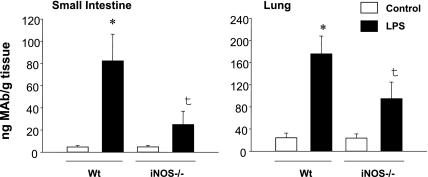
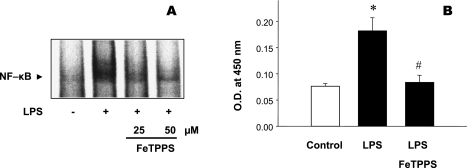
The oxidants that arise from NADPH oxidase activity (e.g., hydrogen peroxide formed by dismutation of superoxide) exert prolonged redox signaling effects that enhance induction of inducible nitric oxide synthase (iNOS) in septic blood vessels and endothelial cells [33, 37, 52, 55–57]. iNOS synthesizes abundant nitric oxide that in turn reacts with superoxide resulting in an excess production of peroxynitrite. This inevitably leads to the further impairment of microvascular function. For instance, iNOS expression in omental arteries of septic patients is associated with a subnormal arteriolar response to norepinephrine that can be normalized by the non-selective NOS inhibitor N(G)-methyl-l-arginine (L-NMMA) [58]. In addition, expression of the vascular adhesion molecule, E-selectin (a marker of vascular pro-inflammatory phenotype) is significantly abridged in the organs of iNOS-deficient mice challenged with LPS (Fig. 2) [59]. Important to note, that interfering with production of peroxynitrite by pretreating cultured endothelial cells with the peroxynitrite decomposition catalyst 5,10,15,20-tetrakis(4-sulfonatophenyl)prophyrinato iron [III] (FeTPPS) also reduces activation of nuclear factor NFκB and subsequent up-regulation of NFκB-dependent expression of E-selectin (Fig. 3) [59].
Fig. 2.
Expression of E-selectin in the small intestine and lungs of LPS-challenged wild-type (WT) and iNOS-deficient (iNOS−/−) mice. Values are mean ± SE (n = 5 in each group). *p<0.05 vs control; †p<0.05 between LPS wild-type and LPS iNOS−/− mice. (Adopted from Lush CW et al., Gastroenterology, 2003. Ref. 59).
Fig. 3.
Pretreatment of cultured endothelial cells (HUVEC) with peroxynitrite decomposition catalyst, FeTPPS for 1 h attenuates inflammatory response with respect to LPS-induced activation of nuclear factor κB, NFκB (EMSA) (A) and subsequent surface expression levels of E-selectin (B). (A) A representative EMSA image from 3 independent experiments. (B) Values are mean ± SE (n = 3). *p<0.05 compared to control. #p<0.05 compared to LPS-treated group. (Adopted from Lush CW et al., Gastroenterology, 2003, Ref. 59).
The oxidants produced in endothelial cells during sepsis also induce expression of tissue factor [30, 60]. This transmembrane glycoprotein (formerly known as thromboplastin) functions as the primary cellular initiator of blood coagulation in vivo [60]. Because endothelial cells and monocytes do not express tissue factor under physiological conditions, there is no appreciable contact of cellular tissue factor with the circulating blood. However, these cells do express tissue factor when exposed to inflammatory cytokines and this response contributes to coagulopathy in sepsis. When tissue factor is exposed to blood, it forms a high-affinity complex with coagulation factors, leading to the formation of an insoluble fibrin clot. Coagulation is activated rapidly after septic insult and results in diffuse microvascular clot formation that may disrupt blood flow in capillary beds [28, 30, 60]. NADPH oxidase-derived oxidants also stimulate the activation of the heterodimeric transcription factor hypoxia inducible factor-1 (HIF-1) in endothelial cells [61]. Normally the HIF-1α subunit has a short half-life because it is covalently modified by HIF-1 prolylhydroxylase, which targets HIF-1α for proteolysis by the ubiquitin-proteosome system. However, oxidants inhibit prolylhydroxylase activity and thereby allow HIF-1α to dimerize with the HIF-1β subunit. The dimerization activates HIF-1 to translocate to the nucleus, bind DNA and promote expression of iNOS and other inflammatory genes [62–64].
Potential Adjunct Therapy for Microvascular Dysfunction in Sepsis
Sepsis is treated by controlling the source of infection, administering antimicrobial (e.g., antibiotic) therapy, assuring hemodynamic support with fluid resuscitation and vasopressor drugs, inducing sedation or analgesia as needed; and providing adequate nutrition [65]. Despite these interventions, approximately one-third of all patients with severe sepsis die before leaving hospital [66]. Therefore, the development of additional therapies is a research topic of urgent priority.
Septic patients may benefit from adjunct therapies that target microvascular dysfunction. This hypothesis is based on the observation that many patients with sepsis die of organ failure despite adequate arterial blood oxygenation and cardiac output [29, 34, 35]. Further, survival is improved by treatments that improve microvascular function (e.g., antioxidants, iNOS deficiency, and tissue factor pathway inhibitor) in animal models of polymicrobial sepsis [33, 67–70]. Several potential therapies that target septic microvascular dysfunction are discussed next.
The administration of activated protein C (APC) to septic patients is a recently introduced therapy. APC exerts anticoagulant effects. Further, the adherence of leukocytes to arteriolar and venular endothelium is strongly diminished by activated protein C infusion, which is associated with an increased density of blood-perfused capillaries in an animal model of SIRS [71]. APC also induces rearrangement of endothelial cells’ actin cytoskeleton and thereby stabilizes endothelial barrier function [72]. Indeed, therapeutic benefit of APC in sepsis may be largely due to augmentation of endothelial barrier function [72].
Unfortunately, any benefit from APC may be outweighed by adverse effects, especially an increased rate of hemorrhage [73]. A recent Cochrane review recommended that APC should not be given to patients at low risk of death or to pediatric patients [74]. The review also concluded that the evidence supporting APC use in patients with severe sepsis and at high risk of death was very weak [74]. However, chemical variants of APC have been synthesized that may retain therapeutic efficacy while reducing the risk of bleeding. Experiments in mouse sepsis models, for example, show that survival is increased by a recombinant APC variant with normal cell signaling activity but <10% anticoagulant activity [75]. Therefore, a therapy may be developed in future that treats septic patients with APC variants that have normal cell signaling but reduced anticoagulant activities.
A second intervention that has been tested in critically ill patients is administration of the nonselective NOS inhibitor N(G)-methyl-l-arginine (L-NMMA). A phase III clinical trial of L-NMMA in patients with septic shock was terminated early because of increased mortality, but post hoc analysis indicated an overall survival benefit for low-dose L-NMMA [76]. The adverse effect of high-dose L-NMMA may have been due to inhibition of the eNOS necessary for microvascular homeostasis [77].
Vitamin C injection is also a candidate therapy [26, 78, 79]. In a randomized, prospective, double-blind, placebo-controlled trial with 226 critically ill patients, 28-day survival was increased in the patients who received combined vitamin C and vitamin E by i.v. infusion compared to those who did not [80]. Another large, randomized, prospective trial demonstrated decreased incidence of organ failure and shortened ICU stay for critically ill patients who began receiving i.v. injections of combined vitamin C (3 g/day for up to 28 days) and vitamin E within 24 h of traumatic injury or major surgery [81]. Yet another randomized, prospective study reported decreased morbidity for severely burned patients who were infused with an even higher dose of vitamin C (1584 mg/kg/day) [82].
Studies of animal models have shown that vitamin C prevents LPS-induced edema and hypotension [83, 84], prolongs survival in experimental bacteremia [85], and improves arteriolar responsiveness, arterial blood pressure, capillary blood flow, liver function and survival in experimental sepsis [26, 31–33, 86]. Therefore, the biological plausibility of this intervention is discussed further in the following paragraphs.
Vitamin C (ascorbic acid) dissociates to form ascorbate at physiological pH [79]. Ascorbate functions as an antioxidant and enzyme cofactor, becoming oxidized to dehydroascorbic acid (DHAA) in the process. SIRS and sepsis lower plasma ascorbate concentration [26, 31, 87–90]. Injection i.v. of vitamin C can increase the amount of ascorbate delivered to endothelial cells to a greater extent than can oral ingestion [79].
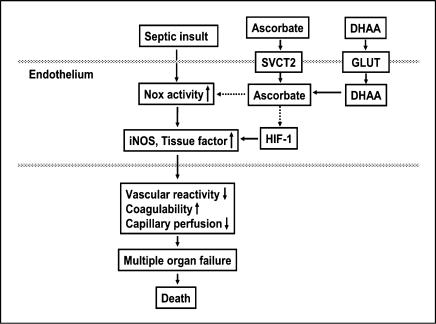
As depicted in Fig. 4, ascorbate is transported into microvascular endothelial cells by the specific transporter SVCT2, while DHAA is taken up through facilitative glucose transporters (GLUT) and then reduced to ascorbate [79, 91–93]. The intracellular concentrations of ascorbate thus achieved are 4–16 mM [37, 94]. This intracellular ascorbate may protect microvascular function in two phases of action: initially by inhibiting NADPH oxidase activation and increasing eNOS activity, and subsequently by suppressing expression of NADPH oxidase, iNOS and tissue factor (Fig. 4).
Fig. 4.
Proposed mechanisms by which ascorbate and DHAA may prevent microvascular dysfunction and death in sepsis. The dotted lines represent inhibition by ascorbate of NADPH oxidase (Nox) activity and HIF-1.
Ascorbate in endothelial cells prevents tetrahydrobiopterin oxidation, increases tetrahydrobiopterin content and elevates synthesis of nitric oxide by eNOS [95, 96]. It is important that ascorbate stimulates eNOS activity while inhibiting iNOS expression. Studies of endotoxin induced shock indicate that iNOS inhibition has little or no beneficial effect in the presence of a failing eNOS system, because some nitric oxide is needed to maintain adequate organ function [97].
An antioxidant role for vitamin C is supported by the observation that ascorbate abrogates stimulation by septic insult of superoxide production in microvascular endothelial cells [37]. Intracellular concentrations of ascorbate inside cells are high enough to prevent the reaction of superoxide and nitric oxide that creates peroxynitrite [98] and to re-reduce the oxidation products that peroxynitrite creates when it reacts with cellular components [99]. Intracellular ascorbate blocks induction by LPS + IFNγ or hydrogen peroxide of endothelial NADPH oxidase activity, NADPH oxidase protein expression and iNOS protein expression [37, 55, 56, 100]. Injection i.v. of vitamin C also diminishes iNOS mRNA expression in microvascular endothelial cells and arterioles in an in vivo model of sepsis [32, 33].
Ascorbate is required for the optimal activity of the proline and asparagine hydroxylases that control the transcription factor HIF-1 [101]. Thus, intracellular ascorbate lowers HIF-1 concentration to non-detectable levels [102]. Expression of HIF-1 sensitive genes, such as iNOS, is similarly inhibited by ascorbate [32, 33, 102].
Bolus i.v. injection of 2 g vitamin C in human subjects does not alter superoxide production by neutrophils [98]. Similarly, incubation of neutrophils with either ascorbic acid or DHAA, to raise intracellular ascorbate concentration, does not inhibit the production of extracellular ROS elicited when these cells are subsequently challenged by inflammatory stimuli such as E. coli [103, 104]. This is important because neutrophils use ROS to combat microbial pathogens.
There is cause for concern about potential adverse effects of high-dose vitamin C injections. Under certain conditions in vitro, ascorbic acid donates electrons to transition metals (e.g. iron) that then catalyze the synthesis of hydrogen peroxide [105, 106]. Injection i.v. of high-dose vitamin C is not followed by elevation of oxidative stress biomarkers in healthy human subjects [107], but its injection prior to surgery increases oxidative modification of plasma lipids in venous blood during the ischemic phase of surgery [108]. Because injection of high doses of vitamin C may increase the risk of hemolysis in glucose-6-phosphate dehydrogenase deficiency [109], patients should be prescreened for this deficiency before administering i.v. vitamin C. Also, because hyperoxaluric responses may occur [110] and predispose susceptible individuals to nephrolithiasis, oxalate measurements are recommended during long-term vitamin C therapy.
In conclusion, therapies targeting microvascular dysfunction may have a significant impact on morbidity and mortality in sepsis. Based on the low ascorbate concentrations in septic patients, the improved microvascular function and survival observed in septic animal models injected with vitamin C, the improved outcomes observed in critically ill patients administered i.v. vitamins C and E as adjuvant therapy, and the preservation of neutrophils’ capacity to produce ROS during exposure to supraphysiological extracellular ascorbate levels, clinical trials of vitamin C injection as an adjunct therapy in sepsis should be undertaken.
Acknowledgements
This work was supported by grants from the Heart and Stroke Foundation of Ontario (HSFO-NA5580 and HSFO-NA6171) (GC) and National Institutes of Health (NIH/NCCAM 1R01AT003643-01A2) (JXW).
Abbreviations
- APC
activated protein C
- DHAA
dehydroascorbic acid
- eNOS
endothelial nitric oxide synthase
- GLUT
glucose transporters
- HIF-1
hypoxia inducible factor-1
- ICAM-1
intercellular adhesion molecule 1
- IFNγ
interferon-γ
- iNOS
inducible nitric oxide synthase
- L-NMMA
N(G)-methyl-l-arginine
- LPS
lipopolysaccharide
- NADPH
nicotinamide adenine dinucleotide phosphate
- NFκB
nuclear factor kappa B
- NOS
nitric oxide synthase
- Nox
NADPH oxidase
- PMN
neutrophils
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SIRS
systemic inflammatory response syndrome
- VCAM-1
vascular cell adhesion molecule 1
References
- 1.O’Brien J.M., Jr., Ali N.A., Abraham E. Year in review in Critical Care, 2004: sepsis and multi-organ failure. Crit. Care. 2005;9:409–413. doi: 10.1186/cc3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cavaillon J.M., Annane D. Compartmentalization of the inflammatory response in sepsis and SIRS. J. Endotoxin Res. 2006;12:151–170. doi: 10.1179/096805106X102246. [DOI] [PubMed] [Google Scholar]
- 3.O’Brien J.M., Jr ., Ali N.A., Aberegg S.K., Abraham E. Sepsis. Am. J. Med. 2007;120:1012–1022. doi: 10.1016/j.amjmed.2007.01.035. [DOI] [PubMed] [Google Scholar]
- 4.Riedemann N.C., Guo R.F., Ward P.A. The enigma of sepsis. J. Clin. Invest. 2003;112:460–467. doi: 10.1172/JCI19523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jean-Baptiste E. Cellular mechanisms in sepsis. J. Intensive Care Med. 2007;22:63–72. doi: 10.1177/0885066606297123. [DOI] [PubMed] [Google Scholar]
- 6.Lush C.W., Kvietys P.R. Microvascular dysfunction in sepsis. Microcirculation. 2000;7:83–101. doi: 10.1038/sj.mn.7300096. [DOI] [PubMed] [Google Scholar]
- 7.Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420:885–891. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- 8.Abraham E., Singer M. Mechanisms of sepsis-induced organ dysfunction. Crit. Care Med. 2007;35:2408–2416. doi: 10.1097/01.ccm.0000282072.56245.91. [DOI] [PubMed] [Google Scholar]
- 9.Basit A., Reutershan J., Morris M.A., Solga M., Rose C.E., Jr., Ley K. ICAM-1 and LFA-1 play critical roles in LPS-induced neutrophil recruitment into the alveolar space. Am. J. Physiol. Lung Cell Mol. Physiol. 2006;291:L200–207. doi: 10.1152/ajplung.00346.2005. [DOI] [PubMed] [Google Scholar]
- 10.Cepinskas G., Savickiene J., Ionescu C.V., Kvietys P.R. PMN transendothelial migration decreases nuclear NFkappaB in IL-1beta-activated endothelial cells: role of PECAM-1. J. Cell Biol. 2003;161:641–651. doi: 10.1083/jcb.200212048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ley K., Reutershan J. Leucocyte-endothelial interactions in health and disease. Handb Exp. Pharmacol. 2006:97–133. doi: 10.1007/3-540-36028-x_4. [DOI] [PubMed] [Google Scholar]
- 12.Rawlingson A. Nitric oxide, inflammation and acute burn injury. Burns. 2003;29:631–640. doi: 10.1016/s0305-4179(03)00079-2. [DOI] [PubMed] [Google Scholar]
- 13.Razavi H.M., Wang L., Weicker S., Quinlan G.J., Mumby S., McCormack D.G., Mehta S. Pulmonary oxidant stress in murine sepsis is due to inflammatory cell nitric oxide. Crit. Care Med. 2005;33:1333–1339. doi: 10.1097/01.ccm.0000165445.48350.4f. [DOI] [PubMed] [Google Scholar]
- 14.Mochida S., Matsura T., Yamashita A., Horie S., Ohata S., Kusumoto C., Nishida T., Minami Y., Inagaki Y., Ishibe Y., Nakada J., Ohta Y., Yamada K. Geranylgeranylacetone Ameliorates Inflammatory Response to Lipopolysaccharide (LPS) in Murine Macrophages: Inhibition of LPS Binding to The Cell Surface. J. Clin. Biochem. Nutrition. 2007;41:115–123. doi: 10.3164/jcbn.2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riedemann N.C., Guo R.F., Ward P.A. Novel strategies for the treatment of sepsis. Nat. Med. 2003;9:517–524. doi: 10.1038/nm0503-517. [DOI] [PubMed] [Google Scholar]
- 16.Liu L., Kubes P. Molecular mechanisms of leukocyte recruitment: organ-specific mechanisms of action. Thromb Haemost. 2003;89:213–220. [PubMed] [Google Scholar]
- 17.Liu S.F., Malik A.B. NF-kappa B activation as a pathological mechanism of septic shock and inflammation. Am. J. Physiol. Lung Cell Mol. Physiol. 2006;290:L622–L645. doi: 10.1152/ajplung.00477.2005. [DOI] [PubMed] [Google Scholar]
- 18.Abraham E. Nuclear factor-kappaB and its role in sepsis-associated organ failure. J. Infect Dis. 2003;187:S364–369. doi: 10.1086/374750. Suppl. 2, [DOI] [PubMed] [Google Scholar]
- 19.Rao R.M., Yang L., Garcia-Cardena G., Luscinskas F.W. Endothelial-dependent mechanisms of leukocyte recruitment to the vascular wall. Circ. Res. 2007;101:234–247. doi: 10.1161/CIRCRESAHA.107.151860b. [DOI] [PubMed] [Google Scholar]
- 20.Janssen-Heininger Y.M., Poynter M.E., Baeuerle P.A. Recent advances towards understanding redox mechanisms in the activation of nuclear factor kappaB. Free Radic. Biol. Med. 2000;28:1317–1327. doi: 10.1016/s0891-5849(00)00218-5. [DOI] [PubMed] [Google Scholar]
- 21.Panacek E.A., Marshall J.C., Albertson T.E., Johnson D.H., Johnson S., MacArthur R.D., Miller M., Barchuk W.T., Fischkoff S., Kaul M., Teoh L., Van Meter L., Daum L., Lemeshow S., Hicklin G., Doig C. Efficacy and safety of the monoclonal anti-tumor necrosis factor antibody F(ab')2 fragment afelimomab in patients with severe sepsis and elevated interleukin-6 levels. Crit. Care Med. 2004;32:2173–2182. doi: 10.1097/01.ccm.0000145229.59014.6c. [DOI] [PubMed] [Google Scholar]
- 22.Bernard G.R., Vincent J.L., Laterre P.F., LaRosa S.P., Dhainaut J.F., Lopez-Rodriguez A., Steingrub J.S., Garber G.E., Helterbrand J.D., Ely E.W., Fisher C.J., Jr. Efficacy and safety of recombinant human activated protein C for severe sepsis. N. Engl. J. Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 23.Abraham E. Effects of recombinant human activated protein C in human models of endotoxin administration. Proc. Am. Thorac. Soc. 2005;2:243–247. doi: 10.1513/pats.200501-004AC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ely E.W., Bernard G.R., Vincent J.L. Activated protein C for severe sepsis. N. Engl. J. Med. 2002;347:1035–1036. doi: 10.1056/NEJM200209263471315. [DOI] [PubMed] [Google Scholar]
- 25.Sumpio B.E., Riley J.T., Dardik A. Cells in focus: endothelial cell. Int. J. Biochem. Cell Biol. 2002;34:1508–1512. doi: 10.1016/s1357-2725(02)00075-4. [DOI] [PubMed] [Google Scholar]
- 26.Armour J., Tyml K., Lidington D., Wilson J.X. Ascorbate prevents microvascular dysfunction in the skeletal muscle of the septic rat. J. Appl. Physiol. 2001;90:795–803. doi: 10.1152/jappl.2001.90.3.795. [DOI] [PubMed] [Google Scholar]
- 27.Goldman D., Bateman R.M., Ellis C.G. Effect of sepsis on skeletal muscle oxygen consumption and tissue oxygenation: interpreting capillary oxygen transport data using a mathematical model. Am. J. Physiol. Heart Circ. Physiol. 2004;287:H2535–2544. doi: 10.1152/ajpheart.00889.2003. [DOI] [PubMed] [Google Scholar]
- 28.Laudes I.J., Chu J.C., Sikranth S., Huber-Lang M., Guo R.F., Riedemann N., Sarma J.V., Schmaier A.H., Ward P.A. Anti-c5a ameliorates coagulation/fibrinolytic protein changes in a rat model of sepsis. Am. J. Pathol. 2002;160:1867–1875. doi: 10.1016/S0002-9440(10)61133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakr Y., Dubois M.J., De Backer D., Creteur J., Vincent J.L. Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit. Care Med. 2004;32:1825–1831. doi: 10.1097/01.ccm.0000138558.16257.3f. [DOI] [PubMed] [Google Scholar]
- 30.Salvemini D., Cuzzocrea S. Oxidative stress in septic shock and disseminated intravascular coagulation. Free Radic. Biol. Med. 2002;33:1173–1185. doi: 10.1016/s0891-5849(02)00961-9. [DOI] [PubMed] [Google Scholar]
- 31.Tyml K., Li F., Wilson J.X. Delayed ascorbate bolus protects against maldistribution of microvascular blood flow in septic rat skeletal muscle. Crit. Care Med. 2005;33:1823–1828. doi: 10.1097/01.ccm.0000172548.34622.de. [DOI] [PubMed] [Google Scholar]
- 32.Wu F., Wilson J.X., Tyml K. Ascorbate inhibits iNOS expression and preserves vasoconstrictor responsiveness in skeletal muscle of septic mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;285:R50–56. doi: 10.1152/ajpregu.00564.2002. [DOI] [PubMed] [Google Scholar]
- 33.Wu F., Wilson J.X., Tyml K. Ascorbate protects against impaired arteriolar constriction in sepsis by inhibiting inducible nitric oxide synthase expression. Free Radic. Biol. Med. 2004;37:1282–1289. doi: 10.1016/j.freeradbiomed.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 34.Poeze M., Greve J.W., Ramsay G. Meta-analysis of hemodynamic optimization: relationship to methodological quality. Crit. Care. 2005;9:R771–779. doi: 10.1186/cc3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rivers E., Nguyen B., Havstad S., Ressler J., Muzzin A., Knoblich B., Peterson E., Tomlanovich M. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N. Engl. J. Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 36.Li J.M., Shah A.M. Endothelial cell superoxide generation: regulation and relevance for cardiovascular pathophysiology. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;287:R1014–1030. doi: 10.1152/ajpregu.00124.2004. [DOI] [PubMed] [Google Scholar]
- 37.Wu F., Schuster D.P., Tyml K., Wilson J.X. Ascorbate inhibits NADPH oxidase subunit p47phox expression in microvascular endothelial cells. Free Radic. Biol. Med. 2007;42:124–131. doi: 10.1016/j.freeradbiomed.2006.10.033. [DOI] [PubMed] [Google Scholar]
- 38.Cepinskas G., Rui T., Kvietys P.R. Interaction between reactive oxygen metabolites and nitric oxide in oxidant tolerance. Free Radic. Biol. Med. 2002;33:433–440. doi: 10.1016/s0891-5849(02)00962-0. [DOI] [PubMed] [Google Scholar]
- 39.Jacobi J., Kristal B., Chezar J., Shaul S.M., Sela S. Exogenous superoxide mediates pro-oxidative, proinflammatory, and procoagulatory changes in primary endothelial cell cultures. Free Radic. Biol. Med. 2005;39:1238–1248. doi: 10.1016/j.freeradbiomed.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 40.Frein D., Schildknecht S., Bachschmid M., Ullrich V. Redox regulation: a new challenge for pharmacology. Biochem. Pharmacol. 2005;70:811–823. doi: 10.1016/j.bcp.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 41.Andrew P.J., Mayer B. Enzymatic function of nitric oxide synthases. Cardiovasc. Res. 1999;43:521–531. doi: 10.1016/s0008-6363(99)00115-7. [DOI] [PubMed] [Google Scholar]
- 42.Boulden B.M., Widder J.D., Allen J.C., Smith D.A., Al-Baldawi R.N., Harrison D.G., Dikalov S. I., Jo H., Dudley S.C., Jr. Early determinants of H2O2-induced endothelial dysfunction. Free Radic. Biol. Med. 2006;41:810–817. doi: 10.1016/j.freeradbiomed.2006.05.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cerwinka W.H., Cooper D., Krieglstein C.F., Feelisch M., Granger D.N. Nitric oxide modulates endotoxin-induced platelet-endothelial cell adhesion in intestinal venules. Am. J. Physiol. Heart Circ. Physiol. 2002;282:H1111–1117. doi: 10.1152/ajpheart.00391.2001. [DOI] [PubMed] [Google Scholar]
- 44.Cerwinka W.H., Cooper D., Krieglstein C.F., Ross C.R., McCord J.M., Granger D.N. Superoxide mediates endotoxin-induced platelet-endothelial cell adhesion in intestinal venules. Am. J. Physiol. Heart Circ. Physiol. 2003;284:H535–541. doi: 10.1152/ajpheart.00311.2002. [DOI] [PubMed] [Google Scholar]
- 45.Eichelbronner O., Sielenkamper A., Cepinskas G., Sibbald W.J., Chin-Yee I.H. Endotoxin promotes adhesion of human erythrocytes to human vascular endothelial cells under conditions of flow. Crit. Care Med. 2000;28:1865–1870. doi: 10.1097/00003246-200006000-00030. [DOI] [PubMed] [Google Scholar]
- 46.Singer G., Urakami H., Specian R.D., Stokes K.Y., Granger D.N. Platelet recruitment in the murine hepatic microvasculature during experimental sepsis: role of neutrophils. Microcirculation. 2006;13:89–97. doi: 10.1080/10739680500466343. [DOI] [PubMed] [Google Scholar]
- 47.Marx G. Fluid therapy in sepsis with capillary leakage. Eur. J. Anaesthesiol. 2003;20:429–442. doi: 10.1017/s0265021503000681. [DOI] [PubMed] [Google Scholar]
- 48.Cirino G., Fiorucci S., Sessa W.C. Endothelial nitric oxide synthase: the Cinderella of inflammation? Trends Pharmacol. Sci. 2003;24:91–95. doi: 10.1016/S0165-6147(02)00049-4. [DOI] [PubMed] [Google Scholar]
- 49.Hutcheson I.R., Whittle B.J., Boughton-Smith N.K. Role of nitric oxide in maintaining vascular integrity in endotoxin-induced acute intestinal damage in the rat. Br. J. Pharmacol. 1990;101:815–820. doi: 10.1111/j.1476-5381.1990.tb14163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wong D., Dorovini-Zis K., Vincent S.R. Cytokines, nitric oxide, and cGMP modulate the permeability of an in vitro model of the human blood-brain barrier. Exp. Neurol. 2004;190:446–455. doi: 10.1016/j.expneurol.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 51.Yang B., Rizzo V. TNF-alpha potentiates protein-tyrosine nitration through activation of NADPH oxidase and eNOS localized in membrane rafts and caveolae of bovine aortic endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H954–962. doi: 10.1152/ajpheart.00758.2006. [DOI] [PubMed] [Google Scholar]
- 52.Gertzberg N., Neumann P., Rizzo V., Johnson A. NAD(P)H oxidase mediates the endothelial barrier dysfunction induced by TNF-alpha. Am. J. Physiol. Lung Cell Mol. Physiol. 2004;286:L37–48. doi: 10.1152/ajplung.00116.2003. [DOI] [PubMed] [Google Scholar]
- 53.Knepler J.L., Jr ., Taher L.N., Gupta M.P., Patterson C., Pavalko F., Ober M.D., Hart C.M. Peroxynitrite causes endothelial cell monolayer barrier dysfunction. American Journal of Physiology. 2001;281:C1064–1075. doi: 10.1152/ajpcell.2001.281.3.C1064. [DOI] [PubMed] [Google Scholar]
- 54.Neumann P., Gertzberg N., Vaughan E., Weisbrot J., Woodburn R., Lambert W., Johnson A. Peroxynitrite mediates TNF-alpha-induced endothelial barrier dysfunction and nitration of actin. Am. J. Physiol. Lung Cell Mol. Physiol. 2006;290:L674–L684. doi: 10.1152/ajplung.00391.2005. [DOI] [PubMed] [Google Scholar]
- 55.Wu F., Cepinskas G., Wilson J.X., Tyml K. Nitric oxide attenuates but superoxide enhances iNOS expression in endotoxin- and IFNgamma-stimulated skeletal muscle endothelial cells. Microcirculation. 2001;8:415–425. doi: 10.1038/sj/mn/7800113. [DOI] [PubMed] [Google Scholar]
- 56.Wu F., Tyml K., Wilson JX. Ascorbate inhibits iNOS induction in microvascular endothelial cells through inhibiting NADPH oxidase activity. Faseb J. 2005;19:A507. [Google Scholar]
- 57.Yu H.P., Lui P.W., Hwang T.L., Yen C.H., Lau Y.T. Propofol improves endothelial dysfunction and attenuates vascular superoxide production in septic rats. Crit. Care Med. 2006;34:453–460. doi: 10.1097/01.ccm.0000198530.68343.21. [DOI] [PubMed] [Google Scholar]
- 58.Stoclet J.C., Martinez M.C., Ohlmann P., Chasserot S., Schott C., Kleschyov A.L., Schneider F., Andriantsitohaina R. Induction of nitric oxide synthase and dual effects of nitric oxide and cyclooxygenase products in regulation of arterial contraction in human septic shock. Circulation. 1999;100:107–112. doi: 10.1161/01.cir.100.2.107. [DOI] [PubMed] [Google Scholar]
- 59.Lush C.W., Cepinskas G., Kvietys P.R. Regulation of intestinal nuclear factor-kappaB activity and E-selectin expression during sepsis: a role for peroxynitrite. Gastroenterology. 2003;124:118–128. doi: 10.1053/gast.2003.50001. [DOI] [PubMed] [Google Scholar]
- 60.Steffel J., Luscher T.F., Tanner F.C. Tissue factor in cardiovascular diseases: molecular mechanisms and clinical implications. Circulation. 2006;113:722–731. doi: 10.1161/CIRCULATIONAHA.105.567297. [DOI] [PubMed] [Google Scholar]
- 61.Wartenberg M., Hoffmann E., Schwindt H., Grunheck F., Petros J., Arnold J.R., Hescheler J., Sauer H. Reactive oxygen species-linked regulation of the multidrug resistance transporter P-glycoprotein in Nox-1 overexpressing prostate tumor spheroids. FEBS Lett. 2005;579:4541–4549. doi: 10.1016/j.febslet.2005.06.078. [DOI] [PubMed] [Google Scholar]
- 62.Haddad J.J., Land S.C. A non-hypoxic, ROS-sensitive pathway mediates TNF-alpha-dependent regulation of HIF-1alpha. FEBS Lett. 2001;505:269–274. doi: 10.1016/s0014-5793(01)02833-2. [DOI] [PubMed] [Google Scholar]
- 63.Hellwig-Burgel T., Rutkowski K., Metzen E., Fandrey J., Jelkmann W. Interleukin-1beta and tumor necrosis factor-alpha stimulate DNA binding of hypoxia-inducible factor-1. Blood. 1999;94:1561–1567. [PubMed] [Google Scholar]
- 64.Natarajan R., Salloum F.N., Fisher B.J., Kukreja R.C., Fowler A.A., 3rd Hypoxia inducible factor-1 activation by prolyl 4-hydroxylase-2 gene silencing attenuates myocardial ischemia reperfusion injury. Circ. Res. 2006;98:133–140. doi: 10.1161/01.RES.0000197816.63513.27. [DOI] [PubMed] [Google Scholar]
- 65.Dellinger R.P., Carlet J.M., Masur H., Gerlach H., Calandra T., Cohen J., Gea-Banacloche J., Keh D., Marshall J.C., Parker M.M., Ramsay G., Zimmerman J.L., Vincent J.L., Levy M.M. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit. Care Med. 2004;32:858–873. doi: 10.1097/01.ccm.0000117317.18092.e4. [DOI] [PubMed] [Google Scholar]
- 66.Rice T.W., Bernard G.R. Therapeutic intervention and targets for sepsis. Annu. Rev. Med. 2005;56:225–248. doi: 10.1146/annurev.med.56.082103.104356. [DOI] [PubMed] [Google Scholar]
- 67.Hollenberg S.M., Broussard M., Osman J., Parrillo J.E. Increased microvascular reactivity and improved mortality in septic mice lacking inducible nitric oxide synthase. Circ. Res. 2000;86:774–778. doi: 10.1161/01.res.86.7.774. [DOI] [PubMed] [Google Scholar]
- 68.Opal S.M., Palardy J.E., Parejo N.A., Creasey A.A. The activity of tissue factor pathway inhibitor in experimental models of superantigen-induced shock and polymicrobial intra-abdominal sepsis. Crit. Care Med. 2001;29:13–17. doi: 10.1097/00003246-200101000-00003. [DOI] [PubMed] [Google Scholar]
- 69.Pearce R.A., Finley R.J., Mustard R.A., Jr., Duff J.H. 2,3-Dihydroxybenzoic acid. Effect on mortality rate in a septic rat model. Arch. Surg. 1985;120:937–940. doi: 10.1001/archsurg.1985.01390320061012. [DOI] [PubMed] [Google Scholar]
- 70.van Veen S.Q., Levi M., van Vliet A.K., Florquin S., van Gulik T.M., Boermeester M.A. Peritoneal lavage with activated protein C alters compartmentalized coagulation and fibrinolysis and improves survival in polymicrobial peritonitis. Crit. Care Med. 2006;34:2799–2805. doi: 10.1097/01.CCM.0000243795.04284.2A. [DOI] [PubMed] [Google Scholar]
- 71.Hoffmann J.N., Vollmar B., Laschke M.W., Inthorn D., Fertmann J., Schildberg F.W., Menger M.D. Microhemodynamic and cellular mechanisms of activated protein C action during endotoxemia. Crit. Care Med. 2004;32:1011–1017. doi: 10.1097/01.ccm.0000120058.88975.42. [DOI] [PubMed] [Google Scholar]
- 72.Jacobson J.R., Garcia J.G. Novel therapies for microvascular permeability in sepsis. Curr. Drug Targets. 2007;8:509–514. doi: 10.2174/138945007780362719. [DOI] [PubMed] [Google Scholar]
- 73.Baillie J.K. Activated protein C: controversy and hope in the treatment of sepsis. Curr. Opin. Investig. Drugs. 2007;8:933–938. [PubMed] [Google Scholar]
- 74.Marti-Carvajal A., Salanti G., Cardona A.F. Human recombinant activated protein C for severe sepsis. Cochrane Database Syst. Rev. 2007:CD004388. doi: 10.1002/14651858.CD004388.pub2. [DOI] [PubMed] [Google Scholar]
- 75.Kerschen E.J., Fernandez J.A., Cooley B.C., Yang X.V., Sood R., Mosnier L.O., Castellino F.J., Mackman N., Griffin J.H., Weiler H. Endotoxemia and sepsis mortality reduction by non-anticoagulant activated protein C. J. Exp. Med. 2007;204:2439–2448. doi: 10.1084/jem.20070404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lopez A., Lorente J.A., Steingrub J., Bakker J., McLuckie A., Willatts S., Brockway M., Anzueto A., Holzapfel L., Breen D., Silverman M.S., Takala J., Donaldson J., Arneson C., Grove G., Grossman S., Grover R. Multiple-center, randomized, placebo-controlled, double-blind study of the nitric oxide synthase inhibitor 546C88: effect on survival in patients with septic shock. Crit. Care Med. 2004;32:21–30. doi: 10.1097/01.CCM.0000105581.01815.C6. [DOI] [PubMed] [Google Scholar]
- 77.Wang W., Mitra A., Poole B., Falk S., Lucia M.S., Tayal S., Schrier R. Endothelial nitric oxide synthase-deficient mice exhibit increased susceptibility to endotoxin-induced acute renal failure. Am. J. Physiol. Renal. Physiol. 2004;287:F1044–1048. doi: 10.1152/ajprenal.00136.2004. [DOI] [PubMed] [Google Scholar]
- 78.Lehr H.A., Germann G., McGregor G.P., Migeod F., Roesen P., Tanaka H., Uhlig C., Biesalski H.K. Consensus meeting on “Relevance of parenteral vitamin C in acute endothelial dependent pathophysiological conditions (EDPC)”. Eur. J. Med. Res. 2006;11:516–526. [PubMed] [Google Scholar]
- 79.Wilson J.X. Regulation of vitamin C transport. Annu. Rev. Nutr. 2005;25:105–125. doi: 10.1146/annurev.nutr.25.050304.092647. [DOI] [PubMed] [Google Scholar]
- 80.Crimi E., Liguori A., Condorelli M., Cioffi M., Astuto M., Bontempo P., Pignalosa O., Vietri M.T., Molinari A.M., Sica V., Della Corte F., Napoli C. The beneficial effects of antioxidant supplementation in enteral feeding in critically ill patients: a prospective, randomized, double-blind, placebo-controlled trial. Anesth. Analg. 2004;99:857–863. doi: 10.1213/01.ANE.0000133144.60584.F6. [DOI] [PubMed] [Google Scholar]
- 81.Nathens A.B., Neff M.J., Jurkovich G.J., Klotz P., Farver K., Ruzinski J.T., Radella F., Garcia I., Maier R.V. Randomized, prospective trial of antioxidant supplementation in critically ill surgical patients. Ann. Surg. 2002;236:814–822. doi: 10.1097/00000658-200212000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tanaka H., Matsuda T., Miyagantani Y., Yukioka T., Matsuda H., Shimazaki S. Reduction of resuscitation fluid volumes in severely burned patients using ascorbic acid administration: a randomized, prospective study. Arch. Surg. 2000;135:326–331. doi: 10.1001/archsurg.135.3.326. [DOI] [PubMed] [Google Scholar]
- 83.Feng N.H., Chu S.J., Wang D., Hsu K., Lin C.H., Lin H.I. Effects of various antioxidants on endotoxin-induced lung injury and gene expression: mRNA expressions of MnSOD, interleukin-1beta and iNOS. Chin. J. Physiol. 2004;47:111–120. [PubMed] [Google Scholar]
- 84.Shen K.P., Lo Y.C., Yang R.C., Liu H.W., Chen I.J., Wu B.N. Antioxidant eugenosedin-A protects against lipopolysaccharide-induced hypotension, hyperglycaemia and cytokine immunoreactivity in rats and mice. J. Pharm. Pharmacol. 2005;57:117–125. doi: 10.1211/0022357055137. [DOI] [PubMed] [Google Scholar]
- 85.Gaut J.P., Belaaouaj A., Byun J., Roberts L.J., 2nd, Maeda. N., Frei B., Heinecke J.W. Vitamin C fails to protect amino acids and lipids from oxidation during acute inflammation. Free Radic. Biol. Med. 2006;40:1494–1501. doi: 10.1016/j.freeradbiomed.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 86.Kim J.Y., Lee S.M. Vitamins C and E protect hepatic cytochrome P450 dysfunction induced by polymicrobial sepsis. Eur. J. Pharmacol. 2006;534:202–209. doi: 10.1016/j.ejphar.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 87.Borrelli E., Roux-Lombard P., Grau G.E., Girardin E., Ricou B., Dayer J., Suter P.M. Plasma concentrations of cytokines, their soluble receptors, and antioxidant vitamins can predict the development of multiple organ failure in patients at risk. Crit. Care Med. 1996;24:392–397. doi: 10.1097/00003246-199603000-00006. [DOI] [PubMed] [Google Scholar]
- 88.Galley H.F., Davies M.J., Webster N.R. Ascorbyl radical formation in patients with sepsis: effect of ascorbate loading. Free Radic. Biol. Med. 1996;20:139–143. doi: 10.1016/0891-5849(95)02022-5. [DOI] [PubMed] [Google Scholar]
- 89.Long C.L., Maull K.I., Krishnan R.S., Laws H.L., Geiger J.W., Borghesi L., Franks W., Lawson T.C., Sauberlich H.E. Ascorbic acid dynamics in the seriously ill and injured. J. Surg. Res. 2003;109:144–148. doi: 10.1016/s0022-4804(02)00083-5. [DOI] [PubMed] [Google Scholar]
- 90.Rumelin A., Humbert T., Luhker O., Drescher A., Fauth U. Metabolic clearance of the antioxidant ascorbic acid in surgical patients. J. Surg. Res. 2005;129:46–51. doi: 10.1016/j.jss.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 91.Best K.A., Holmes M.E., Samson S.E., Mwanjewe J., Wilson J.X., Dixon S.J., Grover A.K. Ascorbate uptake in pig coronary artery endothelial cells. Mol. Cell Biochem. 2005;271:43–49. doi: 10.1007/s11010-005-3442-0. [DOI] [PubMed] [Google Scholar]
- 92.Davis K.A., Samson S.E., Best K., Mallhi K.K., Szewczyk M., Wilson J.X., Kwan C.Y., Grover A.K. Ca2+-mediated ascorbate release from coronary artery endothelial cells. Br. J. Pharmacol. 2006;147:131–139. doi: 10.1038/sj.bjp.0706492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Davis K.A., Samson S.E., Wilson J.X., Grover A.K. Hypotonic shock stimulates ascorbate release from coronary artery endothelial cells by a Ca2+-independent pathway. Eur. J. Pharmacol. 2006;548:36–44. doi: 10.1016/j.ejphar.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 94.Wilson J.X., Dixon S.J., Yu J., Nees S., Tyml K. Ascorbate uptake by microvascular endothelial cells of rat skeletal muscle. Microcirculation. 1996;3:211–221. doi: 10.3109/10739689609148290. [DOI] [PubMed] [Google Scholar]
- 95.Kim H.J., Lee S.I., Lee D.H., Smith D., Jo H., Schellhorn H.E., Boo Y.C. Ascorbic acid synthesis due to L-gulono-1,4-lactone oxidase expression enhances NO production in endothelial cells. Biochem. Biophys. Res. Commun. 2006;345:1657–1662. doi: 10.1016/j.bbrc.2006.05.090. [DOI] [PubMed] [Google Scholar]
- 96.Smith A.R., Visioli F., Hagen T.M. Vitamin C matters: increased oxidative stress in cultured human aortic endothelial cells without supplemental ascorbic acid. Faseb J. 2002;16:1102–1104. doi: 10.1096/fj.01-0825fje. [DOI] [PubMed] [Google Scholar]
- 97.Cuzzocrea S., Mazzon E., Di Paola R., Esposito E., Macarthur H., Matuschak G.M., Salvemini D. A role for nitric oxide-mediated peroxynitrite formation in a model of endotoxin-induced shock. J. Pharmacol. Exp. Ther. 2006;319:73–81. doi: 10.1124/jpet.106.108100. [DOI] [PubMed] [Google Scholar]
- 98.Jackson T.S., Xu A., Vita J.A., Keaney J.F., Jr. Ascorbate prevents the interaction of superoxide and nitric oxide only at very high physiological concentrations. Circ. Res. 1998;83:916–922. doi: 10.1161/01.res.83.9.916. [DOI] [PubMed] [Google Scholar]
- 99.Kirsch M., de Groot H. Ascorbate is a potent antioxidant against peroxynitrite-induced oxidation reactions. Evidence that ascorbate acts by re-reducing substrate radicals produced by peroxynitrite. J. Biol. Chem. 2000;275:16702–16708. doi: 10.1074/jbc.M909228199. [DOI] [PubMed] [Google Scholar]
- 100.Wu F., Tyml K., Wilson J.X. Ascorbate inhibits iNOS expression in endotoxin- and IFN gamma-stimulated rat skeletal muscle endothelial cells. FEBS Lett. 2002;520:122–126. doi: 10.1016/s0014-5793(02)02804-1. [DOI] [PubMed] [Google Scholar]
- 101.Knowles H.J., Raval R.R., Harris A.L., Ratcliffe P.J. Effect of ascorbate on the activity of hypoxia-inducible factor in cancer cells. Cancer Res. 2003;63:1764–1768. [PubMed] [Google Scholar]
- 102.Vissers M.C., Gunningham S.P., Morrison M.J., Dachs G.U., Currie M.J. Modulation of hypoxia-inducible factor-1 alpha in cultured primary cells by intracellular ascorbate. Free Radic. Biol. Med. 2007;42:765–772. doi: 10.1016/j.freeradbiomed.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 103.Carr A.C., Frei B. Human neutrophils oxidize low-density lipoprotein by a hypochlorous acid-dependent mechanism: the role of vitamin C. Biol. Chem. 2002;383:627–636. doi: 10.1515/BC.2002.065. [DOI] [PubMed] [Google Scholar]
- 104.Sharma P., Raghavan S.A., Saini R., Dikshit M. Ascorbate-mediated enhancement of reactive oxygen species generation from polymorphonuclear leukocytes: modulatory effect of nitric oxide. J. Leukoc. Biol. 2004;75:1070–1078. doi: 10.1189/jlb.0903415. [DOI] [PubMed] [Google Scholar]
- 105.Barton J.C., McDonnell S.M., Adams P.C., Brissot P., Powell L.W., Edwards C.Q., Cook J.D., Kowdley K.V. Management of hemochromatosis. Hemochromatosis Management Working Group. Ann. Intern Med. 1998;129:932–939. doi: 10.7326/0003-4819-129-11_part_2-199812011-00003. [DOI] [PubMed] [Google Scholar]
- 106.Chen Q., Espey M.G., Krishna M.C., Mitchell J.B., Corpe C.P., Buettner G.R., Shacter E., Levine M. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: action as a pro-drug to deliver hydrogen peroxide to tissues. Proc. Natl. Acad. Sci. U. S. A. 2005;102:13604–13609. doi: 10.1073/pnas.0506390102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Muhlhofer A., Mrosek S., Schlegel B., Trommer W., Rozario F., Bohles H., Schremmer D., Zoller W.G., Biesalski H.K. High-dose intravenous vitamin C is not associated with an increase of pro-oxidative biomarkers. Eur. J. Clin. Nutr. 2004;58:1151–1158. doi: 10.1038/sj.ejcn.1601943. [DOI] [PubMed] [Google Scholar]
- 108.Bailey D.M., Raman S., McEneny J., Young I.S., Parham K.L., Hullin D.A., Davies B., McKeeman G., McCord J.M., Lewis M.H. Vitamin C prophylaxis promotes oxidative lipid damage during surgical ischemia-reperfusion. Free Radic. Biol. Med. 2006;40:591–600. doi: 10.1016/j.freeradbiomed.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 109.Rees D.C., Kelsey H., Richards J.D. Acute haemolysis induced by high dose ascorbic acid in glucose-6-phosphate dehydrogenase deficiency. BMJ. 1993;306:841–842. doi: 10.1136/bmj.306.6881.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pena de la Vega L., Lieske J.C., Milliner D., Gonyea J., Kelly D.G. Urinary oxalate excretion increases in home parenteral nutrition patients on a higher intravenous ascorbic acid dose. J. Parenter. Enteral. Nutr. 2004;28:435–438. doi: 10.1177/0148607104028006435. [DOI] [PubMed] [Google Scholar]